Background: How the adaptive ER stress response causes cell death remains enigmatic.
Results: cIAP1 prevents ER stress-mediated apoptosis in pancreatic β-cells by acting as an E3 ligase inducing CHOP ubiquitination and degradation.
Conclusion: cIAP1 is a key determinative factor for β-cell survival under ER stress.
Significance: The findings provide a new insight into the mechanistic determination of cell fate following ER stress response.
Keywords: Beta Cell, Cell Death, Diabetes, ER Stress, Lipotoxicity, Ubiquitin Ligase, Unfolded Protein Response, CHOP, cIAP1
Abstract
Lipotoxicity in pancreatic β-cells, arising from excess free fatty acid-induced endoplasmic reticulum (ER) stress response, has been recognized as a key pathogenic factor causing loss of β-cell mass and contributing to type 2 diabetes. However, how the adaptive ER stress response causes cell death remains enigmatic. We report herein a critical role of cellular inhibitor of apoptosis protein-1 (cIAP1) in controlling β-cell survival under ER stress. While both palmitate and palmitoleate induced an overt ER stress response, lipotoxicity was only observed in β-cells exposed to palmitate but not palmitoleate. Interestingly, cells treated with palmitoleate exerted a sustainable level of cIAP1, whereas the protein quickly degraded following palmitate treatment. Enforced overexpression of cIAP1 prevented palmitate-induced cell death. In contrast, siRNA-mediated knockdown of cIAP1 in β-cells or knock-out of cIap1 in mouse embryonic fibroblasts not only increased palmitate-induced apoptosis, but also committed cells to death in response to the nontoxic palmitoleate treatment. Of importance, we found that cIAP1 functions as an E3 ubiquitin ligase promoting ubiquitination and degradation of C/EBP homologous protein (CHOP), a key mediator of ER stress-induced cell death. These findings define a novel mechanism for β-cell survival under ER stress and help to identify targets for therapeutic intervention against lipotoxicity in β-cells.
Introduction
Eukaryotic cells have evolved multiple specific pathways to respond to various environmental challenges for maintaining normal function and cell survival. One of the most important pathways is triggered by endoplasmic reticulum (ER)3 stress, which is referred to as the unfolded protein response (UPR). As a prototypical, professional secretory cell type, pancreatic β-cells have a highly developed ER system to facilitate the folding of large amounts of insulin and various glycoproteins for secretion, and thus are vulnerable to ER stress. Indeed, the process of ER stress response is critically involved in β-cell biology, either under normal physiological or disease conditions (1–3). For instance, under obese and insulin resistant conditions, β-cells are confronted with a dramatically enhanced demand for insulin production, accompanied by multiple pathological factors such as elevated levels of free fatty acids (FFA). Such insults place a strain on the cell and cause prolonged or chronic activation of the UPR pathways, ultimately leading to β-cell dysfunction and cell death. This has been suggested to be a key pathogenic event contributing to the development of type 2 diabetes (T2D) (2, 3).
CHOP (C/EBP homologous protein), a transcription factor induced upon UPR activation, is regarded as a key mediator of cell death in response to ER stress (4–6). Overexpression or microinjection of CHOP was reported to induce apoptosis through a mechanism associated with down-regulation of Bcl-2 or increased Bax translocation to the mitochondrial membrane (7, 8). In addition, the heterodimer of CHOP with C/EBPα binds directly to an element in the first intron of the gene encoding the BH3-only protein Bim, promoting its expression and leading to apoptosis (9). Furthermore, several studies from chop deficient mice indicate that CHOP is required for ER stress-induced apoptosis in a diverse range of cell types, including pancreatic β-cells (6, 10, 11). However, while the important role of CHOP in mediating cell death has been clearly documented, the ER stress-induced CHOP expression appears not to uniformly commit cells to death. For instance, perk−/− and eIF2αS51A knock-in cells that are unable to induce CHOP expression, display a hypersensitivity to ER stress-induced apoptosis (12, 13). Moreover, recent studies suggest that CHOP plays an important protective role to improve the ER protein-folding capacity and help cellular recovery through the transcriptional regulation of target genes or microRNAs, including GADD34 (5), ODZ4 (14), and miR-708 (15). The divergent findings that CHOP plays roles in both cellular recovery and cell death, indicate additional mechanisms are responsible for determining cell fate following UPR-induced CHOP production.
Cellular inhibitor of apoptosis protein-1 (cIAP1/birc2) belongs to the IAP family of anti-apoptotic proteins, which is characterized by the presence of baculoviral IAP repeat (BIR) motifs (16). Like other prototypic IAPs, cIAP1 contains a central CARD (caspase-activating recruitment domain) and a C-terminal RING domain that possesses E3 ubiquitin ligase activity. As such, besides the potent anti-apoptotic property, cIAP1 can also act as an E3 ligase to ubiquitylate and regulate target proteins, thereby participating in the regulation of multiple cellular functions (reviewed in Refs. 16, 17). Several recent studies have reported that ER stress increases cIAP1 expression in cancer cells through the UPR pathway (18, 19). The induction of cIAP1 is suggested to be important for cancerous cell survival under stress conditions (18, 19). However, how cIAP1 promotes ER stress-associated cell survival has not been elucidated and its role in pancreatic β-cells is unknown. In the present study, we have provided substantial evidence showing a critical role of cIAP1 in protecting β-cells against ER stress-mediated lipotoxicity. We found that cIAP1 acts as an E3 ubiquitin ligase promoting ubiquitination and degradation of the pro-apoptotic protein CHOP, thereby preventing cell death. These functional and mechanistic data provide a new insight into the regulation of ER stress-associated cell survival in pancreatic β-cells, and help create a novel strategy for the management of T2D by protecting β-cells against lipotoxicity.
EXPERIMENTAL PROCEDURES
Cell Culture, Transfection, and Treatments
All cell lines were maintained at 37 °C with 5% CO2 in DMEM containing 10% (v/v) FCS (except rat INS-1 β-cells in RPMI1640 medium supplemented with 10% FCS). FuGene HD reagent (Roche) was used for transient transfection according to the manufacturer's protocol. siRNA against CHOP and cIAP1 and their control siRNA (purchased from Ambion) were transfected into cells using HiPerFect (Qiagen) as described previously (20). Palmitate or palmitoleate (Sigma) was dissolved in 0.1 m NaOH by heating at 90 °C for 10min, and then prepared a stock solution containing 5 mm FFA coupled with 5% (w/v) fatty acid-free BSA. For treatment of cells, the stock FFA solution was added into serum-free medium at the indicated concentrations for a desired time period.
Pancreatic Islet Isolation
C57BL/6 mice were housed under conventional conditions and used for the research according to the protocol approved by the Animal Care and Ethics Committee of the University of Sydney. Islets were isolated from adult mice following injection of collagenase through the pancreatic duct as previously described (21). The islets were allowed to recover for 48 h in a humidified cell culture incubator before use.
Cell Viability and Cell Death Assays
Cell viability was measured by using colorimetric MTS assay as described previously (22). For cell death assays, cells were co-stained with propidium iodide (PI) and annexin V-FITC using an Apoptosis and Necrosis Quantification Kit (Biotium), followed by flow cytometry analysis.
Western Blotting and Immunoprecipitations
Standard procedures for Western blotting and immunoprecipitations were followed as previously described (23). Antibodies used in this study include: anti-ubiquitin, anti-cytochrome C, anti-phospho-eIF2α, anti-CHOP, anti-caspase 3, anti-COX IV, anti-HA tag (all from Cell Signaling, Danvers, MA), anti-FLAG tag, anti-β-actin (Sigma), and anti-cIAP1 (gift from Dr. John Silke).
Ubiquitination Assays
For in vivo ubiquitination assays, cells were transiently transfected with various combinations of plasmids as indicated and pre-treated with 10 μm MG132. 10 mm N-ethylmaleimide (Sigma) and 10 μm MG132 (Calbiochem) were added to the lysis buffer. Lysates were cleared by centrifugation and proteins were dissociated by heating at 95 °C for 10 min. Samples were diluted, immunoprecipitated with anti-CHOP antibodies, anti-cIAP1 antibodies, or anti-Flag-conjugated Sepharose (Sigma), and immunoblotted as indicated. For in vitro ubiquitination assays, the reactions were carried out at 37 °C for 30 min in a 20 μl reaction system containing 1× ubiquitin conjugation reaction buffer, 1× PhosSTOP complete phosphatase inhibitor mixture (Roche), 10 mm N-ethylmaleimide, 1× protease inhibitor mixture, 5 mm Mg-ATP, 50 μm ubiquitin, 100 nm E1 ubiquitin-activating enzyme UBE1 (Boston Biochem), and 20 μg/ml of various E2 ubiquitin-conjugating enzymes (all from Boston Biochem). 0.3 μg of recombinant CHOP protein was used as a substrate in the reaction. 0.3 μg of either full-length or truncated cIAP1 recombinant proteins was added as E3 ligase to the reaction. The reactions were stopped by heating the mix with NuPAGE LDS sample buffer at 80 °C for 5 min, and analyzed by Western blotting as indicated.
Statistical Analysis
Data are presented as means ± S.D. from an appropriate number of experiments as indicated in the figure legends, and analyzed with Graph-Pad Prism (Version 5.0). Statistical significance was evaluated with an unpaired two-tailed t test, and differences at values of p < 0.05 were considered significant.
RESULTS
Palmitate-induced Lipotoxicity in Pancreatic β-Cells Is Associated with ER Stress and CHOP Induction
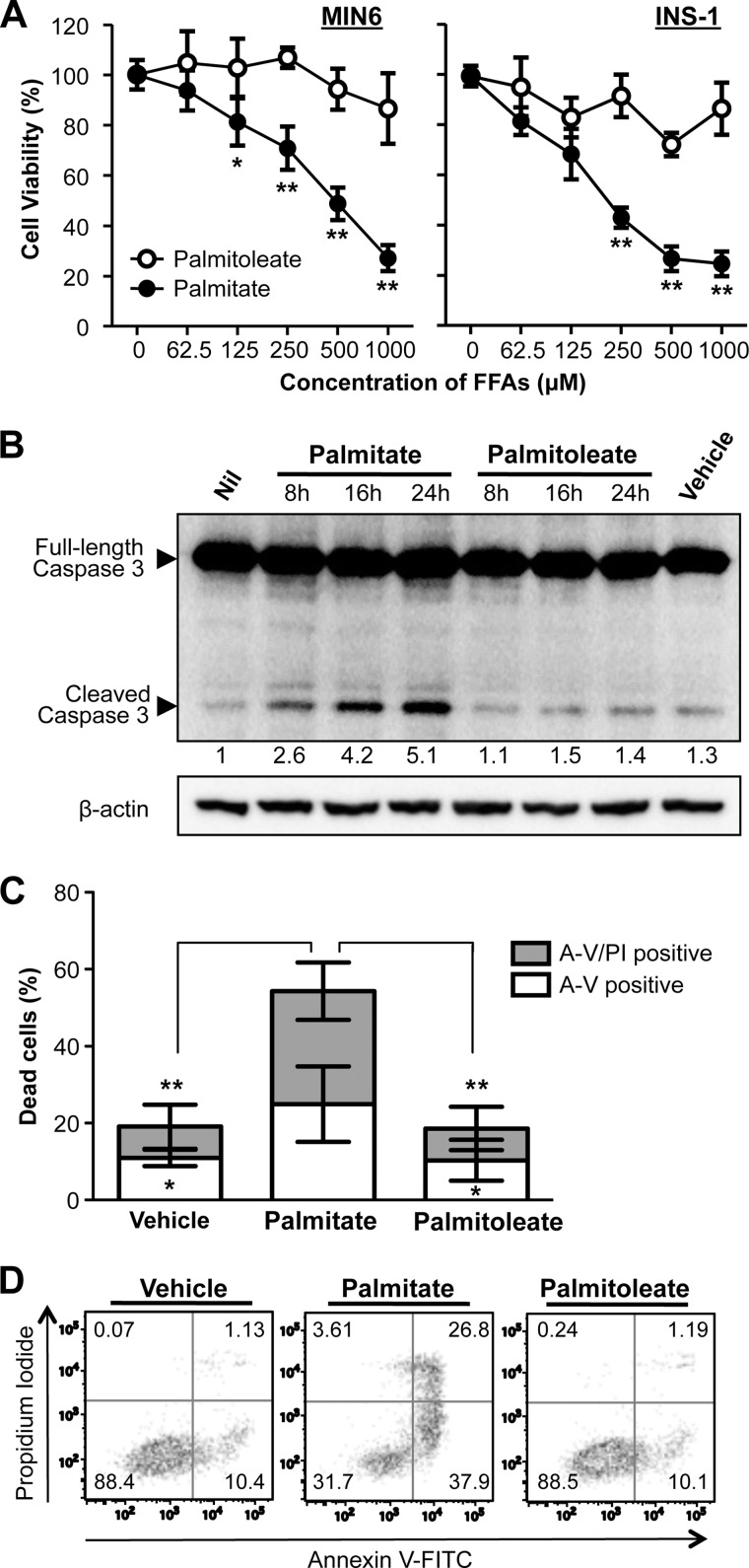
Lipotoxicity arising from chronic exposure of saturated FFA, such as palmitate, has been shown to cause β-cell dysfunction and apoptosis (24, 25). Treatment of MIN-6 β-cells for 24 h with palmitate resulted in a dose-dependent reduction in cell viability (Fig. 1A). In contrast, the unsaturated FFA palmitoleate had no evident cytotoxic effects on β-cells (Fig. 1A). The effects of these FFAs on cell viability were confirmed in INS-1 β-cells (Fig. 1A, right panel). In agreement with previous reports showing that the lipotoxicity was mediated through the apoptotic pathway (21, 26), a time-dependent increase in caspase-3 cleavage was observed in β-cells exposed to palmitate, but not palmitoleate (Fig. 1B). In addition, the palmitate-induced apoptotic cell death was confirmed by using flow cytometry analysis of β-cells co-stained with annexin V and propidium iodide (Fig. 1, C and D).
FIGURE 1.
Palmitate, but not palmitoleate, exhibits lipotoxicity in β-cells. A, cell viability was determined by MTS colorimetric assay in MIN6 and INS-1 β-cells treated for 24 h with palmitate or palmitoleate at increasing concentrations as indicated. Data are expressed as a percentage of untreated control cells. B, MIN6 cells were treated with vehicles (0.5% (w/v) BSA), palmitate or palmitoleate (500 μm) for the indicated time points, and then caspase-3 cleavage was determined by Western blotting. Numbers below lanes indicate band intensity of cleaved caspase 3 relative to that of the loading control β-actin. The blots represent four independent experiments. C, MIN6 cells were treated for 24 h with vehicles, palmitate or palmitoleate (500 μm), and then co-stained with propidium iodide (PI) and annexin V-FITC (A-V) followed by flow cytometric analysis. Dead cells refer to percentage of cells encompassing both A-V single positive and A-V/PI double-positive cells. Representative scatter plots from flow cytometric analysis are shown in D. Error bars represent S.D. of the mean value from three independent experiments (*, p < 0.05, **, p < 0.01).
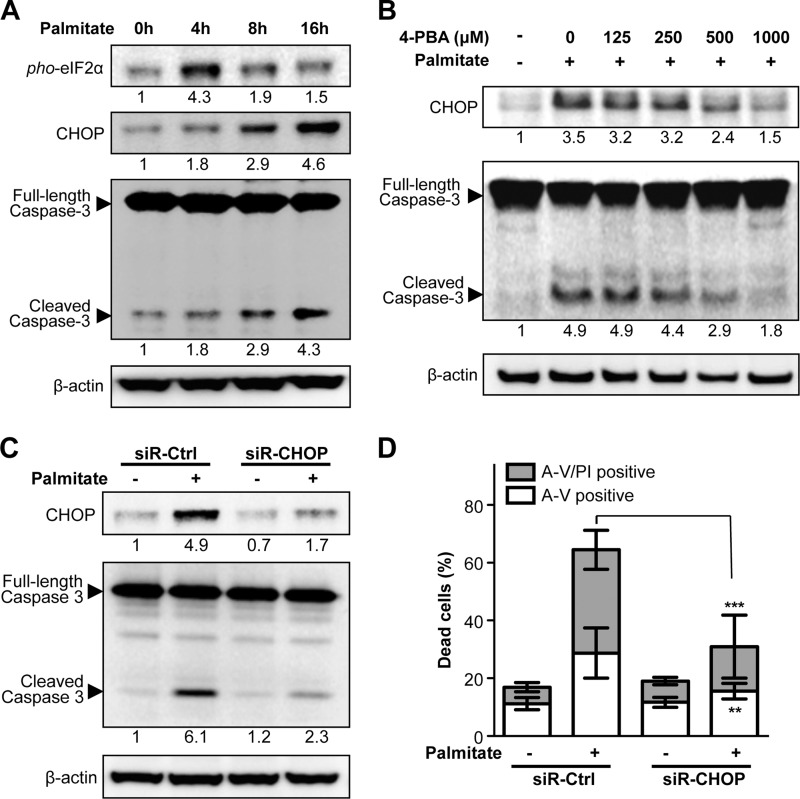
A number of studies have demonstrated an essential role for the UPR in mediating lipotoxicity through CHOP production (as reviewed in Ref. 27). Deletion of the Chop gene has been reported to prevent apoptosis and improve cellular function in β-cells under ER stress conditions in several diabetic models (10, 11), underlining a critical role of CHOP in ER stress-induced β-cell death. Consistent with this notion, palmitate treatment resulted in a significant increase in the levels of phosphorylated eIF2α and CHOP expression in a time-dependent fashion in MIN6 β-cells (Fig. 2A), indicating that the PERK-eIF2α UPR pathway is activated under this condition. In the presence of a chemical ER chaperone, 4-phenyl butyric acid (4-PBA), palmitate-induced CHOP expression was strikingly attenuated, with a concomitant reduction in caspase-3 activity (Fig. 2B), suggesting an important role of UPR-dependent CHOP expression in the lipotoxicity. Furthermore, knockdown of CHOP expression by using its specific siRNA in MIN6 β-cells significantly inhibited palmitate-induced caspase-3 activity (Fig. 2C) and cell death (Fig. 2D). Collectively, these data support the notion that CHOP induction plays an important role in ER stress-mediated lipotoxicity in β-cells.
FIGURE 2.
Palmitate-induced lipotoxicity is mediated through ER stress response and depends on CHOP expression. A, MIN6 cells were treated with 500 μm palmitate for the indicated time points, and changes in levels of phosphorylated eIF2α, CHOP, and cleaved caspase 3 were analyzed by Western blotting (β-actin was used as a loading control). B, MIN6 cells were treated for 1 h with 4-PBA at the indicated concentrations prior to palmitate (500 μm) treatment for an additional 24 h. C, MIN6 cells were transfected with control siRNA or siRNA against CHOP for 48 h prior to palmitate (500 μm) treatment for an additional 24 h. CHOP induction and cleaved caspase 3 were analyzed by Western blotting, and (D) cell death was determined by flow cytometry analysis, displaying both A-V single positive and A-V/PI double positive cells. Data are mean ± S.D. (n = 3). **, p < 0.01, ***, p < 0.001, CHOP-siRNA versus control siRNA. Numbers below lanes (A, B, and C) indicate band intensity relative to that of the loading control β-actin. Representative blots from at least three independent experiments are shown.
ER Stress-mediated Lipotoxicity Is Associated with cIAP1 Degradation
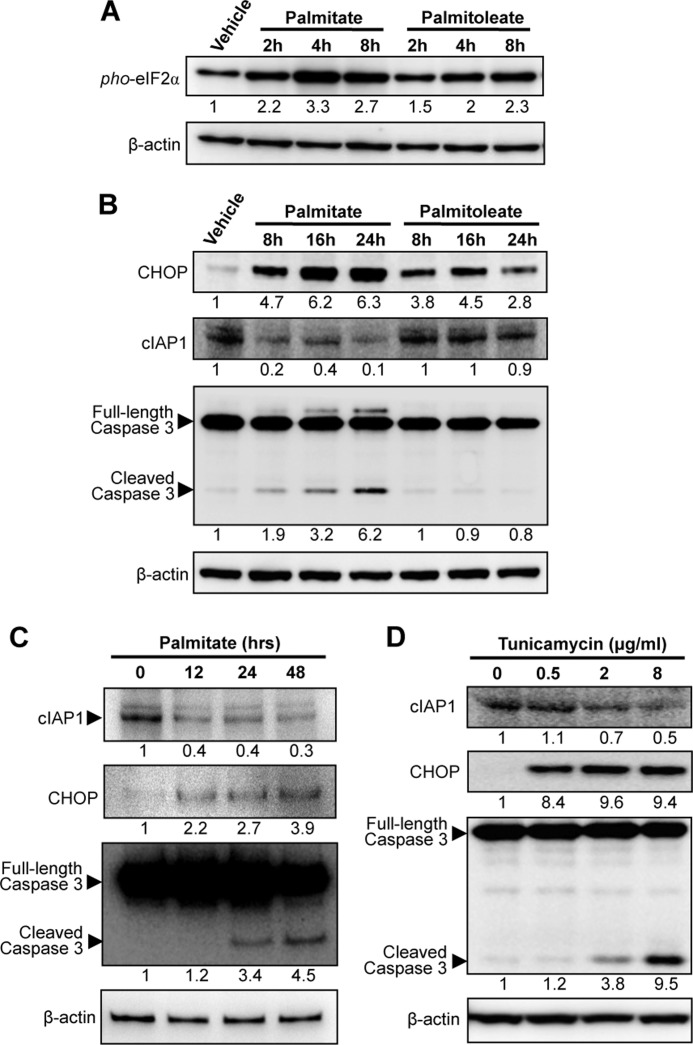
Because palmitoleate had no apoptotic effect on β-cells, we thought that this unsaturated FFA might be unable to induce CHOP expression. Surprisingly, palmitoleate was indeed able to activate the PERK-eIF2α UPR pathway, as reflected by an increase in both eIF2α phosphorylation (Fig. 3A) and CHOP induction (Fig. 3B), albeit to a lower level than that observed in palmitate-treated cells. Remarkably, palmitoleate-induced CHOP expression declined sharply after 24 h treatment, whereas a prolonged increase in CHOP expression was observed after palmitate treatment for at least 24 h (Fig. 3B). These observations suggest that additional factors, dependent or independent of CHOP, are involved in regulating β-cell death following the PERK-eIF2α UPR pathway activation.
FIGURE 3.
Down-regulation of cIAP1 is associated with CHOP expression in cells under ER stress. MIN6 cells were treated with 500 μm palmitate or palmitoleate for the indicated time points, and then changes in levels of (A) phosphorylated eIF2α, (B) CHOP, cIAP1, and cleaved caspase 3 were analyzed by Western blots. C, murine islets were treated with 500 μm palmitate for the indicated time points and (D) MIN6 cells treated for 24 h with tunicamycin at increasing concentrations, and then immunoblotted as indicated. Numbers below lanes (A--D) indicate band intensity relative to that of the loading control β-actin. Shown are representative blots from at least three independent experiments.
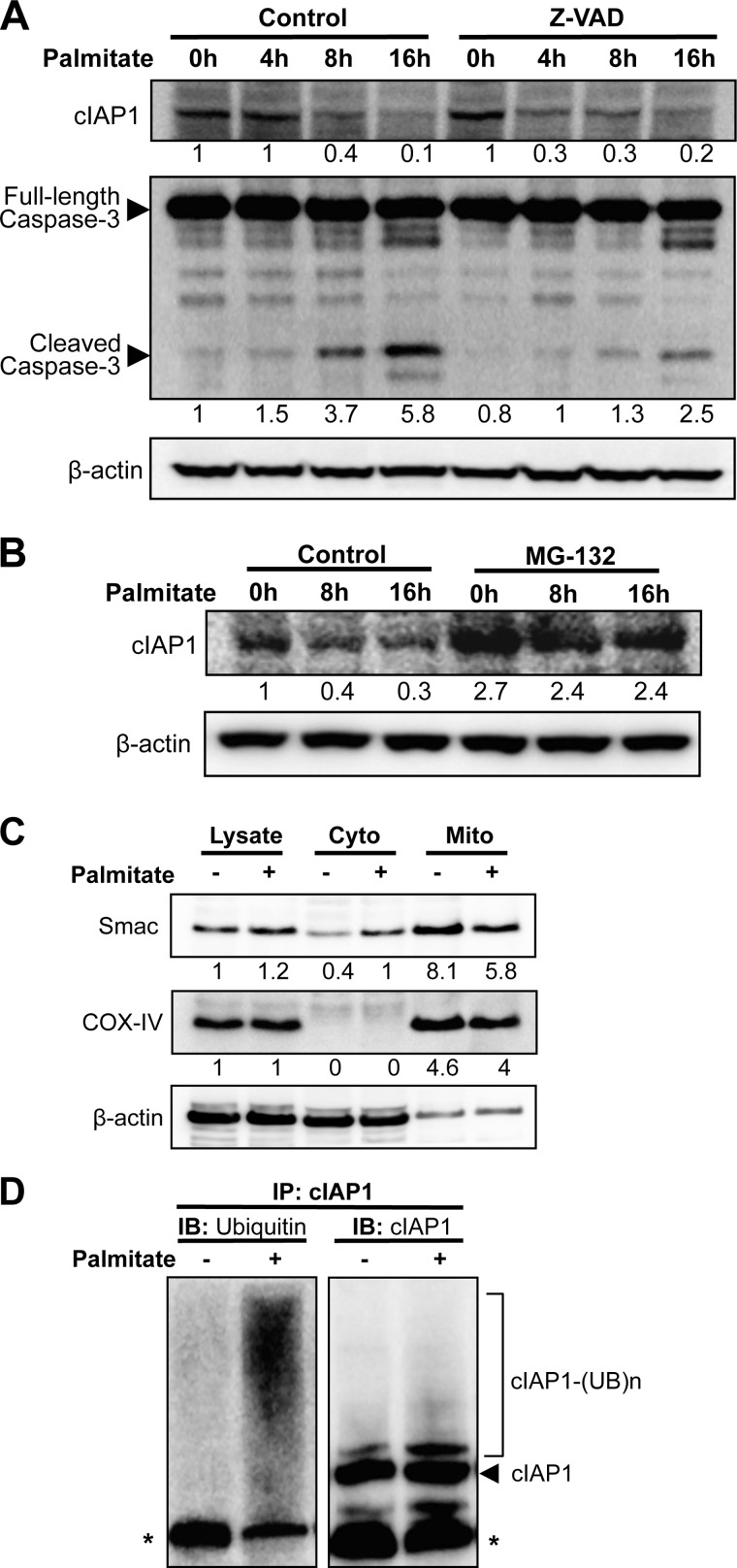
In an attempt to further elucidate the mechanisms of ER stress-mediated lipotoxicity, we sought to examine the role of cIAP1 that was reportedly up-regulated by ER stress response (19). Interestingly, treatment of β-cells with palmitate, but not palmitoleate, resulted in a significant decrease in cIAP1 expression in a time-dependent manner (Fig. 3B, middle panel). The palmitate-induced time-dependent reduction of cIAP1 and a concomitant increase in CHOP expression and cleaved caspase-3 were further confirmed in primary pancreatic islets isolated from mice (Fig. 3C). Furthermore, treatment of MIN6 β-cells with tunicamycin, a known chemical ER stressor, also resulted in a dose-dependent reduction of cIAP1, coinciding with an increase in CHOP expression and caspase-3 cleavage (Fig. 3D). These data illustrate that cIAP1 degradation is correlated with ER stress-mediated cell death. However, pre-treatment with the pan-caspase inhibitor Z-VAD-fmk did not prevent cIAP1 reduction, although caspase-3 activation was blocked by the treatment (Fig. 4A), indicating that the loss of cIAP1 was not a consequence of apoptosis in palmitate-treated cells. Strikingly, addition of the proteasomal inhibitor MG-132 completely restored cIAP1 levels (Fig. 4B), suggesting that proteasome-mediated degradation is, at least in part, responsible for the palmitate-induced cIAP1 reduction.
FIGURE 4.
Palmitate induces cIAP1 ubiquitination and proteasomal degradation. A, MIN6 cells were treated for 1h with 20 μm Z-VAD, or (B) 20 μm MG-132 prior to exposure to 500 μm palmitate for the indicated additional time points, and then cIAP1 expression levels were determined by Western blots. C, Western-blot analysis of Smac expression in total lysate or cytosolic (Cyto) and mitochondrial (Mito) fractions isolated from MIN6 cells treated with or without 500 μm palmitate for 24 h. COX-V was used as a mitochondrial marker. Numbers below lanes (A--C) indicate band intensity relative to that of the loading control β-actin. Shown are representative blots from at least three independent experiments. D, in vivo ubiquitination of cIAP1 was analyzed in MIN6 cells treated for 6 h with or without palmitate (500 μm) in the presence of MG-132 (20 μm). The endogenous cIAP1 was immunoprecipitated and then immunoblotted with antibodies against ubiquitin and cIAP1 (* indicates IgG heavy chains).
Because a peptide derived from a mitochondrial protein, Smac (second mitochondria-derived activator of caspases, also known as DIABLO), was demonstrated to induce cIAP1 autoubiquitination and proteasome-mediated degradation (28, 29), we asked whether palmitate might activate this pathway. Indeed, treatment of MIN6 β-cells with palmitate resulted in a significant reduction of Smac expression in the mitochondrial fractions and a simultaneous increase in cytosolic levels of the protein (Fig. 4C), showing an ability of palmitate to induce Smac release from mitochondria. We then examined the effect of palmitate on cIAP1 ubiquitination. As shown in Fig. 4D, treatment of cells with palmitate caused robust ubiquitination of cIAP1 as determined by in vivo ubiquitination assays. Collectively, these data demonstrate that the Smac/cIAP1 pathway is activated by palmitate treatment and associated with the ER stress-mediated lipotoxicity in β-cells.
cIAP1 Induces CHOP Ubiquitination and Degradation
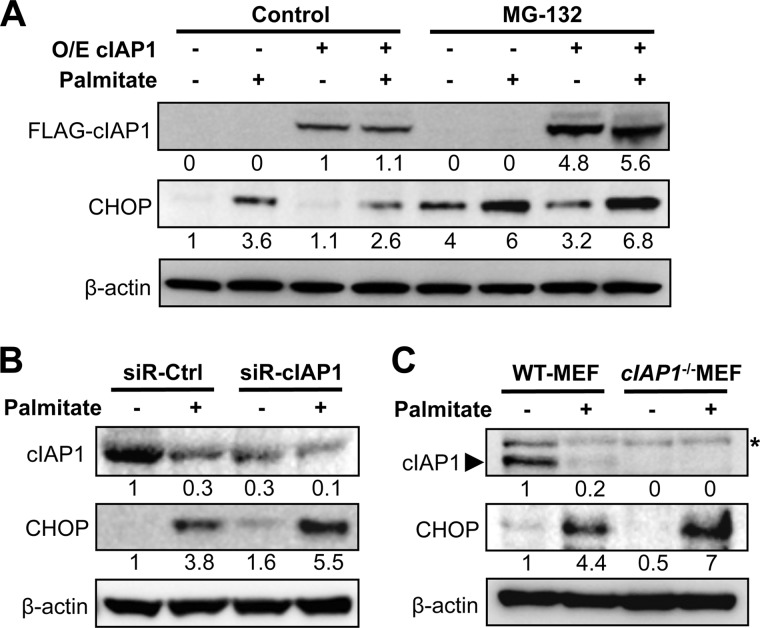
It was observed that while CHOP expression was induced by ER stressors, including palmitate, palmitoleate, or tunicamycin, high levels of CHOP were sustained under conditions that induced cIAP1 degradation, i.e. palmitate or tunicamycin treatment in either β-cell lines or primary islets (Fig. 3, B, C, and D). To further verify the relationship between cIAP1 and CHOP expression, we manipulated cIAP1 levels by overexpression of the gene or knockdown of the protein expression using specific siRNA. Cells overexpressing cIAP1 exhibited a significant reduction in palmitate-induced CHOP expression (Fig. 5A). This reduction was completely prevented by the proteasome inhibitor MG-132, suggesting a post-translational effect of cIAP1 on CHOP expression. In contrast, the palmitate-induced CHOP expression was significantly enhanced by the siRNA-mediated knockdown of cIAP1 in β-cells (Fig. 5B). Moreover, cIap−/− mouse embryonic fibroblasts (MEFs) exhibited higher levels of CHOP expression in response to palmitate treatment than that in wild-type cells (Fig. 5C). Together, these data suggest that cIAP1 may regulate cellular levels of CHOP following its induction by ER stress.
FIGURE 5.
cIAP1 regulates CHOP degradation under ER stress conditions. A, MIN6 cells were transfected with FLAG-tagged cIAP1 (O/E) or empty vectors for 30 h, and (B) transfected with siRNA targeting cIAP1 or control siRNA for 48 h. After the treatment, cells were exposed to 500 μm palmitate for an additional 24 h, 20 μm of MG-132 was introduced for the last 6 h of palmitate treatment, then immunoblotted. C, wild-type and cIap1−/− MEFs were treated with or without 500 μm palmitate for 24 h and immunoblotted as indicated (*, nonspecific bands). Numbers below lanes (A--C) indicate band intensity relative to that of the loading control β-actin. Shown are representative blots from at least three independent experiments.
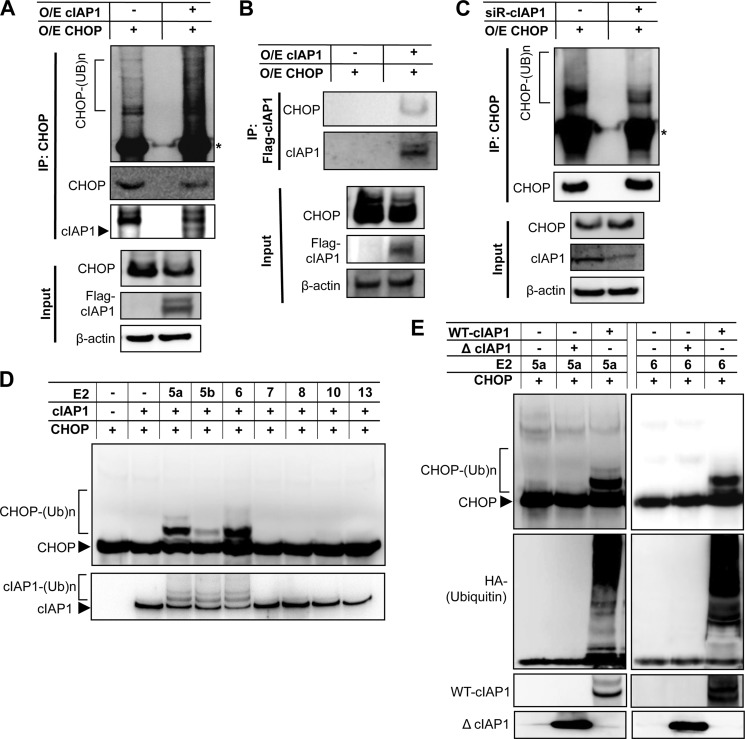
As cIAP1 has been recently identified as an E3 ubiquitin ligase that regulates ubiquitin- mediated proteasomal degradation of its substrates (17, 30), we investigated whether CHOP is a substrate of cIAP1. To this end, we transiently co-transfected HEK293 cells with FLAG-tagged cIAP1 and CHOP. Co-expression of CHOP and cIAP1 resulted in a marked increase in the levels of CHOP ubiquitination, as detected by immunoblotting after immunoprecipitation of CHOP (Fig. 6A). In addition, FLAG-tagged cIAP1 was coimmunoprecipitated with CHOP, and conversely, CHOP was detected in the immunocomplex of anti-FLAG (Fig. 6B), indicating an interaction between cIAP1 and CHOP. Furthermore, in vivo ubiquitination assays show that ubiquitinated CHOP was significantly reduced by the siRNA-mediated knockdown of cIAP1 (Fig. 6C), further supporting the role of cIAP1 in CHOP ubiquitination. To directly assess E3 ubiquitin ligase activity of cIAP1 toward CHOP, we carried out in vitro ubiquitination assays using recombinant CHOP protein as substrate, in the presence of GST-fused cIAP1, along with different E2 ubiquitin-conjugating enzymes. As shown in Fig. 6D, cIAP1 was capable of inducing CHOP ubiquitination in vitro in the presence of UbcH5a, UbcH5b and UbcH6, but not other E2 enzymes, including UbcH7, UbcH8, UbcH10, and UbcH13. Moreover, while the full-length wild-type cIAP1 induced CHOP ubiquitination in the presence of UbcH5a or UbcH6, the truncated cIAP1 mutant (ΔcIAP1) that lacks E3 ligase activity failed to do so (Fig. 6E), indicating that the E3 ligase activity of cIAP1 is required for CHOP ubiquitination.
FIGURE 6.
cIAP1 promotes CHOP ubiquitination. HEK293 cells were co-transfected with Flag-tagged cIAP1 and CHOP for 24 h and treated with 10 μm MG-132 for an additional 16 h. Cell lysates were (A) immunoprecipitated by anti-CHOP antibodies followed by immunoblots to detect in vivo ubiquitination of CHOP using antibodies against ubiquitin, CHOP and Flag, respectively (*, IgG heavy chains), and (B) immunoprecipitated by anti-Flag antibodies and immunoblotted with antibodies against CHOP and cIAP1, showing an interaction between these two proteins. C, cells were co-transfected with CHOP and siRNA against cIAP1, and then in vivo ubiquitination of CHOP was analyzed as described above. D, CHOP ubiquitination was determined by in vitro ubiquitination assays with an assay system containing purified recombinant full-length cIAP1, recombinant CHOP, HA-tagged ubiquitin, and a series of E2 enzymes as indicated. E, CHOP ubiquitination was measured by the assays containing either recombinant full-length cIAP1 or ΔcIAP1 that lacks a RING domain in the presence of the E2 enzyme UbcH5a (left panels) or UbcH6 (right panels). Shown are representative blots from at least three independent experiments.
cIAP1 Prevents ER Stress-mediated Lipotoxicity
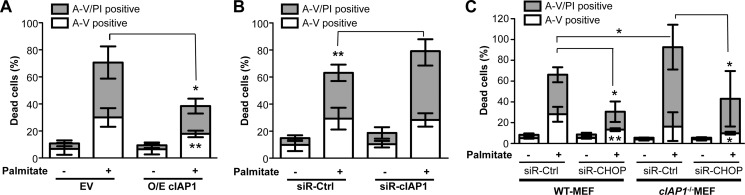
Considering both the known pro-apoptotic activity of CHOP and anti-apoptotic property of cIAP1, the finding that cIAP1 promotes CHOP ubiquitination and degradation led us as to explore the role of cIAP1 in β-cell survival under lipotoxic stress conditions. As expected, overexpression of cIAP1 significantly inhibited palmitate-induced cell death (Fig. 7A), whereas lipotoxicity was enhanced by the siRNA-mediated knockdown of cIAP1 in β-cells (Fig. 7B). Furthermore, cIap1−/− MEFs exhibited significantly more cell death than wild-type cells in response to palmitate treatment (Fig. 7C), underlining an important role of cIAP1 in protecting the ER stress-mediated lipotoxicity. Moreover, the enhanced lipotoxicity in palmitate-treated cIap1−/− MEFs was prevented by the siRNA-mediated knockdown of CHOP (Fig. 7C), further supporting the mechanistic connections between cIAP1 and CHOP in protection against cell death under ER stress.
FIGURE 7.
cIAP1 protects β-cells against ER stress-mediated cell death. Dead cells were quantified by flow cytometric analysis with PI and annexin V (A–V) co-staining, after treatment with palmitate for 24 h at the indicated concentrations in (A) MIN6 cells transfected with FLAG-tagged cIAP1 or empty vectors (EV) for 36 h, (B) MIN6 cells, and (C) wild-type or cIap1−/− MEFs transfected with siRNA against CHOP or control siRNA for 48 h. Data are shown as mean ± S.D. (n ≥ 3). *, p < 0.05; **, p < 0.01.
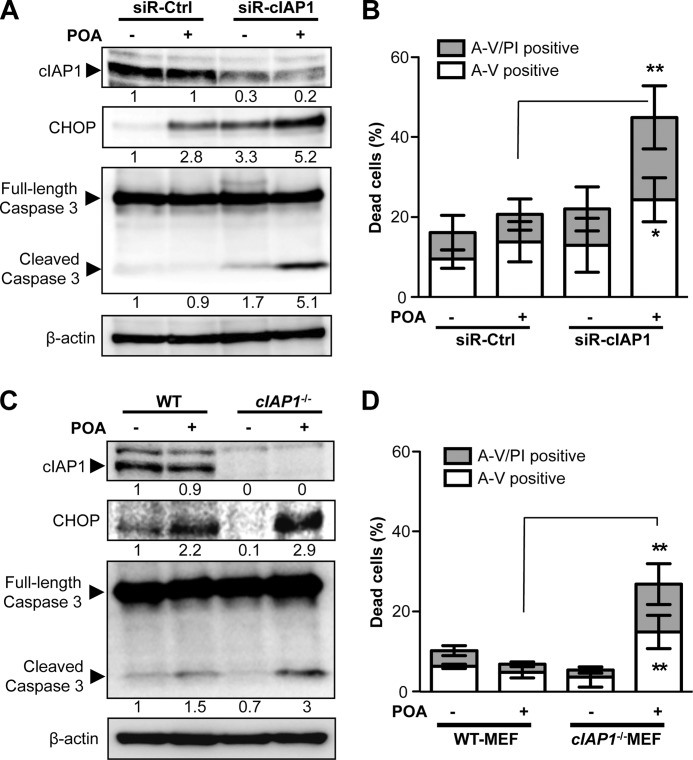
As aforementioned, the unsaturated FFA palmitoleate had no significant cytotoxic effect on β-cells (Fig. 1), although it was capable of inducing the PERK-eIF2α UPR pathway activation and CHOP expression (Fig. 3, A and B). We wanted to further explore the role of cIAP1 in cell survival under this setting. By knocking down cIAP1 expression, the cIAP-targeted siRNA induced a significant increase in CHOP expression and caspase-3 cleavage in β-cells exposed to palmitoleate (Fig. 8A). Consequently, a significant proportion of cells treated with the cIAP1-siRNA underwent cell death in response to palmitoleate treatment, compared with the control siRNA-treated cells (Fig. 8B). In line with these findings, treatment of cIap1−/− MEFs with palmitoleate also resulted in a significant increase in caspase-3 activity and cell death (Fig. 8, C and D), whereas the palmitoleate-treated wild-type MEFs displayed no evident cytotoxicity. Collectively, these data further support the critical role of cIAP1 in β-cell survival under FFA-induced ER stress.
FIGURE 8.
cIAP1 promotes cell survival in palmitoleate-treated cells. A and B, MIN6 cells transfected with siRNA against cIAP1 or control siRNA for 48 h and (C and D) wild-type or cIap1−/− MEFs were treated with 500 μm palmitoleate for 24 h. After the treatment, changes in expression levels of cIAP1, CHOP, and cleaved caspase 3 were analyzed by Western blots (A, C), and cell death was determined by flow cytometric analysis with PI and annexin V (A-V) co-staining (B, D). Shown are data of mean ± S.D. from at least three independent experiments (*, p < 0.05, **, p < 0.01). Numbers below lanes (A and C) indicate band intensity relative to that of the loading control β-actin.
DISCUSSION
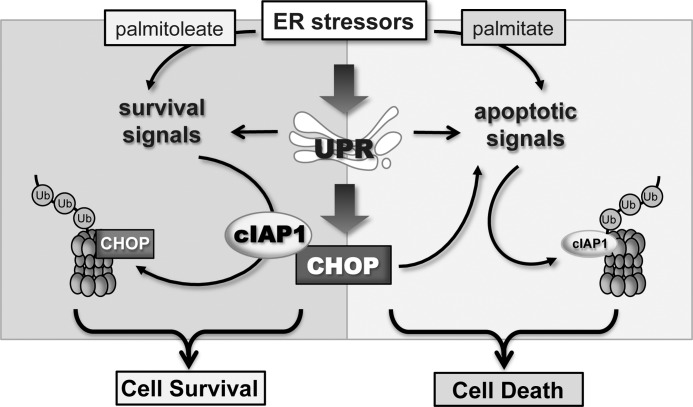
The UPR, which consists of multiple pathways leading to the coordinate transcriptional activation of a set of genes encoding ER chaperones and certain cell death signals, occurs as a result of ER stress. It has been generally believed that cell death is an inevitable event when ER stress is persistent or overwhelming, as demonstrated in pancreatic β-cell death induced by exposure to excess saturated FFA (1, 3, 31). The mechanistic link of UPR to the cellular apoptotic program has been intensively investigated, and the transcription factor CHOP is one of the best-characterized pro-apoptotic molecules accounting for the induction of lipotoxicity in β-cells (5, 8). However, what is less clear is how cells are able to escape death when both pro-survival and pro-apoptotic pathways are simultaneously activated following UPR-induced CHOP production. In the present study, we demonstrate a key protective role of cIAP1 against ER stress-mediated lipotoxicity in β-cells. The findings, for the first time, uncover that cIAP1 functions as an E3 ubiquitin ligase that directly promotes CHOP ubiquitination and degradation, preferentially leading to cell survival under ER stress. This delineates a new signaling model, whereby cIAP1 plays a central role in controlling ER stress-associated cell fate by eliminating the pro-apoptotic activity of CHOP (Fig. 9).
FIGURE 9.
Model depicting the role of cIAP1 in regulation of cell fate under ER stress. For adaptation to ER stress, the UPR (e.g. induced by palmitoleate) activates survival pathways and increases the expression of cIAP1. cIAP1 functions through its antiapoptotic properties and the E3 ligase activity causing CHOP ubiquitination and degradation, ultimately leading to cell survival. Under certain ER stress conditions (e.g. exposure to palmitate) cIAP1 is quickly degraded through the apoptotic pathways, which relieves CHOP degradation and preserves its pro-apoptotic activity, resulting in cell death.
A number of studies have demonstrated that palmitate causes apoptotic cell death through CHOP-dependent mechanisms in pancreatic β-cells (21, 25, 26). This ER stress-mediated event has been implicated in the pathology of obesity and T2D both in animal models and in the clinic (25, 27). Recent studies suggest that multiple pathways can contribute to CHOP-induced apoptosis, whereas the mitochondria-mediated intrinsic apoptotic pathway appears to play a predominant role in this process (32, 33). The intrinsic apoptotic pathway is precisely regulated by the Bcl-2 family, including both anti-apoptotic members such as Bcl-2, Bcl-XL, and Mcl-1, and pro-apoptotic members such as Bax and Bak (reviewed in (34). These proteins govern mitochondrial membrane integrity by either promoting or suppressing the release of apoptogenic proteins from these organelles. Among the mitochondrial proteins released into the cytosol during apoptosis is cytochrome c, which binds and activates Apaf-1 leading to caspase-9 and consequently caspase-3 activation. The ability of CHOP to induce expression of Bim (9) and down-regulation of Bcl-2 (8) has been documented, providing a mechanistic link between ER stress and mitochondria-dependent apoptosis. In addition, another mitochondrial protein, Smac is also released into the cytosol during apoptosis. Upon its release from mitochondria, Smac directly binds the IAP family proteins and induces their autoubiquitination and degradation, facilitating apoptosis (29, 35). Although the Smac/IAP pathway is predominantly associated with the death receptor-mediated extrinsic apoptotic pathway, it can also participate in the late stage of mitochondria-dependent apoptosis (16, 36). However, whether the IAP family proteins are actively involved in ER stress-induced apoptosis remains to be elucidated.
In the current study, we found that treatment of pancreatic β-cells with palmitate or the chemical ER stressor tunicamycin significantly reduced cIAP1 expression in both a time-dependent and a dose-dependent manner. This is inconsistent with the previous reports showing that ER stressors increased the expression levels of cIAP1, cIAP2, and XIAP in transformed and cancer cell lines (18, 19). The conflicting findings could be attributed to the difference between experimental models applied in the current study and others. However, it is noted that although the mRNA levels of these IAPs were indeed increased in the ER stressor-treated β-cells (date not shown), their protein levels were significantly reduced following treatment for 24 h, when cells underwent apoptosis, suggesting that the reduced levels of IAPs could be a consequence of apoptosis. However, the reduction of cIAP1 occurred as early as 4 h after palmitate treatment in β-cells where no evident apoptosis was observed (Fig. 3C). Furthermore, cIAP1 reduction was insensitive to the pan-caspase inhibitor Z-VAD-fmk, placing it upstream of the caspase activation cascades and suggesting an active role of cIAP1 in ER stress-mediated apoptosis. Indeed, enforced overexpression of cIAP1 significantly prevented palmitate-induced β-cell death, whereas lipotoxicity was augmented in the cIAP1-knockdown β-cells or cIap1-deficient MEFs (Fig. 7). Collectively, these data demonstrate that cIAP1 is a key endogenous protector against ER stress-mediated apoptosis in β-cells.
It is of interest to note that although both the saturated FFA palmitate and the unsaturated palmitoleate caused ER stress response and CHOP induction in β-cells, only palmitate was capable of inducing cell death. The different fate of these stressed cells suggests that additional signals are required for the CHOP-UPR pathway to commit apoptosis. A key finding of the current study illustrated that down-regulation of cIAP1 is essential to induce cell death under ER stress, which is supported by the following evidence: (i) the lipotoxic reagent palmitate or the chemical ER stressor tunicamycin, but not the nontoxic analog palmitoleate, induced significant cIAP1 reduction (Fig. 3); (ii) both endogenous and enforced overexpressed levels of cIAP1 were negatively correlated with CHOP expression (Fig. 3 and Fig. 5); (iii) either the siRNA-mediated knockdown of cIAP1 in β-cells or knock-out of cIap1 in MEFs not only increased palmitate-induced apoptosis (Fig. 7), but also committed cells to death in response to the nontoxic palmitoleate treatment (Fig. 8), highlighting an essential role of cIAP1 for β-cell survival under ER stress.
Because of the ability to directly or indirectly inhibit caspase activity, the IAP family has been thought of as primarily intrinsic inhibitors of apoptosis (37). More recently, cIAP1 has emerged as an E3 ubiquitin ligase that catalyzes both trans- and auto-ubiquitination, playing important roles in mitotic chromosome segregation, cellular morphogenesis and cell signaling (16, 38). Interestingly, cIAP1 can mediate either non-degradative Lys-63 polyubiquitination or degradative Lys-48 polyubiquitination of protein targets. For example, Lys-63 polyubiquitination of RIP1 mediates TNFα-evoked NF-κB activation (30), whereas Lys-48 auto- or trans-ubiquitination mediates proteasomal degradation of cIAP1 itself, or of a cIAP1-binding protein such as a caspase or Smac/DIABLO (38, 39). Whether these activities of cIAP1 may also account for its anti-apoptotic effect in ER stress-induced apoptosis remains to be investigated. Besides these known anti-apoptotic properties, we revealed that cIAP1 functions as an E3 ubiquitin ligase, causing CHOP ubiquitination and degradation, illustrating a new mechanism for the protective role of cIAP1 against ER stress-induced apoptosis. It has been previously reported that the C/EBP family transcription factors, including CHOP and C/EBPγ, are polyubiquitinated and subsequently degraded (40). However, the mechanisms and enzymes responsible for CHOP degradation are unknown. Our data demonstrate that cIAP1 directly binds to and induces CHOP ubiquitination in the presence of UbcH5a, UbcH5b, and UbcH6 E2 enzymes. Because other E2 enzymes that we tested were unable to mediate the cIAP1-dependent ubiquitination of CHOP, it is likely that the key reaction is Lys-48 ubiquitination, by which cIAP1 promotes proteasomal degradation of CHOP. However, we cannot exclude the possibility that cIAP1 may also produce other ubiquitin linkages on CHOP, which requires further investigation. Nevertheless, the findings that the siRNA-mediated knockdown of cIAP1 resulted in a sharp reduction of CHOP ubiquitination in vivo (Fig. 6C), coinciding with an increased level of CHOP in β-cells (Fig. 5), indicate that cIAP1 functions as a CHOP ubiquitin ligase, triggering its degradation under physiological circumstances. These findings thus provide mechanistic insights into the cellular pathways utilized by cIAP1 to prevent β-cells from ER stress-induced cell death (Fig. 9). It is tempting to speculate that cIAP1 or its regulators may represent novel targets for therapeutic intervention of lipotoxicity in β-cells, providing an additional way to prevent T2D.
Acknowledgments
We thank Dr. Mikihiko Naito for providing cIAP1 constructs, Dr. Xudong Zhang for CHOP constructs, Dr. John Silke for cIAP1−/− MEFs and antibodies, Dr. Jingbiao Chen for technical assistance, Dr. Carol Wadham, Mathew Vada, and Andrew Holmes for critical reading of the manuscript.
This work was supported by grants from the Australian National Health and Medical Research Council (NHMRC, Project #349348 and Program #571408).
- ER
- endoplasmic reticulum
- 4-PBA
- 4-PBA 4-phenyl butyric acid
- CHOP
- transcription factor C/EBP homologous protein
- FFA
- free fatty acids
- IAP
- inhibitor of apoptosis protein
- IRE1
- inositol-requiring enzyme
- MEFs
- mouse embryonic fibroblasts
- PERK
- PKR-like ER kinase
- UPR
- unfolded protein response.
REFERENCES
- 1. Ron D., Walter P. (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. 8, 519–529 [DOI] [PubMed] [Google Scholar]
- 2. Eizirik D. L., Cardozo A. K., Cnop M. (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 29, 42–61 [DOI] [PubMed] [Google Scholar]
- 3. Hotamisligil G. S. (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zinszner H., Kuroda M., Wang X., Batchvarova N., Lightfoot R. T., Remotti H., Stevens J. L., Ron D. (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12, 982–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marciniak S. J., Yun C. Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H. P., Ron D. (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tabas I., Ron D. (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 13, 184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsumoto M., Minami M., Takeda K., Sakao Y., Akira S. (1996) Ectopic expression of CHOP (GADD153) induces apoptosis in M1 myeloblastic leukemia cells. FEBS Lett. 395, 143–147 [DOI] [PubMed] [Google Scholar]
- 8. McCullough K. D., Martindale J. L., Klotz L. O., Aw T. Y., Holbrook N. J. (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 21, 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Puthalakath H., O'Reilly L. A., Gunn P., Lee L., Kelly P. N., Huntington N. D., Hughes P. D., Michalak E. M., McKimm-Breschkin J., Motoyama N., Gotoh T., Akira S., Bouillet P., Strasser A. (2007) ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129, 1337–1349 [DOI] [PubMed] [Google Scholar]
- 10. Oyadomari S., Mori M. (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 11, 381–389 [DOI] [PubMed] [Google Scholar]
- 11. Song B., Scheuner D., Ron D., Pennathur S., Kaufman R. J. (2008) Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Investig. 118, 3378–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harding H. P., Zhang Y., Bertolotti A., Zeng H., Ron D. (2000) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5, 897–904 [DOI] [PubMed] [Google Scholar]
- 13. Scheuner D., Song B., McEwen E., Liu C., Laybutt R., Gillespie P., Saunders T., Bonner-Weir S., Kaufman R. J. (2001) Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7, 1165–1176 [DOI] [PubMed] [Google Scholar]
- 14. Wang X. Z., Kuroda M., Sok J., Batchvarova N., Kimmel R., Chung P., Zinszner H., Ron D. (1998) Identification of novel stress-induced genes downstream of chop. EMBO J. 17, 3619–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Behrman S., Acosta-Alvear D., Walter P. (2011) A CHOP-regulated microRNA controls rhodopsin expression. J. Cell Biol. 192, 919–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Srinivasula S. M., Ashwell J. D. (2008) IAPs: what's in a name? Mol. Cell 30, 123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gyrd-Hansen M., Meier P. (2010) IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat. Rev. Cancer 10, 561–574 [DOI] [PubMed] [Google Scholar]
- 18. Hu P., Han Z., Couvillon A. D., Exton J. H. (2004) Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J. Biol. Chem. 279, 49420–49429 [DOI] [PubMed] [Google Scholar]
- 19. Hamanaka R. B., Bobrovnikova-Marjon E., Ji X., Liebhaber S. A., Diehl J. A. (2009) PERK-dependent regulation of IAP translation during ER stress. Oncogene 28, 910–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang N., Qi Y., Wadham C., Wang L., Warren A., Di W., Xia P. (2010) FTY720 induces necrotic cell death and autophagy in ovarian cancer cells: a protective role of autophagy. Autophagy 6, 1157–1167 [DOI] [PubMed] [Google Scholar]
- 21. Laybutt D. R., Preston A. M., Akerfeldt M. C., Kench J. G., Busch A. K., Biankin A. V., Biden T. J. (2007) Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50, 752–763 [DOI] [PubMed] [Google Scholar]
- 22. Sukocheva O., Wadham C., Holmes A., Albanese N., Verrier E., Feng F., Bernal A., Derian C. K., Ullrich A., Vadas M. A., Xia P. (2006) Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J. Cell Biol. 173, 301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xia P., Wang L., Moretti P. A., Albanese N., Chai F., Pitson S. M., D'Andrea R. J., Gamble J. R., Vadas M. A. (2002) Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J. Biol. Chem. 277, 7996–8003 [DOI] [PubMed] [Google Scholar]
- 24. Robertson R. P., Harmon J., Tran P. O., Poitout V. (2004) Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 53, S119–S124 [DOI] [PubMed] [Google Scholar]
- 25. Cnop M., Igoillo-Esteve M., Cunha D. A., Ladrière L., Eizirik D. L. (2008) An update on lipotoxic endoplasmic reticulum stress in pancreatic beta-cells. Biochem. Soc Trans. 36, 909–915 [DOI] [PubMed] [Google Scholar]
- 26. Karaskov E., Scott C., Zhang L., Teodoro T., Ravazzola M., Volchuk A. (2006) Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology 147, 3398–3407 [DOI] [PubMed] [Google Scholar]
- 27. Eizirik D. L., Cnop M. (2010) ER stress in pancreatic beta cells: the thin red line between adaptation and failure. Sci. Signal 3, pe7. [DOI] [PubMed] [Google Scholar]
- 28. Kandasamy K., Srinivasula S. M., Alnemri E. S., Thompson C. B., Korsmeyer S. J., Bryant J. L., Srivastava R. K. (2003) Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release. Cancer Res. 63, 1712–1721 [PubMed] [Google Scholar]
- 29. Yang Q. H., Du C. (2004) Smac/DIABLO selectively reduces the levels of c-IAP1 and c-IAP2 but not that of XIAP and livin in HeLa cells. J. Biol. Chem. 279, 16963–16970 [DOI] [PubMed] [Google Scholar]
- 30. Bertrand M. J., Milutinovic S., Dickson K. M., Ho W. C., Boudreault A., Durkin J., Gillard J. W., Jaquith J. B., Morris S. J., Barker P. A. (2008) cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 [DOI] [PubMed] [Google Scholar]
- 31. Xu C., Bailly-Maitre B., Reed J. C. (2005) Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Investig. 115, 2656–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Szegezdi E., Logue S. E., Gorman A. M., Samali A. (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 7, 880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim I., Xu W., Reed J. C. (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 7, 1013–1030 [DOI] [PubMed] [Google Scholar]
- 34. Youle R. J., Strasser A. (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nature Rev. 9, 47–59 [DOI] [PubMed] [Google Scholar]
- 35. Du C., Fang M., Li Y., Li L., Wang X. (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42 [DOI] [PubMed] [Google Scholar]
- 36. Maas C., Verbrugge I., de Vries E., Savich G., van de Kooij L. W., Tait S. W., Borst J. (2010) Smac/DIABLO release from mitochondria and XIAP inhibition are essential to limit clonogenicity of Type I tumor cells after TRAIL receptor stimulation. Cell Death Differ. 17, 1613–1623 [DOI] [PubMed] [Google Scholar]
- 37. Hunter A. M., LaCasse E. C., Korneluk R. G. (2007) The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis 12, 1543–1568 [DOI] [PubMed] [Google Scholar]
- 38. Mace P. D., Shirley S., Day C. L. (2010) Assembling the building blocks: structure and function of inhibitor of apoptosis proteins. Cell Death Differ. 17, 46–53 [DOI] [PubMed] [Google Scholar]
- 39. Yang Y., Fang S., Jensen J. P., Weissman A. M., Ashwell J. D. (2000) Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 288, 874–877 [DOI] [PubMed] [Google Scholar]
- 40. Hattori T., Ohoka N., Inoue Y., Hayashi H., Onozaki K. (2003) C/EBP family transcription factors are degraded by the proteasome but stabilized by forming dimer. Oncogene 22, 1273–1280 [DOI] [PubMed] [Google Scholar]