Background: Phosphorylation and acetylation are ubiquitous post-translational modifications of bacterial proteins.
Results: Glucose-induced cpxP transcription requires acetyl phosphate. This activity is inhibited by lysine 291 of the RNA polymerase α subunit, which becomes acetylated under inhibitory conditions.
Conclusion: Phosphorylation and acetylation co-regulate the cpxP promoter.
Significance: Central metabolism is implicated in the regulation of the stress-responsive promoter cpxP.
Keywords: Acetyl Coenzyme A, Bacterial Signal Transduction, Bacterial transcription, Phosphorylation, RNA Polymerase, CpxAR, Phos-Tag, Acetyl Phosphate, Central Metabolism, Lysine Acetylation
Abstract
The ability of bacteria to adapt to environmental changes has allowed these organisms to thrive in all parts of the globe. By monitoring their extracellular and intracellular environments, bacteria assure their most appropriate response for each environment. Post-translational modification of proteins is one mechanism by which cells respond to their changing environments. Here, we report that two post-translational modifications regulate transcription of the extracytoplasmic stress-responsive promoter cpxP: (i) acetyl phosphate-dependent phosphorylation of the response regulator CpxR and (ii) acetyl coenzyme A-dependent acetylation of the α subunit of RNA polymerase. Together, these two post-translational modifications fine-tune cpxP transcription in response to changes in the intracellular environment.
Introduction
The response regulator CpxR and its cognate sensor kinase CpxA constitute the two-component signal transduction pathway CpxAR (Fig. 1A). This signal pathway is highly conserved in Enterobacteriaceae and can be found in other proteobacteria. In Escherichia coli, the CpxAR pathway regulates transcription of at least 50 genes (1, 2). The CpxAR pathway also has been implicated in regulation of some virulence factors. In enteropathogenic E. coli and in Legionella pneumophila, the pathway regulates the type IV bundle-forming pilus and some components of type IV secretion, respectively (reviewed in Ref. 3). In Haemophilus ducreyi, it regulates proteins involved in resistance to phagocytosis, affecting the ability of H. ducreyi to infect humans (4).
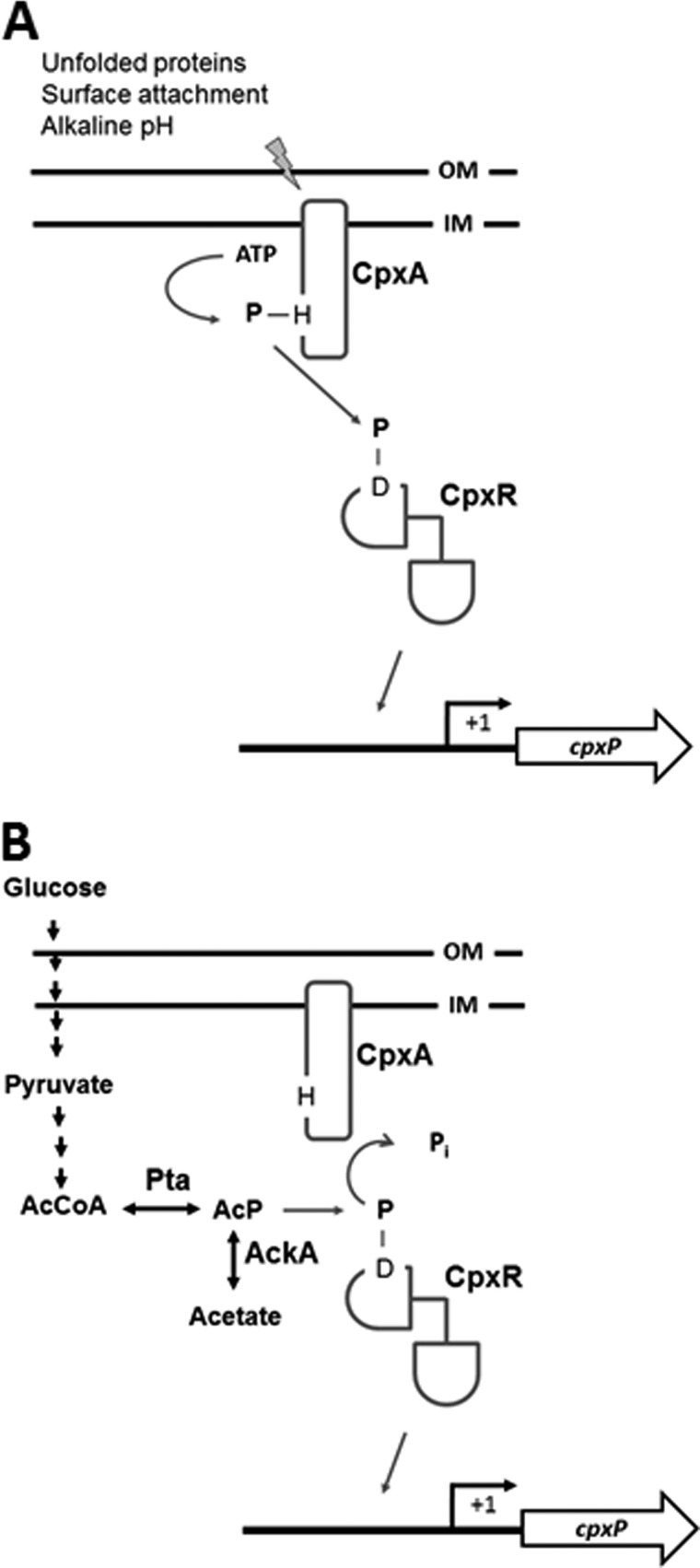
FIGURE 1.
The two-component response regulator CpxR can become activated by two independent mechanisms. A, activation by the cytoplasmic membrane sensor kinase CpxA. Upon activation by diverse extracytoplasmic signals, CpxA autophosphorylates on a conserved histidine residue, using ATP as its phosphoryl donor. Phospho-CpxA then acts as a phosphoryl donor to the response regulator CpxR, which autophosphorylates on the conserved aspartate residue (Asp-51). By homology to OmpR, phosphorylation of CpxR is predicted to expose its DNA-binding domain, which promotes binding to its target genes (2, 6). B, activation by AcP. In the absence of extracytoplasmic stimuli, CpxA functions as a net phosphatase, removing inorganic phosphate (Pi) from CpxR-P. Under these conditions, CpxR can become phosphorylated using as its phosphoryl donor AcP, the intermediate of the Pta-AckA pathway (5, 8). Phosphotransacetylase (Pta) converts acetyl-coenzyme A (AcCoA) to AcP, whereas acetate kinase (AckA) converts AcP to acetate (44). Bent arrow, cpxP promoter. +1, transcription initiation site.
In response to certain extracytoplasmic signals (e.g. alkaline pH, outer and inner membrane perturbation, surface attachment, or misfolded proteins) (reviewed in Ref. 3), CpxA is thought to autophosphorylate on a conserved histidine residue (His-248), using ATP as its phosphoryl donor. Phosphorylated CpxA then donates its phosphoryl group to a conserved aspartate residue (Asp-51) of CpxR. The latter phosphorylation event regulates CpxR-dependent transcription (5–7) (Fig. 1A).
In the absence of such extracytoplasmic cues (for example, during growth in tryptone broth buffered at pH 7), CpxA functions as an inhibitor of the Cpx pathway. This inhibition is thought to be accomplished by the removal of phosphoryl groups from phospho-CpxR (Fig. 1B) (7, 8), an activity that has been demonstrated in vitro (9, 10). In vivo, the existence of this activity is supported by the apparent accumulation of phospho-CpxR by cpxA mutants of Yersinia pseudotuberculosis (11).
Under such conditions (Fig. 1B), acetyl phosphate (AcP),2 the intermediate of the Pta-AckA pathway, has been proposed to be the phosphoryl donor to CpxR (5, 12). AcP is often used to phosphorylate response regulators in vitro and much evidence now exists to support the hypothesis that this process contributes to in vivo activation of a subset of those response regulators (reviewed in Ref. 12). For example, the response regulator RcsB can be induced to activate capsule biosynthesis and repress flagellar biogenesis in an AcP-dependent manner (13).
Although many behaviors associated with disruption of the Pta-AckA pathway correlate with AcP concentrations, some do not (reviewed in Ref. 12). One such behavior is the CpxA-independent induction of cpxP transcription that occurs when glucose is added to tryptone-based growth media (8, 14, 15). If a behavior strictly depends on AcP to donate its phosphoryl group to a response regulator, then an ackA mutant, which accumulates AcP (16), should elicit behavior opposite to that exhibited by a pta mutant, which cannot synthesize AcP (16). Thus, if glucose-induced cpxP transcription depended strictly on AcP, then an ackA mutant should respond more robustly to glucose than its wild-type (WT) parent, whereas the pta (or pta ackA) mutant should respond more poorly. Although the latter is true (14), the former is not (8); ackA mutants do not elicit the predicted robust response, even though purified CpxR readily autophosphorylates in the presence of AcP (6, 11). This puzzling observation could mean that AcP is not the in vivo phosphoryl donor to CpxR. Alternatively, glucose-induced cpxP transcription might be inhibited in an ackA mutant (8).
We previously proposed that glucose-induced cpxP transcription is regulated by a reversible acetylation event mediated by the protein acetyltransferase YfiQ (also known as Pka and PatZ in E. coli) and the protein deacetylase CobB. We also presented evidence that nearly 30 lysine residues on three different RNAP subunits are acetylated in a glucose- and YfiQ-dependent manner. One of these acetylated lysines (the surface-exposed Lys-298 of the α subunit) is required for glucose-induced cpxP transcription (15).
In addition to YfiQ and Lys-298, glucose-induced cpxP transcription also requires CpxR (8), a member of the OmpR/PhoB family of DNA-binding response regulators (reviewed in Ref. 17) that does not appear to be an acetylation target (15). On the basis of OmpR homology, phosphorylation of Asp-51 of CpxR is predicted to induce a conformational change that promotes gene regulation (17). Indeed, Asp-51 is required for AcP-dependent phosphorylation of CpxR purified from Y. pseudotuberculosis (11) and for CpxA-induced cpxP transcription (18).
In this report, we investigated the role of CpxR phosphorylation in glucose-induced cpxP transcription. We provide evidence that, in addition to YfiQ and Lys-298 of α, glucose-induced cpxP transcription requires AcP and Asp-51 of CpxR. We further demonstrate that the unexpectedly low level of cpxP transcription exhibited by glucose-exposed ackA mutant cells (8) requires Lys-291, a surface-exposed residue on the carboxyl-terminal domain of α (αCTD) that becomes acetylated when ackA mutants are grown in the presence of glucose. Taken together, these observations are consistent with a model in which AcP-dependent phosphorylation of CpxR is required for glucose-induced activation of cpxP transcription, a process that appears to be modulated by differential acetylation of RNAP.
MATERIALS AND METHODS
Bacterial Strains, Plasmids, and Bacteriophage
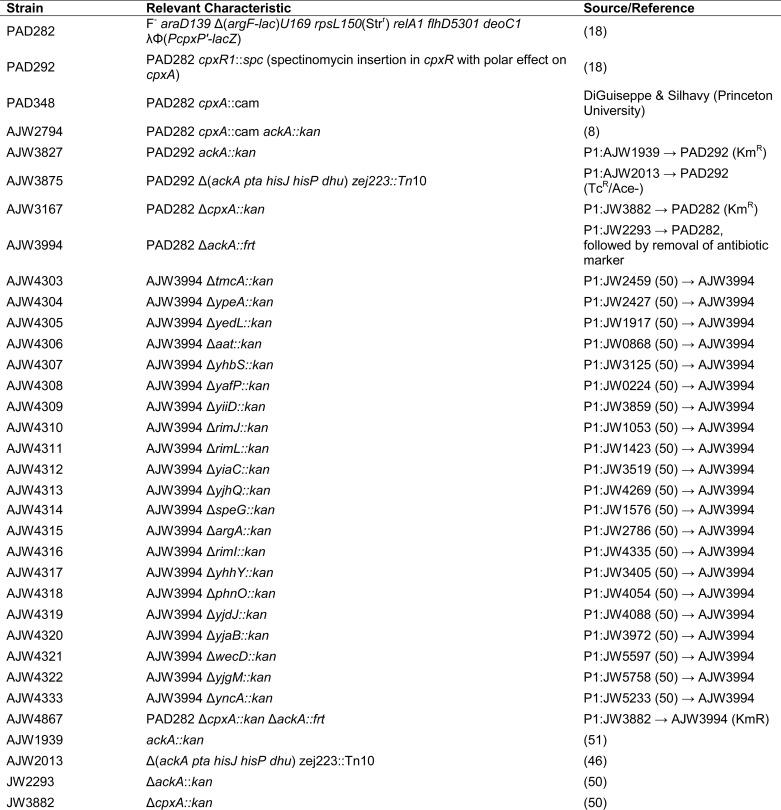
All bacterial strains used in this study are listed in Table 1. Derivatives were constructed by generalized transduction with P1kc, as described previously (19). The transcriptional fusion Φ(PcpxP-lacZ), carried by λPcpxP as described previously (14), was a generous gift from Thomas Silhavy (Princeton University, Princeton, NJ). Construction of monolysogens was performed and verified as described previously (20, 21).
TABLE 1.
Strains and plasmid used in this study
Culture Conditions
For strain construction, cells were grown in LB containing 1% (w/v) tryptone, 0.5% (w/v) yeast extract, and 0.5% (w/v) sodium chloride; LB plates also contained 1.5% agar. For promoter activity assays, cells were grown in TB7, which contains 1% (w/v) tryptone buffered at pH 7.0 with potassium phosphate (100 mm). Transformation was performed using transformation and storage solution, as previously described (22). Cell growth was monitored spectrophotometrically (DU640; Beckman Instruments, Fullerton, CA) by determining the optical density at 600 nm (A600). Kanamycin (40 μg/ml), tetracycline (15 μg/ml), spectinomycin (100 μg/ml), chloramphenicol (25 μg/ml), and ampicillin (100 μg/ml) were added to growth media when needed. Five μm isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to induce gene expression from plasmid vectors, unless otherwise mentioned.
Promoter Activity Assays
To monitor promoter activity from Φ(PcpxP-lacZ), cells were grown aerobically at 37 °C with agitation at 250 rpm in TB7 for 8 h. At regular intervals, 50-μl aliquots were harvested and added to 50 μl of All-in-One β-galactosidase reagent (Pierce Biochemical). β-Galactosidase activity was determined quantitatively using a microtiter format, as described previously (23). Promoter activity was plotted versus A600; however, only the last time point is shown. Each experiment included three independent measurements unless otherwise mentioned. All experiments were performed at least twice.
Site-directed Mutagenesis
Site-directed mutagenesis of CpxR and α were conducted using a QuikChange® II XL Site-directed Mutagenesis Kit (Stratagene) in accordance with the manufacturer's instructions.
Western Immunoblot Analysis
Western immunoblot analysis was conducted on 1-ml samples of cell culture harvested after 7.5 h incubation at 37 °C with aeration. Samples were treated as reported previously (18). Rabbit polyclonal antibody raised against an MBP-CpxR fusion protein was used at a 1:30,000 dilution into 5% milk solution. Both the anti-α (NeoClone Biotechnology) and anti-His6 (Cell Signaling) antibodies were used at a 1:2,000 dilution. Detection was achieved with HRP-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies. The blot was incubated with the primary antibody overnight at 4 °C and then with the secondary antibody for 1 h at room temperature.
CpxR Cloning, Expression, and Purification
The WT cpxR allele was amplified from pCA24n-CpxR using primers cpxRFNdeI and cpxRRHindIII. The resulting amplicon was ligated into pJET1.2, using the CloneJETTM PCR Cloning Kit (Fermentas). The ligated product was cut out of pJET1.2 with NdeI and HindIII, gel-extracted, ligated into pET28 downstream of the IPTG-inducible promoter, and fused to a C-terminal His6 tag. The resulting plasmid was named pBPL001. For purification, expression of CpxR was carried out in BL21 cells transformed with pBPL001. An overnight culture of the transformants was used to inoculate 1 liter of LB at an initial A600 of 0.05. The culture was incubated at 37 °C with shaking at 250 rpm. Once the A600 reached 0.6, 250 μm IPTG was added to the culture to induce CpxR expression. After 3 h of induction, the cell pellet was collected by centrifugation at 4 °C and stored at −20 °C overnight.
Cell lysis was done by resuspending the pellet with 10 ml of BugBuster® (Novagen) and 10 μl of LysonaseTM (Novagen). The resuspended cell pellet was incubated for 30 min at room temperature with gentle shaking. Following this room temperature incubation, 30 ml of pH 8.0 resuspension buffer (50 mm Na2HPO4, 1.4 m NaCl, 20 mm imidazole, 0.1% Tween 20, 5% ethanol, and 15 mm β-mercaptoethanol) was added to the resuspended pellet. The cell debris was pelleted by centrifugation at 15,000 × g, at 4 °C for 30 min. The supernatant was loaded onto a 500 μl of TALON® Metal Affinity Resin (Clontech) column, washed with 10 ml of wash buffer (50 mm Na2HPO4, 0.3 m NaCl, 30 mm imidazole, 0.1% Tween 20, and 0.5% ethanol) buffered at pH 8.0. CpxR elution was performed in a stepwise manner by flowing 500 μl of wash buffer with increasing imidazole concentration from 35 to 85 mm in 5 mm increments. Fractions 6–11 were collected, combined, and dialyzed overnight at 4 °C into storage buffer (10 mm Tris-HCl, pH 8.0, 100 mm KCl, 50% glycerol, 10 mm MgCl2, 0.1 mm EDTA, and 1 mm DTT). The dialyzed protein was aliquoted and stored at −80 °C for future use.
In Vitro Phosphorylation
In vitro phosphorylation was carried out by incubating lithium potassium AcP (Sigma) with purified His-tagged CpxR at 30 °C for 15 min in buffer containing (40 mm Tris-HCl, pH 8.0, 10 mm MgCl2, 40 mm KCl, and 1 mm DTT).
Detection of Phosphorylated CpxR
Whether from cell lysates or from in vitro phosphorylation, phosphorylated CpxR was detected by first separating phosphorylated from nonphosphorylated CpxR using zinc(II) Phos-TagTM SDS-PAGE (10% acrylamide (29:1), 350 mm Tris, pH 6.8, 0.1% SDS, 75 μm Phos-Tag and 150 μm Zn(NO3)2) (NARD Institute LTD).3 Purified protein was detected by staining the gel with SimplyBlueTM (Invitrogen), whereas protein from cell lysate was visualized by Western immunoblot with anti-His6 antibody (Cell Signaling). Cell lysis for Phos-Tag analysis was done at 4 °C with 2× SDS loading buffer. Prior to transfer of the protein onto the membrane, the gel was incubated at room temperature with gentle shaking for 15 min in Towbin buffer containing 1 mm EDTA to chelate the zinc and then for another 15 min in only Towbin buffer.
Immunoprecipitation
For immunoprecipitation, 50 ml of buffered TB cultures were collected after 7.5 h of incubation at 37 °C, pelleted by centrifugation, resuspended in 5 ml of TE buffer, and lysed by sonication. 1 ml of lysate was used for immunoprecipitation of RNAP with anti-RNAP β mouse monoclonal antibodies (NeoClone Biotechnology) and rotated overnight at 4 °C. Protein G-Sepharose® (Sigma) was used to pull down antibodies from cell lysates. Pulled down beads were washed three times with TE buffer and once with wash buffer containing 100 mm NaCl and 50 mm Tris-HCl, pH 7.2. Loading buffer was added directly to the beads and samples were heated to 95 °C for 5 min. Samples were resolved by SDS-PAGE and stained with NOVEX® Colloidal Blue Stain (Invitrogen), according to the manufacturer's instructions.
LC-MS/MS Analysis Using an LTQ Orbitrap Mass Spectrometer and Identification of Lysine Acetylation Sites
The α, β, and β′ bands were excised and subjected to tryptic digestion, as described previously (24). Tryptic peptides were separated and measured online by ESI-mass spectrometry using a nanoACQUITY UPLCTM system (Waters, Milford, MA) coupled to an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Waltham, MA). A trap column (Symmetry® C18, 5 μm, 180 μm inner diameter × 20 mm, Waters) was used for desalting. Elution was performed onto an analytical column (BEH130 C18, 1.7 μm, 100 μm inner diameter × 100 mm, Waters) by a binary gradient of buffers A (0.1% (v/v) acetic acid) and B (99.9% (v/v) acetonitrile, 0.1% (v/v) acetic acid) over a period of 80 min with a flow rate of 400 nl/min. The Orbitrap XL was operated in data-dependent MS/MS mode using the lockmass option for real time recalibration.
Proteins were identified by searching all MS/MS spectra in “dta” format against an E. coli database (extracted from the Uniprot-KB database) using SorcererTM-SEQUEST® (Sequest version 2.7 rev. 11, Thermo Electron including Scaffold_3_00_05, Proteome Software Inc., Portland, OR). The Sequest search was carried out considering the following parameters: a parent ion mass tolerance of 10 ppm and fragment ion mass tolerances of 1.00 Da. Up to two tryptic miscleavages was allowed. Methionine oxidation (+15.99492 Da), cysteine carbamidomethylation (+57,021465 Da), and lysine acetylation (+42.010571 Da) were set as variable modifications. Proteins were identified by at least two peptides applying a stringent SEQUEST filter. Sequest identifications required at least ΔCn scores of greater than 0.10 and XCorr scores of greater than 1.9, 2.2, 3.3, and 3.8 for singly, doubly, triply, and quadruply charged peptides. Acetylated peptides that passed these filter criteria were examined manually and accepted only when b− or y− ions confirmed the acetylation site.
RESULTS
Glucose-induced cpxP Transcription Requires Asp-51, the Conserved Phosphoryl Acceptor Residue of CpxR
We recently proposed that glucose-induced cpxP transcription requires Lys-298 on the surface of the αCTD and seems to be regulated by a reversible acetylation event mediated by the acetyltransferase YfiQ and the deacetylase CobB (15). The knowledge that glucose-induced cpxP transcription does not require the sensor kinase CpxA, but requires the cognate response regulator CpxR (8) led us to re-investigate the role of CpxR in this response.
We first asked whether this behavior requires phospho-CpxR. To address this question, we attempted to complement the cpxR1 mutant allele in a strain that carries the transcriptional fusion λΦ(PcpxP′-lacZ) (strain PAD 292; Table 1). cpxR1 is polar on the downstream gene cpxA; thus, this strain expresses neither CpxR nor CpxA (5). Complementation was attempted by transformation with either plasmid-borne WT cpxR (pCA24n-cpxR; Table 1) or plasmid-borne cpxRD51A (pCA24n-cpxRD51A; Table 1). The latter encodes a mutant protein that lacks the conserved aspartyl residue that serves as the phosphoacceptor. Both alleles were expressed from an IPTG-inducible promoter.
We grew the resultant transformants in the absence or presence of 0.4% glucose and measured β-galactosidase activity as a reporter of cpxP promoter function. The transformants that expressed WT CpxR responded to glucose. In contrast, the transformants that expressed CpxRD51A did not (Fig. 2A, left). Because Western immunoblot analysis indicated that the steady state levels of the WT and mutant forms of CpxR proteins were similar (Fig. 2A, inset), the lack of cpxP transcription by cells that express the mutant protein cannot be explained by a difference in protein expression levels. Instead, this lack of response likely resulted from the inability of the mutant protein to become phosphorylated (Fig. 3B). We therefore conclude that the response to glucose by cpxP requires Asp-51 and propose that this response requires phosphorylation of CpxR.
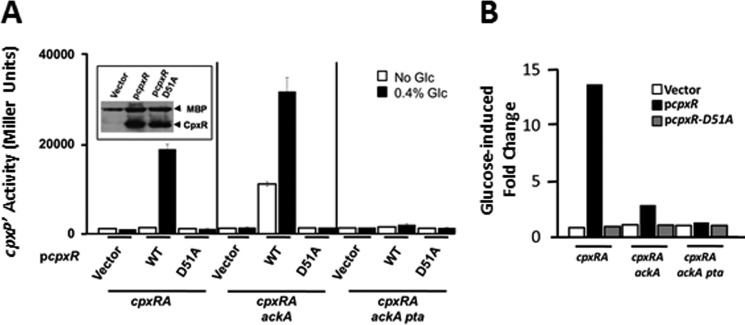
FIGURE 2.
The glucose response requires Asp-51 and AcP. A, λcpxP lysogens of the cpxR1 mutant (strain PAD292), the cpxR1 ackA mutant (strain AJW3827), and the cpxR1 ackA pta mutant (strain AJW3875) were transformed with plasmids pCA24n (vector), pCA24n-cpxR (WT), or pCA24n-cpxR-D51A (D51A). Transformants were grown at 37 °C with shaking in TB7 (open bars), or the same medium supplemented with 0.4% glucose (closed bars). Cells were harvested at regular intervals and A600 and β-galactosidase activity were measured. Only the values at the last time point are shown. The bars indicate the means of triplicate independent cultures, and the error bars indicate the S.D. Inset, Western immunoblot analysis of steady state levels of plasmid-expressed CpxR and CpxR-D51A from whole cell lysates of PAD292 using polyclonal antibody generated against MBP-CpxR fusion protein (gift from Thomas Silhavy). Endogenous MBP serves as a loading control. B, histogram representing the fold-change in cpxP transcription observed by exposure to glucose. The fold-change was calculated dividing the β-galactosidase activity measured in the presence of glucose by the β-galactosidase activity measured in the absence of glucose from A.
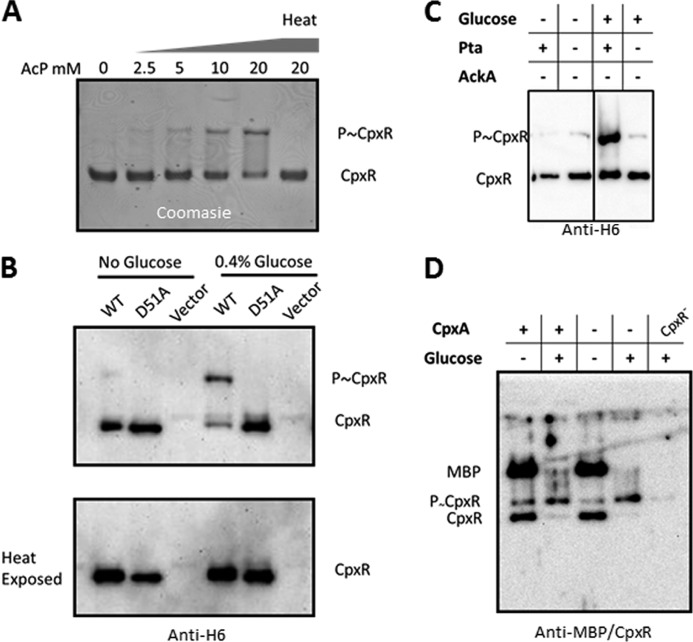
FIGURE 3.
Detection of phosphorylated CpxR. A, purified His6-CpxR was incubated with increasing concentrations of AcP at 30 °C for 15 min and then resolved on a Phos-Tag SDS-PAGE. A fraction of the in vitro phosphorylation reaction carried out with 20 mm AcP was incubated at 95 °C for 15 min to promote hydrolysis of the phosphoryl group. The protein was detected by Coomassie Brilliant Blue. B, λcpxP lysogens of the cpxR1 mutant (strain PAD292) transformed with plasmids pCA24n (vector), pCA24n-cpxR (WT), or pCA24n-cpxR-D51A (D51A) were grown in TB7 in the absence or presence of 0.4% glucose at 37 °C with shaking. 50 mm IPTG was added to the cultures to induce CpxR expression after cultures reached an A600 of 0.6. After 3 h of induction, cell lysates were collected and resolved by Phos-Tag SDS-PAGE before and after incubation at 95 °C for 15 min. CpxR was visualized by Western immunoblot with anti-His6 antibody. C, λcpxP lysogens of the cpxR1 ackA mutant (strain AJW3827), and the cpxR1 ackA pta mutant (strain AJW3875) transformed with the plasmid pCA24n-cpxR were grown in TB7 in the absence or presence of 0.4% glucose at 37 °C with shaking. After cultures reached an A600 of 0.6, 50 mm IPTG was added to the cultures to induce CpxR expression. After 3 h of induction, cell lysates were collected and resolved by Phos-Tag SDS-PAGE. CpxR was visualized by Western immunoblot with anti-His6 antibody. D, λcpxP lysogens of the WT strain (strain PAD282), the cpxA mutant (strain PAD348), and the cpxR1 mutant (strain PAD292) were grown in TB7 in the absence and presence of 0.4% glucose at 37 °C with shaking for 7.5 h. Cell lysate was collected and resolved by Phos-Tag SDS-PAGE. Endogenous WT CpxR was detected using polyclonal antibody generated against the MBP-CpxR fusion protein. Note that MBP is catabolite-repressed. Therefore, its expression is inhibited in the presence of glucose.
The Response to Glucose Requires Acetyl Phosphate
Because the response to glucose occurs independently of the cognate sensor CpxA, but seems to require phospho-CpxR, we sought the source of the phosphoryl group elsewhere. One alternative source of phosphoryl groups is AcP (Fig. 1B), which has been shown to function in vitro as a phosphoryl donor to CpxR (6), and has been proposed to do likewise in vivo (5, 14). If AcP could function as the phosphoryl donor, then the cpxR1 ackA double mutant (strain AJW3827; Table 1), which accumulates AcP (12, 16), should respond to the addition of glucose in a Asp-51-dependent manner. In contrast, we predicted that the cpxR1 pta ackA triple mutant (strain AJW3875; Table 1), which cannot synthesize AcP (12, 16), would not respond to exogenous glucose regardless of the status of Asp-51. Indeed, the cpxR1 ackA double mutant responded to glucose in a Asp-51-dependent manner, whereas the cpxR1 pta ackA triple mutant did not respond to glucose (Fig. 2A, middle and right, respectively). Similar results were obtained when we used oligo-mediated recombineering (25, 26) to alter the endogenous cpxR allele, such that it encodes the CpxRD51A mutant (data not shown). Thus, in the absence of CpxA and in the presence of glucose, cpxP transcription requires both Asp-51 and AcP.
In Vivo Phospho-CpxR Status Correlates with AcP but Not with cpxP Transcription
The simplest explanation for the requirement of both AcP and Asp-D51 is that the CpxA-independent, glucose-induced cpxP transcription requires AcP-dependent phosphorylation of CpxR. Despite this requirement for AcP, the AcP-accumulating ackA mutant failed to promote the predicted proportional glucose-induced increase in cpxP transcription, eliciting an ∼3-fold instead of the ∼14-fold increase exhibited by its WT parent (Fig. 2B). This behavior, displayed by cells that overexpress CpxR, is almost identical to the behavior of cells that express CpxR endogenously (8). To understand why an ackA mutant strain that accumulates high levels of AcP responds less robustly to glucose than its AckA+ parent, we assessed whether in vivo levels of phospho-CpxR correlate with those of AcP in strains WT or mutant for the Pta-AckA pathway.
To monitor phospho-CpxR, we took advantage of a previously reported method used for the detection of phosphorylated proteins (27, 28), including bacterial response regulators (11, 29). This method uses a dinuclear metal complex (i.e. 1,3-bis[bis(pyridin-2-yl-methyl)amino] propan-2-olato dizinc (II)) that has affinity for phosphomonoester dianions, such as the aspartyl phosphate of response regulators. When included in an SDS-PAGE, this phosphate-binding tag (Phos-Tag) slows the migration of the phosphorylated protein, allowing it to be distinguished from the nonphosphorylated protein by mobility shift.
To verify the report that AcP can function in vitro as a phosphoryl donor to CpxR (6) and to optimize the assay, we incubated purified His6-CpxR with AcP, resolved the proteins by Phos-Tag SDS-PAGE and stained the gel with SimplyBlue (Invitrogen). We detected an AcP-dependent shift in CpxR migration that disappeared when the sample was exposed to 95 °C for 15 min, a condition that induces hydrolysis of phosphorylated aspartyl residues (Fig. 3A).
To test the hypothesis that glucose induces cpxP transcription by increasing phospho-CpxR concentration, we assessed the cpxR1 mutant (strain PAD292) transformed with plasmids that expressed either His6-CpxR or His6-CpxR D51A. We grew the transformants in the absence or presence of 0.4% glucose, harvested cells as the cultures entered stationary phase, separated the cell lysates by Phos-Tag SDS-PAGE, and performed a Western immunoblot analysis with anti-His6 antibody. Consistent with the idea that glucose-induced cpxP transcription requires phospho-CpxR, we detected a glucose- and Asp-51-dependent shift in CpxR that was sensitive to heat (Fig. 3B).
To test the role of AcP, we performed a similar experiment on cpxR1 ackA (strain AJW3827) and cpxR1 pta ackA (strain AJW3875) mutants transformed with the His6-CpxR expression plasmid. Consistent with the hypothesis that AcP mediates CpxR phosphorylation, the shifted band was mostly absent in the cells that cannot synthesize AcP (cpxR1 pta ackA) but abundant in the cells that accumulate AcP (cpxR1 ackA) (Fig. 3C).
To facilitate detection of shifted phospho-CpxR, the aforementioned Phos-Tag experiments were performed with cells that overexpressed His6-CpxR. Because we were aware that overexpression of His-tagged proteins often causes artifacts, we performed a similar experiment with WT and cpxA mutant cells that expressed WT CpxR from the native locus (PAD282 and PAD348, respectively, Table 1). In response to glucose, the phosphorylated fraction of native CpxR increased substantially, regardless of the status of CpxA (Fig. 3D). On the basis of these experiments, we conclude that glucose promotes an AcP-mediated phosphorylation of CpxR that is independent of the sensor kinase CpxA.
Disruption of the Pta-AckA Pathway Alters the Acetylation Profile of RNA Polymerase
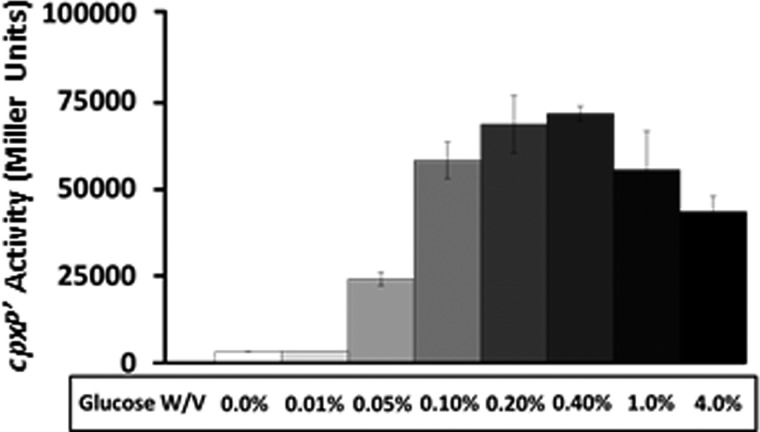
The observation that AcP acts as the primary phosphoryl donor to CpxR (under the tested growth conditions) raises the following question: why does the cpxA ackA mutant respond less robustly to glucose than its WT parent? We previously hypothesized that disruption of the Pta-AckA pathway could lead to the accumulation of an inhibitor of glucose-induced cpxP transcription (8). For several reasons, we considered the possibility that AcCoA could function as this inhibitor, perhaps by acting as an acetyl-donor. First, the concentration of AcCoA has been shown to rapidly increase when E. coli cells are exposed to glucose (30) and growth in the presence of 0.4% glucose causes a general increase in protein acetylation (15). Second, pta mutants excrete large amounts of pyruvate and lactate in an AcCoA-sensitive manner (31), suggesting that disruption of the Pta-AckA pathway causes a metabolic imbalance that could affect the AcCoA pool. Third, cpxP transcription increases when cells are exposed to glucose concentrations up to 0.4%, but decreases progressively in the presence of larger amounts, e.g. 4.0% (Fig. 4), an observation that supports the hypothesis that cpxP transcription is sensitive to central metabolic imbalances. Because AcCoA can provide acetyl groups for protein acetylation (reviewed in Refs. 32–34), because exposure of E. coli to 0.4% glucose promotes acetylation of multiple lysines on multiple subunits of RNAP, and because one of those lysines (Lys-298 of α) is required for glucose-induced cpxP transcription (15), we hypothesized that disruption of the Pta-AckA pathway could shift the pattern of RNAP acetylation and that this altered acetylation profile could affect cpxP transcription.
FIGURE 4.
Excessive glucose inhibits cpxP transcription. λcpxP lysogens of the cpxA mutant (strain PAD348) were grown for 7.5 h at 37 °C with shaking in TB7 supplemented with increasing concentrations of glucose. Cells were harvested at regular intervals and A600 and β-galactosidase activity were measured. Only the values at the last time point are shown. The bars indicate the means of triplicate independent cultures, and the error bars indicate the S.D.
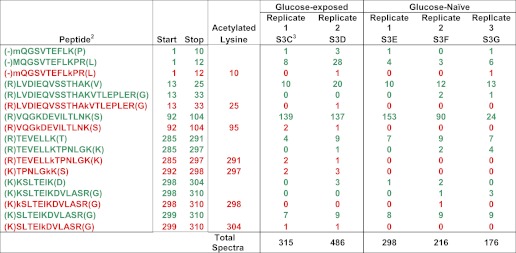
To determine whether disruption of the Pta-AckA pathway alters the RNAP acetylation profile, we grew cpxA ackA mutant cells (strain AJW 2794, Table 1) in the presence or absence of glucose, purified RNAP subunits β, β′, and α by immunoprecipitation (data not shown), and applied liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis using an Orbitrap XL mass spectrometer to detect lysine acetylation sites of these RNAP subunits. From cells grown in the presence of glucose, we mapped 19 acetylation sites to β (Table 2, supplemental Table S1, A–F), of which 12 were detected in at least two biological replicates. We mapped an additional 6 acetylation sites to β′, of which 4 were detected in two biological replicates (Table 2, supplemental Table S2, A–D). Finally, we mapped 7 acetylation sites to α, of which 4 were detected in two biological replicates (Table 3, Fig. 5, supplemental Table S3, A–D). None of the reproducibly acetylated α sites was acetylated when ackA cells were grown in the absence of glucose (Tables 2 and 3, supplemental Table S3, E–G). In support of our hypothesis that disruption of the Pta-AckA pathway could alter the RNAP acetylation profile, most α lysines acetylated in the glucose-grown ackA mutant were not acetylated in the WT parent and vice versa (compare Fig. 5 in this report to Fig. 7 of Ref. 8): Lys-298 was acetylated in the WT parent, but not in the ackA mutant; Lys-10, Lys-25, and Lys-95 on the α amino-terminal and both Lys-291 and Lys-304 on the αCTD were acetylated in the ackA mutant, but not in the WT parent; and Lys-297 was acetylated in both the mutant and the parent.
TABLE 2.
The number of acetylated β and β′ detected from ackA mutant cells
Red, acetylated peptides detected in at least two biological replicates; Black, acetylated peptides detected in one biological replicate; m, oxidized methionine; M, methionine; k, acetylated lysine; K, lysine.

TABLE 3.
Acetylated α peptides from ackA cells1
1 Green, unacetylated peptides; red, acetylated peptides.
2 m, oxidized methionine; M, methionine; k, acetylated lysine; K, lysine.
3 Supplemental Table in which the raw data are presented.
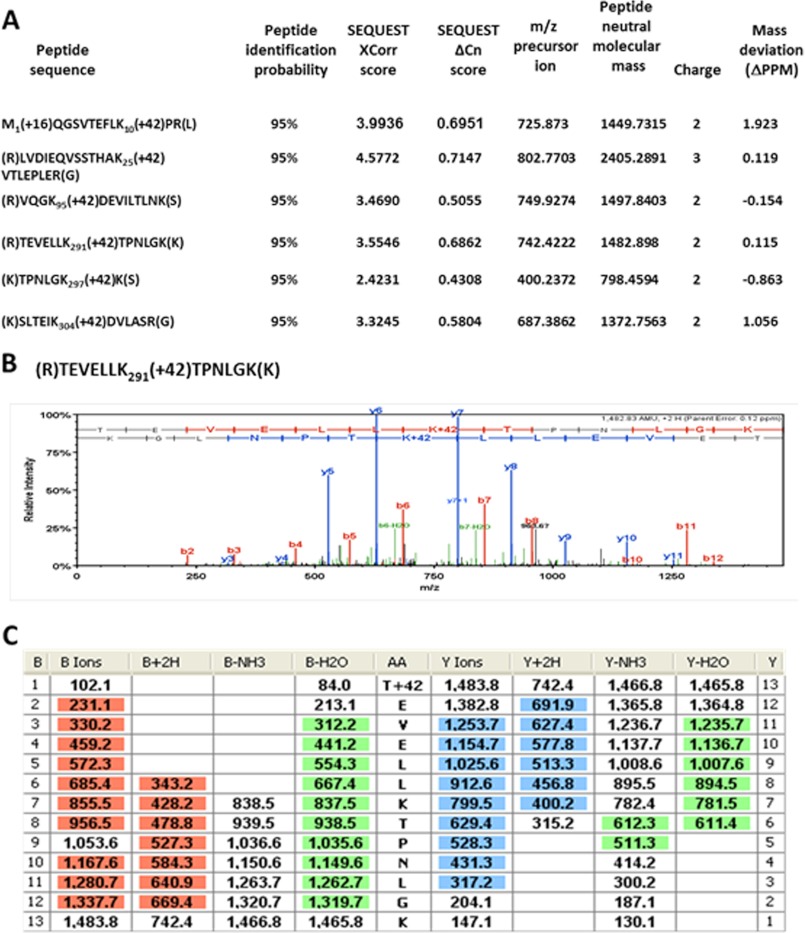
FIGURE 5.
In the glucose-exposed ackA mutant, the α subunit of RNAP is acetylated on Lys-291 and five other lysines. Immunoprecipitated subunits of RNAP were separated by SDS-PAGE. The α bands were excised and tryptically digested as described previously (53). The resulting peptides were analyzed in a LTQ OrbitrapXL mass spectrometer as described under ”Materials and Methods.“ The double- or triple-charged acetyllysine-modified peptides M1(+16)QGSVTEFLK10(+42)PR, LVDIEQVSSTHAK25(+42)VTLEPLER, VQGK95(+42)DEVILTLNK, TEVELLK291(+42)TPNLGK, TPNLGK297(+42)K, and SLTEIK304(+42)DVLASR were detected in the digested α sample as mass peaks of m/z = 725.873, 802.7703, 749.9274, 742.4222, 400.2372, and 687.3862, respectively. A, the Xcorr and ΔCn scores of the six acetylated peptides. B, the corresponding CID MS/MS spectrum for the Lys-291-containing peptide TEVELLK291(+42)TPNLGK. C, complete b and y fragment ion series for the Lys-291-containing peptide TEVELLK291(+42)TPNLGK.
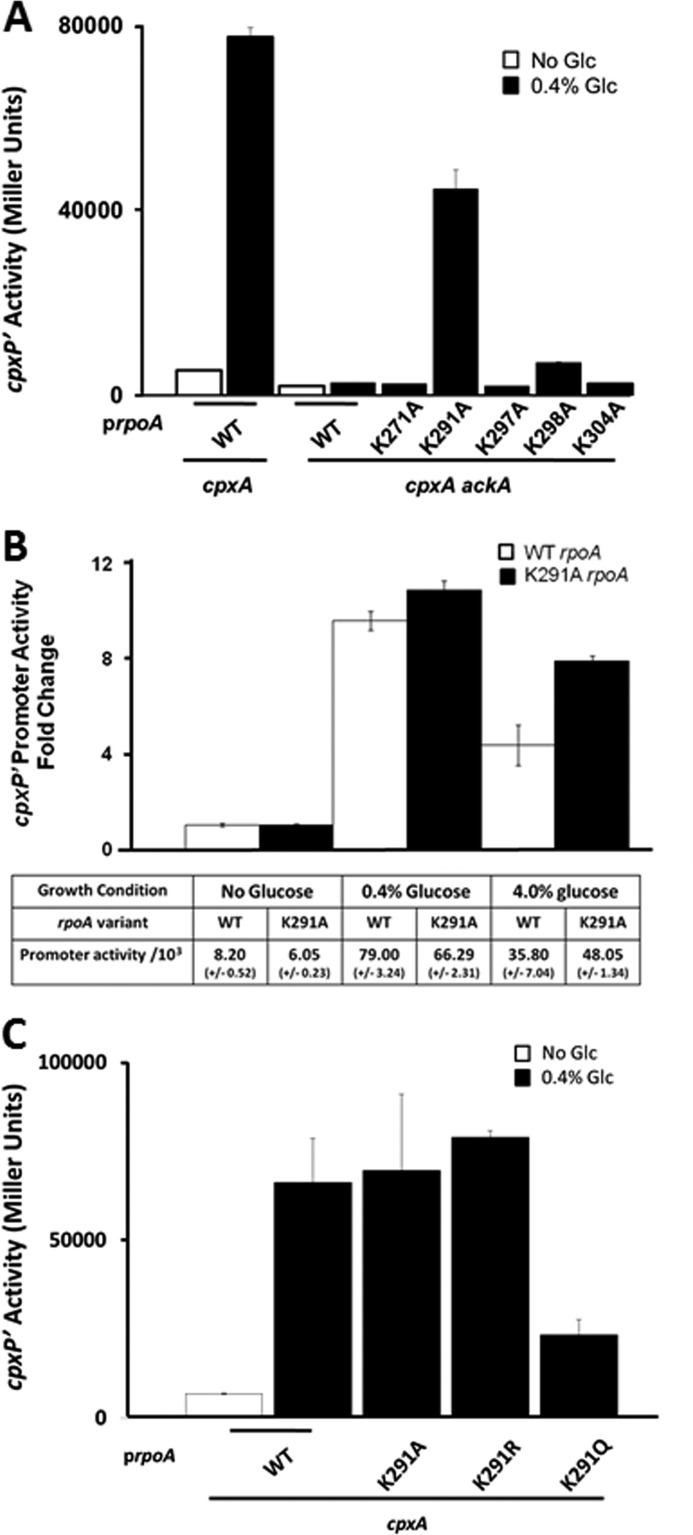
FIGURE 7.
Lys-291 inhibits cpxP transcription. A, λcpxP lysogen of the cpxA mutant (strain PAD348) was transformed with plasmid pREII carrying the WT allele of rpoA, whereas the isogenic cpxA ackA mutant (strain AJW3827) was transformed with pREII carrying the WT allele of rpoA or lysine to alanine mutant derivatives of residues 271, 291, 297, 298, and 304. The resultant transformants were grown for 7.5 h at 37 °C with shaking in TB7 (white bars) or the same medium supplemented with 0.4% glucose (black bars). Cells were harvested at regular intervals and both A600 and β-galactosidase activity was measured. The bars indicate the means of triplicate independent cultures, and the error bars indicate the S.D. B, β-galactosidase activity of λcpxP lysogens of the cpxA mutant (strain PAD348) transformed with plasmid pREII carrying either the WT allele of rpoA (white bars), or the rpoAK291A mutant allele (black bars). The resultant transformants were grown at 37 °C with shaking in TB7. The same medium was supplemented with 0.4 or 4.0% glucose. Cells were harvested at regular intervals and both A600 and β-galactosidase activity were measured. The fold-changes in β-galactosidase activity from cells grown in the presence of glucose relative to those grown in the absence of glucose are reported in the histogram. The bars indicate the means of triplicate independent cultures, and the error bars indicate the S.D. Mean Miller units and S.D. from each condition are reported in the table. C, λcpxP lysogens of the cpxA mutant (strain PAD348) transformed with plasmid pREII carrying the WT allele of rpoA, lysine to alanine, lysine to arginine, and lysine to glutamine mutant derivatives of residue 291. Transformants were grown for 7.5 h at 37 °C with shaking in TB7 in the absence (white bars) or presence of 0.4% glucose (black bars). Cells were harvested at regular intervals and both A600 and β-galactosidase activity was measured. The bars indicate the means of triplicate independent cultures, and the error bars indicate the S.D.
We were particularly intrigued by the lack of acetylated Lys-298 in glucose-exposed ackA mutant cells and wondered if that could explain their weak response to glucose. Alternatively, acetylation of one of the other lysines might inhibit glucose-induced cpxP transcription.
Overexpression of YfiQ Fails to Induce cpxP Transcription in ackA Mutants
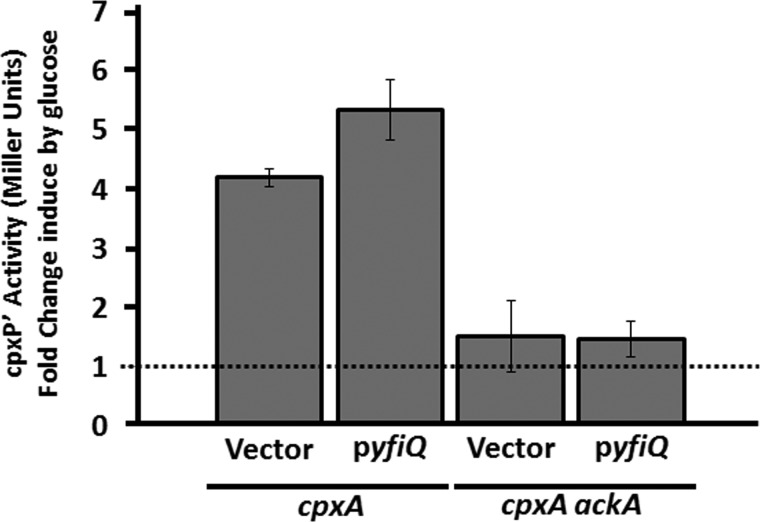
We previously reported that glucose-induced acetylation of Lys-298 on the αCTD required the acetyltransferase YfiQ (15). To test if the weak cpxP transcription exhibited by the ackA mutant was due to the lack of Lys-298 acetylation, we asked whether YfiQ overexpression could suppress the weak response to glucose by cpxA ackA mutants. Into an ackA cpxA λΦ(PcpxP′-lacZ) lysogen (strain AJW4867; Table 1), we introduced a plasmid carrying yfiQ or the vector control (Table 1). We grew the resultant transformants in the absence or presence of 0.4% glucose, monitored β-galactosidase activity, and found that yfiQ overexpression had no effect on the weak glucose response of the cpxA ackA double mutant (Fig. 6).
FIGURE 6.
Overexpression of YfiQ does not induce cpxP transcription in the ackA mutant. λcpxP lysogens of the cpxA mutant (strain AJW3167) and the isogenic cpxA ackA double mutant (strain AJW4867) were transformed with plasmid pCA24n or with pCA24n carrying the WT allele of yfiQ. Transformants were grown in TB7 in the presence or absence of 0.4% glucose. Cells were harvested at regular intervals and A600 and β-galactosidase activity were measured. Only the values at the last time point are shown. The bars indicate fold-increase in β-galactosidase of three independent cultures grown in the presence of 0.4% glucose compared with β-galactosidase of samples grown in the absence of glucose.
Lys-291 on RNAP αCTD Contributes to Transcription Inhibition
That YfiQ overexpression does not promote glucose-induced cpxP transcription in the cpxA ackA mutant background led us to test the alternative hypothesis: that this weak response requires one of the lysines that became acetylated in the absence of ackA. Because the gene that encodes α (rpoA) is essential for bacterial survival, we used a previously reported partial-diploid system (35) to test the hypothesis that one of the other acetylated α lysines inhibits cpxP transcription. Into an ackA cpxA λΦ(PcpxP′-lacZ) lysogen that carries the WT rpoA gene in its native location on the chromosome (strain AJW2794), we introduced plasmids carrying either the WT rpoA allele or Lys to Ala point mutation derivative alleles (Table 1) with the goal of determining if any lysine functional group contributes to this phenotype. We grew the resultant transformants in the absence or presence of 0.4% glucose and monitored β-galactosidase activity. cpxA ackA mutant cells that overexpressed the α K291A mutant responded robustly to the presence of glucose. In contrast, we observed little or no response from cpxA ackA mutants that overexpressed WT α, Lys to Ala mutations of the other 4 lysines of the αCTD (Fig. 7A) or Lys to Ala mutations of the 3 acetylated lysines on the α amino-terminal (data not shown).
We also investigated whether Lys-291 could inhibit 4.0% glucose-induced cpxP transcription in cpxA mutant cells (strain PAD348) whose Pta-AckA pathway remains intact. Indeed, cells that overexpressed the K291A variant responded more robustly to the presence of 4% glucose than did cells that overexpressed WT α (Fig. 7B). In contrast, Lys-291 was not required for Lys-298-dependent, glucose-induced cpxP transcription, as cpxA mutant cells that overexpressed either WT α or the K291A mutant responded robustly to 0.4% glucose (Fig. 7C), as reported previously (15). We conclude that inhibition, whether caused by excessive glucose or by disruption of the Pta-AckA pathway, acts through Lys-291.
To determine whether the requirement for Lys-291 could involve acetylation, we took advantage of a genetic strategy commonly used in studies of eukaryotic protein acetylation (36). In this approach, the lysine is converted to either a glutamine or an arginine. The KQ substitution mimics the neutral acetylated lysine, whereas the KR substitution mimics the positively charged unacetylated lysine. Consistent with the hypothesis that Lys-291 acetylation inhibits glucose-induced cpxP transcription, cpxA mutant cells overexpressing the K291Q mutant variant of α responded to glucose with less intensity than did cells that overexpressed WT α or its K291A or K291R mutant variants (Fig. 7C). This decrease in cpxP transcription, however, cannot be explained by a decrease in steady state levels of protein (data not shown). These results lend support to the hypothesis the Lys-291 acetylation decreases the response to glucose by cpxP.
Is an Acetyltransferase Required for the Weak Response to Glucose by ackA mutants?
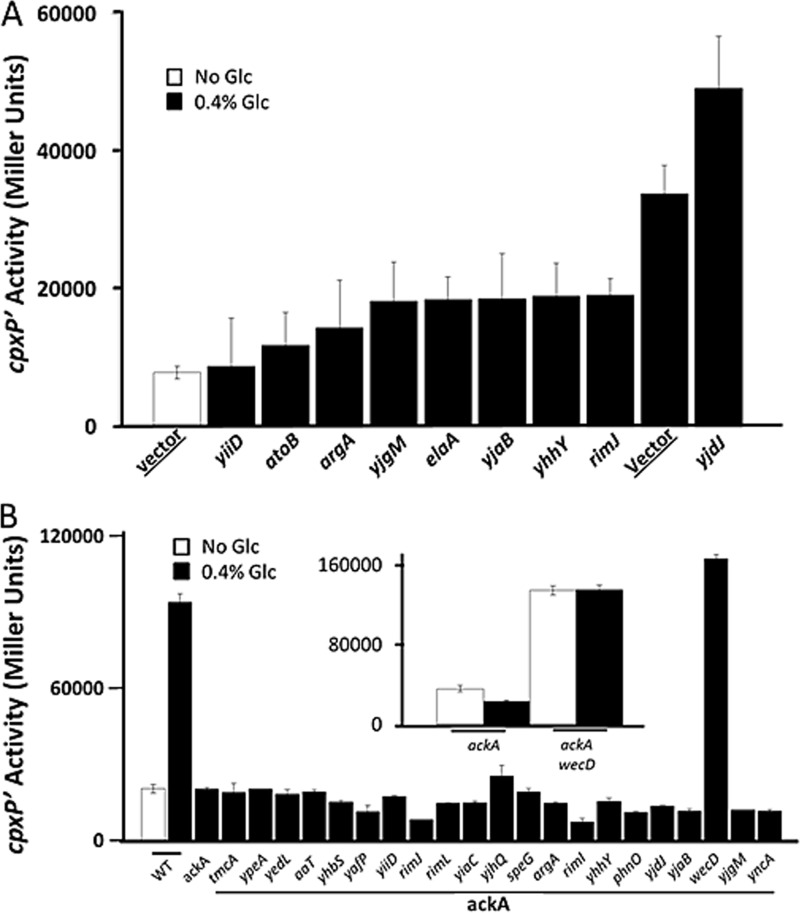
To identify an acetyltransferase that participates in Lys-291-dependent inhibition of cpxP transcription, we transformed WT cells with a set of multicopy plasmids; each plasmid expressed one of 22 known or predicted E. coli acetyltransferases (Table 1). We grew the resultant transformants in the presence of glucose and IPTG to induce acetyltransferase expression, and measured β-galactosidase activity. Relative to the WT strain carrying the vector control, overexpression of 8 different acetyltransferases substantially inhibited the glucose response, whereas overexpression of only one increased the response (Fig. 8A and data not shown).
FIGURE 8.
Overexpression and deletion screens do not identify an acetyltransferase responsible for ackA-dependent inhibition. A, λcpxP lysogens (strain PAD282) were transformed with the vector pCA24n or with pCA24n derivatives each carrying the ORF of a known or predicted acetyltransferase (52). Transformants carrying the vector control were grown for 7.5 h at 37 °C with shaking in TB7 (white bars) or the same medium supplemented with 0.4% glucose (black bars). Transformants carrying genes encoding known or predicted acetyltransferases were grown in medium supplemented with 0.4% glucose. Transformants were harvested at regular intervals and A600 and β-galactosidase activity were measured. Only the values at the last time point are shown. The bars indicate the means of two experiments, each conducted with duplicate independent cultures. The error bars indicate the S.D. B, λcpxP lysogens of an ackA mutant (strain AJW3994) and a set of double mutants, in which the ackA mutation was combined with deletions of known or predicted acetyltransferases (strains AJW4303–4323). All mutants were grown in TB7 supplemented with 0.4% glucose (black bars). λcpxP lysogens of the WT (strain PAD282) were grown in TB7 in the absence (white bars) or presence of 0.4% glucose (black bars). Cells were harvested at regular intervals and A600 and β-galactosidase activity were measured. Only the values at the last time point are shown. The bars indicate the means two experiments, each conducted with duplicate independent cultures. The error bars indicate the S.D. Inset, λcpxP lysogens of the ackA wecD double mutant grown in TB7 in the absence (open bars) or presence of 0.4% glucose (close bars). Cells were harvested at regular intervals and A600 and β-galactosidase activity were measured. Only the values at the last time point are shown. The bars indicate the means of triplicate independent cultures, and the error bars indicate the S.D.
To test if one of these acetyltransferases was required for the weak response to glucose by the ackA mutant, we deleted each acetyltransferase from the ackA mutant (strain AJW3994) and screened for double mutants whose cpxP promoter could respond robustly to glucose. Only the ackA wecD double exhibited robust cpxP transcription (Fig. 8B). However, this expression is unlikely to involve Lys-291 acetylation as it occurred even in the absence of glucose (Fig. 8B, inset). On the basis of these results, we conclude that multiple protein acetyltransferases can inhibit glucose-induced cpxP transcription, but that no single acetyltransferase may be required.
DISCUSSION
Phosphorylation and Acetylation Regulate CpxA-independent, Glucose-induced cpxP Transcription
We propose that the two high-energy central metabolites, AcP and AcCoA, function together to activate transcription from the cpxP promoter. We base this proposal on the following observations. (i) The intracellular pools of both AcP and AcCoA are dynamic. They fluctuate throughout growth and upon exposure to different carbon sources (16, 30). Furthermore, both central metabolites can be used as donors for post-translational modification of proteins: AcP by donating its phosphoryl group for protein phosphorylation, and AcCoA by donating its acetyl group for protein acetylation (12, 32). (ii) Glucose-induced, CpxA-independent cpxP transcription requires both the phosphoacceptor site of CpxR (Asp-51) and the ability of cells to synthesize AcP (Fig. 2). Indeed, Phos-Tag mobility shift assays of cell lysates clearly show that AcP functions as the primary phosphoryl donor to CpxR (Fig. 3) under the tested growth conditions. (iii) Glucose-induced, CpxA-independent cpxP transcription also requires an acetylation event involving AcCoA, the acetyltransferase YfiQ, and the deacetylase CobB. In addition, glucose-induced cpxP transcription involves a lysine (Lys-298) located on the surface of αCTD that is acetylated in a YfiQ- and glucose-dependent manner (15).
Evidence That AcCoA Inhibits AcP-dependent cpxP Transcription
Given that glucose-induced cpxP transcription requires AcP to phosphorylate CpxR, we were initially surprised that the AcP-accumulating ackA mutant (16) responded to 0.4% glucose with only a modest increase in cpxP transcription (Fig. 2B). We previously proposed that this weak response resulted from accumulation of a central metabolic intermediate with inhibitory properties (8). Despite evidence that AcCoA-dependent acetylation contributes to glucose-induced cpxP transcription, we considered AcCoA to be an obvious candidate for the inhibitory compound. (i) E. coli tightly limits CoA synthesis (37). (ii) The limited supply of CoA would be overwhelmed by exposure to large amounts of glucose (e.g. 4.0%), resulting in a large AcCoA-to-CoA ratio (16, 30). (iii) Disruption of the Pta-AckA pathway also would be expected to put pressure on the limited CoA pool. Although deletion of pta or ackA does not appear to alter the AcCoA to CoA ratio (16), the deletion of pta clearly alters the ratio of excreted central metabolic intermediates: WT cells primarily excrete acetate; in contrast, the mutant cells excrete large amounts of pyruvate and lactate (31). Evidence that disruption of the Pta-AckA pathway perturbs the CoA pool is provided by heterologous expression of a pathway that synthesizes the polyhydroxybutyrate from AcCoA. When induced, this pathway restores pyruvate and lactate excretion to WT levels (31). Finally, of course, (iv) AcCoA can regulate protein function by donating its acetyl group to lysine residues (32, 33).
A Potential Role for Lys-291
The acetylation profile of α isolated from the cpxA ackA mutant differed from the acetylation profile of α isolated from its WT parent (compare Fig. 5 in this report to Fig. 7 of Ref. 8). One α acetylation was common to both strains. In contrast, five acetylations were specific to the cpxA ackA mutant including Lys-291, whereas only one was specific to WT (i.e. Lys-298). Our genetic screen suggests that Lys-291 is definitely involved in weakening the response to 0.4% glucose by the ackA mutant (Fig. 7A) or to 4.0% glucose by its WT parent (Fig. 7B). We currently do not possess definitive evidence that acetylation of Lys-291 causes these behaviors; however, support for such a mechanism is provided by the dramatic reduction in glucose-induced cpxP transcription by the K291Q mutation (Fig. 7C), which is predicted to exhibit properties that mimic acetylated Lys-291 (36). Although the underlying molecular mechanism remains to be determined, it is distinctly possible that Lys-291 acetylation could affect one of the many interactions made by α and, as a result, decrease the stability of the transcription complex. Lys-291 is located on the αCTD helix-hairpin-helix motif, which is reported to contribute to both nucleic acid binding and protein-protein interaction (38). To the best of our knowledge, no evidence exists that Lys-291 makes direct contact with DNA. In contrast, Lys-291 has been reported to make direct contact with activation region 2 of the transcription antiterminator NusA (39). Furthermore, Lys-291 is located near the 287 determinant, an αCTD surface known to make direct contact with the catabolite activator protein (also known as CRP) (reviewed in Ref. 40). Although no direct evidence exists for an αCTD-CpxR interaction, our report that the αCTD is required for glucose-induced cpxP transcription (15) hints that an interaction between these two proteins might exist, perhaps in a manner similar to that reported for OmpR, another member of the winged helix-turn-helix family of response regulators (reviewed in Ref. 41).
Using the well characterized CRP-αCTD interaction as a guide, we hypothesize that Lys-291 might participate in an interaction between CpxR and the αCTD. If so, then the acetylation of Lys-291 could affect this interaction and therefore impact transcription. Efforts are underway to identify the mechanism by which Lys-291 and its acetylation affect cpxP transcription.
YfiQ Is Not Involved
We found no evidence that YfiQ plays a role in Lys-291-dependent inhibition of cpxP transcription. Although overexpression of YfiQ promoted a small increase in glucose-induced cpxP transcription, it did not alter the weakened response by the ackA mutant (Fig. 6). This weakened response also was unaffected by deletion of yfiQ (data not shown). At present, we do not know how Lys-291 becomes acetylated. Although the overexpression of several acetyltransferases can inhibit cpxP transcription in the WT parent, deletion of any single enzyme did not permit the ackA mutant to respond robustly to glucose (Fig. 8). A simple explanation for these results is that multiple acetyltransferases could acetylate Lys-291. Alternatively, Lys-291 could become acetylated independently of the action of an acetyltransferase. Efforts to distinguish between these two mechanisms are underway.
AcP Functions as a Phosphoryl Donor in Vivo
Much evidence supports the hypothesis that AcP can serve as a phosphoryl donor to two-component response regulators, e.g. CpxR (12, 42, 43). (i) Many purified response regulators autophosphorylate when exposed to AcP (44). (ii) In response to diverse carbon sources and growth phase, the intracellular AcP concentration varies more than 100-fold (16, 45). (iii) In WT cells, the AcP pool can reach at least 3 mm (16), a concentration that permits efficient in vitro autophosphorylation. (iv) The regulation of about 100 E. coli genes correlates with the status of AcP (46). This regulation depends on response regulators, e.g. RcsB (13) and CpxR (8); however, this dependence does not extend to the cognate sensor kinases RcsC (13) and CpxA (8). (v) AcP-dependent behaviors have been reported in diverse bacteria, including pathogens (for example, Refs. 47 and 48). (vi) A specificity determinant that limits AcP-dependent autophosphorylation of a response regulator has been identified in Camplyobacter (49). Here, we have shown that in vivo phosphorylation of CpxR does not require CpxA. Instead, it requires Pta (Fig. 3). This observation provides the definitive evidence that AcP functions as the primary phosphoryl donor to CpxR, at least under the tested conditions, which were designed to dampen extracytoplasmic stimuli (e.g. alkaline pH) known to activate CpxA kinase activity (8). This observation also fulfills a major prediction made by the hypothesis that AcP can act as a global signal, in vivo evidence that AcP donates its phosphoryl group directly to at least one response regulator.
Finally, we acknowledge the existence of a weak glucose- and AcP-independent signal by the pta ackA double mutant. Currently, we do not know the origin of this weak signal. Perhaps, under the conditions tested, a small percentage of CpxR is phosphorylated by a noncognate sensor kinase and/or another small phospho-donor.
Concluding Remarks
In summary, we propose that AcP can function as a phosphoryl donor to CpxR, both in vitro and in vivo. The output of this phosphorylation event, at the level of the cpxP promoter, seems to be modulated by acetylation of two lysine residues on the surface of RNAP αCTD. Our work suggests that acetylation of Lys-298 somehow contributes to promoter activity, whereas acetylation of Lys-291 dampens this activation.
Acknowledgments
We thank Thomas Silhavy, Patricia DiGiuseppe-Champion, Susan Gottesman, and Rick Gourse for their generous donations of strains, plasmids, and/or antibodies. We thank Richard M. Schultz, Stanley M. Spinola, and members of the Visick and the Wolfe labs for critical discussions and/or reading of the manuscript. We thank members of the Gourse lab, especially Christopher W. Lennon for help with CpxR purification. We also thank Andrew R. Morris and Karen L. Visick, Alice Boulanger Castaing, and Deborah M. Hinton, Kyle Wayne, and Malcolm Winkler for sharing Phos-Tag information prior to publication. Special thanks to David S. Thach and Linda I. Hu for the initial idea that acetylation could inhibit cpxP transcription.
This work was supported, in whole or in part, by National Institutes of Health Grant GM066130 and Loyola University Chicago Potts Foundation Award LU11200 (to A. J. W.) and Deutsche Forschungsgemeinschaft Project AN746/2-1 (to H. A.).

This article contains supplemental Tables S1–S3.
A. Boulanger-Castaing and D. M. Hinton, personal communication.
- AcP
- acetyl phosphate
- αCTD
- α carboxyl-terminal
- IPTG
- isopropyl β-d-1-thiogalactopyranoside.
REFERENCES
- 1. Price N. L., Raivio T. L. (2009) Characterization of the Cpx regulon in Escherichia coli strain MC4100. J. Bacteriol. 191, 1798–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Wulf P., McGuire A. M., Liu X., Lin E. C. (2002) Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 277, 26652–26661 [DOI] [PubMed] [Google Scholar]
- 3. Vogt S. L., Raivio T. L. (2012) Just scratching the surface. An expanding view of the Cpx envelope stress response. FEMS Microbiol. Lett. 326, 2–11 [DOI] [PubMed] [Google Scholar]
- 4. Spinola S. M., Fortney K. R., Baker B., Janowicz D. M., Zwickl B., Katz B. P., Blick R. J., Munson R. S., Jr. (2010) Activation of the CpxRA system by deletion of cpxA impairs the ability of Haemophilus ducreyi to infect humans. Infect. Immun. 78, 3898–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Danese P. N., Snyder W. B., Cosma C. L., Davis L. J., Silhavy T. J. (1995) The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 9, 387–398 [DOI] [PubMed] [Google Scholar]
- 6. Pogliano J., Lynch A. S., Belin D., Lin E. C., Beckwith J. (1997) Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 11, 1169–1182 [DOI] [PubMed] [Google Scholar]
- 7. Raivio T. L., Silhavy T. J. (1997) Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 179, 7724–7733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolfe A. J., Parikh N., Lima B. P., Zemaitaitis B. (2008) Signal integration by the two-component signal transduction response regulator CpxR. J. Bacteriol. 190, 2314–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fleischer R., Heermann R., Jung K., Hunke S. (2007) Purification, reconstitution, and characterization of the CpxRAP envelope stress system of Escherichia coli. J. Biol. Chem. 282, 8583–8593 [DOI] [PubMed] [Google Scholar]
- 10. Miot M., Betton J. M. (2011) Reconstitution of the Cpx signaling system from cell-free synthesized proteins. Nat. Biotechnol. 28, 277–281 [DOI] [PubMed] [Google Scholar]
- 11. Liu J., Obi I. R., Thanikkal E. J., Kieselbach T., Francis M. S. (2011) Phosphorylated CpxR restricts production of the RovA global regulator in Yersinia pseudotuberculosis. PLoS One 6, e23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolfe A. J. (2010) Physiologically relevant small phosphodonors link metabolism to signal transduction. Curr. Opin. Microbiol. 13, 204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fredericks C. E., Shibata S., Aizawa S., Reimann S. A., Wolfe A. J. (2006) Acetyl phosphate-sensitive regulation of flagellar biogenesis and capsular biosynthesis depends on the Rcs phosphorelay. Mol. Microbiol. 61, 734–747 [DOI] [PubMed] [Google Scholar]
- 14. Danese P. N., Silhavy T. J. (1998) CpsP, a stress-combative member of the Cpx regulon. J. Bacteriol. 180, 831–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lima B. P., Antelmann H., Gronau K., Chi B. K., Becher D., Brinsmade S. R., Wolfe A. J. (2011) Involvement of protein acetylation in glucose-induced transcription of a stress-responsive promoter. Mol. Microbiol. 81, 1190–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klein A. H., Shulla A., Reimann S. A., Keating D. H., Wolfe A. J. (2007) The intracellular concentration of acetyl phosphate in Escherichia coli is sufficient for direct phosphorylation of two-component response regulators. J. Bacteriol. 189, 5574–5581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao R., Mack T. R., Stock A. M. (2007) Bacterial response regulators. Versatile regulatory strategies from common domains. Trends Biochem. Sci. 32, 225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DiGiuseppe P. A., Silhavy T. J. (2003) Signal detection and target gene induction by the CpxRA two-component system. J. Bacteriol. 185, 2432–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silhavy T. J., Berman M. L., Enquist L. W. (1984) Experiments with Gene Fusions, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 20. Simons R. W., Houman F., Kleckner N. (1987) Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53, 85–96 [DOI] [PubMed] [Google Scholar]
- 21. Powell B. S., Rivas M. P., Court D. L., Nakamura Y., Turnbough C. L., Jr. (1994) Rapid confirmation of single copy λ prophage integration by PCR. Nucleic Acids Res. 22, 5765–5766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chung C. T., Niemela S. L., Miller R. H. (1989) One-step preparation of competent Escherichia coli. Transformation and storage of bacterial cells in the same solution. Proc. Natl. Acad. Sci. U.S.A. 86, 2172–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beatty C. M., Browning D. F., Busby S. J., Wolfe A. J. (2003) Cyclic AMP receptor protein-dependent activation of the Escherichia coli acsP2 promoter by a synergistic class III mechanism. J. Bacteriol. 185, 5148–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chi B. K., Gronau K., Mader U., Hessling B., Becher D., Antelmann H. (2011) S. bacillithiolation protects against hypochlorite stress in Bacillus subtilis as revealed by transcriptomics and redox proteomics. Mol. Cell. Proteomics 10, M111.009506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Swingle B., Markel E., Costantino N., Bubunenko M. G., Cartinhour S., Court D. L. (2010) Oligonucleotide recombination in Gram-negative bacteria. Mol. Microbiol. 75, 138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sawitzke J. A., Costantino N., Li X. T., Thomason L. C., Bubunenko M., Court C., Court D. L. (2011) Probing cellular processes with oligo-mediated recombination and using the knowledge gained to optimize recombineering. J. Mol. Biol. 407, 45–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kinoshita E., Kinoshita-Kikuta E., Takiyama K., Koike T. (2006) Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteomics 5, 749–757 [DOI] [PubMed] [Google Scholar]
- 28. Kinoshita E., Kinoshita-Kikuta E. (2011) Improved Phos-tag SDS-PAGE under neutral pH conditions for advanced protein phosphorylation profiling. Proteomics 11, 319–323 [DOI] [PubMed] [Google Scholar]
- 29. Barbieri C. M., Stock A. M. (2008) Universally applicable methods for monitoring response regulator aspartate phosphorylation both in vitro and in vivo using Phos-tag-based reagents. Anal. Biochem. 376, 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chohnan S., Izawa H., Nishihara H., Takamura Y. (1998) Changes in size of intracellular pools of coenzyme A and its thioesters in Escherichia coli K-12 cells to various carbon sources and stresses. Biosci. Biotechnol. Biochem. 62, 1122–1128 [DOI] [PubMed] [Google Scholar]
- 31. Chang D. E., Shin S., Rhee J. S., Pan J. G. (1999) Acetate metabolism in a pta mutant of Escherichia coli W3110. Importance of maintaining acetyl coenzyme A flux for growth and survival. J. Bacteriol. 181, 6656–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang X. J., Seto E. (2008) Lysine acetylation. Codified cross-talk with other post-translational modifications. Mol. Cell 31, 449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu L. I., Lima B. P., Wolfe A. J. (2010) Bacterial protein acetylation. The dawning of a new age. Mol. Microbiol. 77, 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thao S., Chen C. S., Zhu H., Escalante-Semerena J. C. (2010) Nϵ-lysine acetylation of a bacterial transcription factor inhibits Its DNA-binding activity. PLoS One 5, e15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gaal T., Ross W., Blatter E. E., Tang H., Jia X., Krishnan V. V., Assa-Munt N., Ebright R. H., Gourse R. L. (1996) DNA-binding determinants of the α subunit of RNA polymerase. Novel DNA-binding domain architecture. Genes Dev. 10, 16–26 [DOI] [PubMed] [Google Scholar]
- 36. Dang W., Steffen K. K., Perry R., Dorsey J. A., Johnson F. B., Shilatifard A., Kaeberlein M., Kennedy B. K., Berger S. L. (2009) Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature 459, 802–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jackowski S., Rock C. O. (1986) Consequences of reduced intracellular coenzyme A content in Escherichia coli. J. Bacteriol. 166, 866–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shao X., Grishin N. V. (2000) Common fold in helix-hairpin-helix proteins. Nucleic Acids Res. 28, 2643–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schweimer K., Prasch S., Sujatha P. S., Bubunenko M., Gottesman M. E., Rösch P. (2011) NusA interaction with the α subunit of E. coli RNA polymerase is via the UP element site and releases autoinhibition. Structure 19, 945–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Busby S., Ebright R. H. (1999) Transcription activation by catabolite activator protein (CAP). J. Mol. Biol. 293, 199–213 [DOI] [PubMed] [Google Scholar]
- 41. Kenney L. J. (2002) Structure/function relationships in OmpR and other winged-helix transcription factors. Curr. Opin. Microbiol. 5, 135–141 [DOI] [PubMed] [Google Scholar]
- 42. McCleary W. R., Stock J. B., Ninfa A. J. (1993) Is acetyl phosphate a global signal in Escherichia coli? J. Bacteriol. 175, 2793–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wanner B. L. (1993) Gene regulation by phosphate in enteric bacteria. J. Cell. Biochem. 51, 47–54 [DOI] [PubMed] [Google Scholar]
- 44. Wolfe A. J. (2005) The acetate switch. Microbiol. Mol. Biol. Rev. 69, 12–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Keating D. H., Shulla A., Klein A. H., Wolfe A. J. (2008) Optimized two-dimensional thin layer chromatography to monitor the intracellular concentration of acetyl phosphate and other small phosphorylated molecules. Biol. Proced. Online 10, 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wolfe A. J., Chang D. E., Walker J. D., Seitz-Partridge J. E., Vidaurri M. D., Lange C. F., Prüss B. M., Henk M. C., Larkin J. C., Conway T. (2003) Evidence that acetyl phosphate functions as a global signal during biofilm development. Mol. Microbiol. 48, 977–988 [DOI] [PubMed] [Google Scholar]
- 47. Xu H., Caimano M. J., Lin T., He M., Radolf J. D., Norris S. J., Gherardini F., Wolfe A. J., Yang X. F. (2010) Role of acetyl phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi. PLoS Pathog. 6, e1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Park D., Ciezki K., van der Hoeven R., Singh S., Reimer D., Bode H. B., Forst S. (2009) Genetic analysis of xenocoumacin antibiotic production in the mutualistic bacterium Xenorhabdus nematophila. Mol. Microbiol. 73, 938–949 [DOI] [PubMed] [Google Scholar]
- 49. Boll J. M., Hendrixson D. R. (2011) A specificity determinant for phosphorylation in a response regulator prevents in vivo cross-talk and modification by acetyl phosphate. Proc. Natl. Acad. Sci. U.S.A. 108, 20160–20165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants. The Keio collection. Mol. Syst. Biol. 2, 2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kumari S., Beatty C. M., Browning D. F., Busby S. J., Simel E. J., Hovel-Miner G., Wolfe A. J. (2000) Reguation of acetyl coenzyme A synthetase in Escherichia coli. J. Bacteriol. 182, 4173–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kitagawa M., Ara T., Arifuzzaman M., Ioka-Nakamichi T., Inamoto E., Toyonaga H., Mori H. (2005) Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive). Unique resources for biological research. DNA Res. 12, 291–299 [DOI] [PubMed] [Google Scholar]
- 53. Chi B. K., Albrecht D., Gronau K., Becher D., Hecker M., Antelmann H. (2010) The redox-sensing regulator YodB senses quinones and diamide via a thiol-disulfide switch in Bacillus subtilis. Proteomics 10, 3155–3164 [DOI] [PubMed] [Google Scholar]