Background: The mechanism whereby IL-1β induces islet PGE2 production and inhibition of insulin secretion is unclear.
Results: Basal COX-2 protein levels are stimulated by IL-1β; mPGES-1 levels are not.
Conclusion: COX-2, not mPGES-1, is the final regulatory enzyme for PGE2 production.
Significance: COX-2, not mPGES-1, is the pharmacologic target to protect islet function from IL-1β in type 2 diabetes.
Keywords: Cyclooxygenase (COX) Pathway, Gene Knockout, Insulin Secretion, Interleukin, Prostaglandins, COX-2, Interleukin-1β, Prostaglandin E2, mPGES-1
Abstract
Arachidonic acid is converted to prostaglandin E2 (PGE2) by a sequential enzymatic reaction performed by two isoenzyme groups, cyclooxygenases (COX-1 and COX-2) and terminal prostaglandin E synthases (cPGES, mPGES-1, and mPGES-2). mPGES-1 is widely considered to be the final enzyme regulating COX-2-dependent PGE2 synthesis. These generalizations have been based in most part on experiments utilizing gene expression analyses of cell lines and tumor tissue. To assess the relevance of these generalizations to a native mammalian tissue, we used isolated human and rodent pancreatic islets to examine interleukin (IL)-1β-induced PGE2 production, because PGE2 has been shown to mediate IL-1β inhibition of islet function. Rat islets constitutively expressed mRNAs of COX-1, COX-2, cPGES, and mPGES-1. As expected, IL-1β increased mRNA levels for COX-2 and mPGES-1, but not for COX-1 or cPGES. Basal protein levels of COX-1, cPGES, and mPGES-2 were readily detected in whole cell extracts but were not regulated by IL-1β. IL-1β increased protein levels of COX-2, but unexpectedly mPGES-1 protein levels were low and unaffected. In microsomal extracts, mPGES-1 protein was barely detectable in rat islets but clearly present in human islets; however, in neither case did IL-1β increase mPGES-1 protein levels. To further assess the importance of mPGES-1 to IL-1β regulation of an islet physiologic response, glucose-stimulated insulin secretion was examined in isolated islets of WT and mPGES-1-deficient mice. IL-1β inhibited glucose-stimulated insulin secretion equally in both WT and mPGES-1−/− islets, indicating that COX-2, not mPGES-1, mediates IL-1β-induced PGE2 production and subsequent inhibition of insulin secretion.
Introduction
In the 1970s, cyclooxygenase (COX)2 was considered to be the central regulatory enzyme for arachidonic acid metabolism, formation of endoperoxides, and subsequent prostaglandin synthesis. In the 1990s, it was first recognized that two forms of COX exist (1–5). COX-1 mRNA is constitutively expressed in most tissues, whereas COX-2 expression is highly regulated by cytokines, endotoxins, growth factors, chemokines, and environmental stress. Thus, it has been envisioned that COX-1 generates prostaglandin E2 (PGE2) for housekeeping functions whereas COX-2 produces PGE2 in response to stimuli. Exceptions to this generalization in which constitutive expression of COX-2 mRNA is prominent include pancreatic islets, brain, renal glomerular tissue, and lung (6–10).
Prostaglandin E synthases (PGESs) were identified a decade later (11–16). These were named cPGES, mPGES-1, and mPGES-2. cPGES is located in the cytosol, whereas mPGES-1 and mPGES-2 are found in microsomes. In general, using primarily cell lines and tumor tissues, gene expression of cPGES and mPGES-2 has been reported to be constitutive, whereas mPGES-1 is regulated. Co-transfection and antisense experiments indicated that cPGES is capable of converting COX-1-, but not COX-2-, derived prostaglandin H2 to PGE2 in cells (17). In contrast, co-transfection experiments suggest that mPGES-1 has a marked preference for COX-2 over COX-1 products (18). Interestingly, mPGES-2 can couple with either COX-1 or COX-2, although it has a preference for COX-1 (19). Knock-out mice for all three genes have been generated. mPGES-1-deficient mice exhibited decreased response to inflammatory stimuli (20), supporting the concept of mPGES-1 regulating inflammation-induced PGE2 production. cPGES deficiency is perinatally lethal with the mice exhibiting poor lung development, delayed skin maturation, and growth retardation (20). No function under physiological or pathophysiological conditions has been ascribed to mPGES-2 deficiency (20). Similarly to the COX enzymes, it is generalized that cPGES generates PGE2 for housekeeping functions whereas mPGES-1 produces PGE2 in response to stimuli. Exceptions to these generalizations were subsequently noted, especially in studies of inflamed and tumor tissues (19, 22–25). However, the importance of the PGESs as key regulators of PGE2 synthesis in islets has not been examined previously.
Interleukin (IL)-1β is a major proinflammatory cytokine that inhibits function and promotes apoptosis of islet β-cells (26). IL-1β has been reported in islets from humans with type 2 diabetes, and antagonism of IL-1β receptors has been reported to improve glucose control in type 2 diabetic humans (27, 28). An anti-IL-1β receptor antagonist reduced β-cell apoptosis and increased β-cell proliferation in a diet-induced obesity mouse model (29). We have reported previously that lL-1β stimulates PGE2 synthesis by the β-cell and that PGE2 inhibits insulin secretion (7, 30). We have also observed that the anti-inflammatory drug sodium salicylate decreases PGE2 synthesis by the islet (31), decreases IL-1β-induced inhibition of insulin secretion (32), and improves insulin secretion and glucose disposal in type 2 diabetic humans (33).
These studies were designed to ascertain whether 1) isolated pancreatic islets express cPGES and mPGESs at the mRNA and protein levels, 2) cPGES or mPGESs are regulated by IL-1β, and 3) IL-1β-induced PGE2 production and consequent inhibition of glucose-stimulated insulin secretion (GSIS) require mPGES-1.
MATERIALS AND METHODS
Islet Preparations
Pancreata from male C57B6/J mice and Wistar rats were infused with 10 ml of 0.75 and 1.5 mg/ml collagenase type V (Sigma), respectively, with 1% FBS, and 2 units/ml RQ1 DNase (Promega, Madison, WI) in M199/Earle's balanced salt solution (ThermoScientific, Waltham, MA), pH 7.4. After surgical removal, pancreata were incubated for 10 min at 37 °C and then shaken 30 times. Undigested acinar tissue was removed by two washes with ice-cold Hanks' balanced salt solution followed by centrifugation at 250 × g for 4 min. For rat isolation a Histopaque gradient was used. Briefly, islet pellets were resuspended in 10 ml of Histopaque (Sigma), and a layer was gently added that consisted of 10 ml of M199/Earle's balanced salt solution containing 1% FBS, followed by 25-min centrifugation. Islets were handpicked and preincubated in RPMI 1640 medium containing 10% FBS and penicillin streptomycin (Sigma) for 24 h. The following day, islets were picked and subcultured for 20–24 h in medium containing 0.2% FBS, with and without 10 ng/ml IL-1β (R&D Systems, Minneapolis, MN). Human pancreatic islets were obtained from the Integrated Islet Distribution Program and had a purity of 80% or greater and were purified further by handpicking to remove acinar tissue. Preparations from three separate human cadaveric donors were studied. All experiments were performed using intact islets in culture. Thereafter, cells were lysed, and total protein or mRNA was extracted for analysis.
PGE2 Production
Isolated mouse, rat, and cadaveric human islets were used in static incubations for measurements of PGE2 production. After purification, islets were placed in 12 × 75-mm polypropylene tubes. These test tubes were modified by removing their bottoms and fusing nylon mesh to the end of the tubes to allow drainage of buffer from the islets with retention of the islets by the mesh. These tubes containing the mesh were loaded with 100 handpicked islets and then placed into a larger tube containing 2.8 mm glucose for 14 h. They were then transferred into fresh media containing dimethyl sulfoxide and IL-1β (10 ng/ml) with or without NS-398 (10 μm, Enzo Life Sciences, Farmingdale, NY) for increasing time intervals from 0 to 24 h. Incubation buffer was collected at time epochs of 0–5 min, 5 min–1 h, 1–2 h, 2–4 h, 4–12 h, and 12–24 h. Islets were rinsed in RPMI 1640 medium containing 2.8 mm glucose between each subsequent experimental period. Samples were frozen at −20 °C and used later for determination of PGE2 concentration (Cayman Chemical, Ann Arbor, MI).
Insulin Secretion
Islets from wild type and mPGES-1-deficient mice were used for studies of GSIS. Islets from wild type and mPGES-1−/− mice (generously provided by Dr. Satoshi Uematsu (34)) were incubated for 2 h in Krebs-Ringer buffer in 2.8 mm or 16.7 mm glucose with and without IL-1β (10 ng/ml) or 10−6 m PGE2 or 10−9 m epinephrine or 10−9 m somatostatin. Insulin was measured by radioimmunoassay (32).
RNA Isolation and Real-time Fluorescence-based Reverse Transcription (RT-PCR)
RNA was extracted from aliquots of 500 islets using the RNeasy Mini kit (Qiagen, Valencia, CA). PCR primers and probes for the COX-1, COX-2, cPGES, and mPGES-1 genes were designed using the Primer Express software program (Applied Biosystems, Carlsbad, CA). Sequences (5′-3′) are as follows: COX-1 probe, 6FAM-CCGCTTTGGCCTCGACAACTACCAGT-TAMRA; COX-1 forward primer, GCCAGAACCAGGGTGTCTGT; COX-1 reverse primer, GTAGCCCGTGCGAGTACAATC; COX-2 probe, 6FAM-TCCATGGCCCAGTCCTCGGGT-TAMRA; COX-2 forward primer, CCAGCACTTCACCCATCAGTT; COX-2 reverse primer, AAGGCGCAGTTTATGTTGTCTGT; cPGES probe, 6FAM-CCACTTTGCAGAAGCAGGCTGCATT-TAMRA; cPGES forward primer, CCCCTGCCCCGTTCA; cPGES reverse primer, TGAAGACATAGTCCCTTCGATCG; mPGES-1 probe, 6FAM-CCGTGTGGTACACACCGTGGCC-TAMRA; mPGES-1 forward primer, ATACATTTCCTCGTGGTCCTCACA; and mPGES-1 reverse primer, GGGTTCATTTTGCCCAGGTA. One-step RT-PCR was carried out using the TaqMan PCR core kit (Applied Biosystems) and an ABI Prism 7700 sequence detector as described previously (35).
Western Analysis
Whole cell extracts were obtained by harvesting islets in lysis buffer (140 mm NaCl, 10 mm Tris, pH 7.4, 1 mm CaCl2, 1 mm MgCl2, 10% glycerol, 1% Nonidet P-40, 1 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride, 2 ng/ml aprotinin, and 20 ng/ml leupeptin). For microsomal preparations, islets (2,000–3,000) were resuspended in 800 μl of homogenization buffer (0.1 m potassium phosphate buffer, pH 7.4, 0.25 m sucrose, and complete ULTRA protease inhibitor mixture (Roche Applied Science)) and sonicated (40%) for 30-s pulses. Samples were centrifuged at 1,000 × g for 10 min at 4 °C. Supernatants were centrifuged at 10,000 × g for 15 min at 4 °C. These supernatants were then centrifuged at 170,000 × g for 60 min at 4 °C. Pellets were resuspended in 50 μl of homogenization buffer and stored at −80 °C. Cellular proteins were separated on 10% polyacrylamide Criterion gels (Bio-Rad) and electrotransferred onto nitrocellulose membranes (Bio-Rad). All primary antibodies were purchased from Cayman Chemical. Membranes were immunoblotted with antisera: COX-1 (160109; 1:200) for mouse and rat islets and COX-1 (160108; 1:500) for human islets; COX-2 (160106; 1:200); cPGES (160150, 1:300); mPGES-1 (160140; 1:200); and mPGES-2 (160145; 1:400) overnight at 4 °C in 10% nonfat dry milk in phosphate-buffered saline with 0.5% Tween 20. The membranes were washed multiple times with phosphate-buffered saline with 0.5% Tween 20 and then immunoblotted with anti-rabbit IgG (1:4000) (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h at room temperature. Proteins were detected using ECL Western blotting detection kit (Amersham Biosciences). Membranes were stripped and reprobed with anti-TFIID (1:200 N-12, Santa Cruz Biotechnology, Santa Cruz, CA) to control for protein loading. OptiQuant image analysis software (Packard) was used for densitometry measurements of the autoradiograms.
Statistics
Intergroup differences among means were analyzed by ANOVA and Dunnett multiple comparisons tests.
RESULTS
IL-1β Stimulation of PGE2 Synthesis by Islets
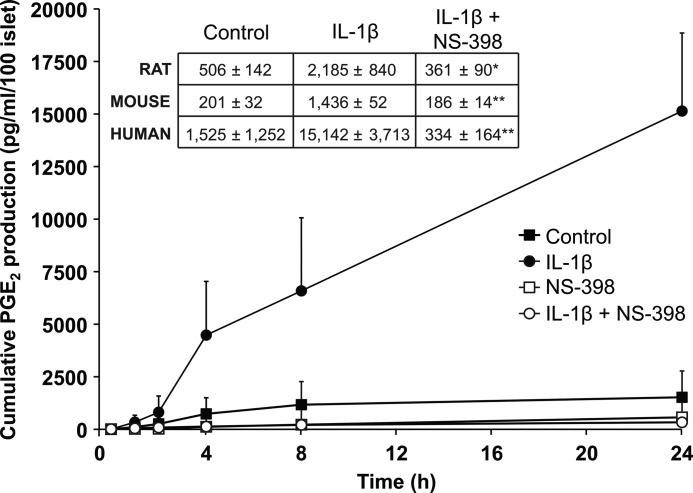
Rodent and human islets were incubated for increasing amounts of time (0, 1, 2, 4, 8, and 24 h) in the presence of IL-1β (10 ng/ml) with or without NS-398, 10 μm, a specific inhibitor of COX-2. Time-related, linear increases in IL-1β-stimulated-PGE2 (p < 0.01–0.001) were observed, effects that were completely abrogated by NS-398 (Fig. 1). These results demonstrate a role for Il-1β as a stimulator of PGE2 production in islets across species.
FIGURE 1.
Stimulation by IL-1β of PGE2 production by rodent and human islets. Cultured human islets produced increasing levels of PGE2 in the presence of IL-1β in a time-related, linear manner, and PGE2 production stimulated by IL-1β was completely inhibited by the specific COX-2 antagonist, NS-398 (p < 0.01–0.001). Islets were obtained from three different donors; individual experiments were performed in triplicate. Inset, 24-h incubation results from experiments using mice and rat isolated islets were comparable with the experiments using human islets, i.e. NS-398 inhibited IL-1β-induced PGE2 production (*, p < 0.05; **, p < 0.01).
Gene Expression of COXs and PGESs
Basal levels of COX-1, cPGES, and mPGES-2 mRNA were readily detectable in islets, but none was stimulated by IL-1β (Fig. 2). Basal levels of COX-2 and mPGES-1 mRNA were detected, and both were stimulated ∼10-fold by IL-1β. These results demonstrate that, as with other tissues, gene expression of COX-2 and mPGES-1 in islets is regulated by IL-1β.
FIGURE 2.
Basal and stimulated levels of COX-1, COX-2, cPGES, and mPGES-1 mRNAs in rat pancreatic islets incubated with or without IL-1β. mRNA levels were quantified by triplicate determinations using real-time RT-PCR, and data were normalized to levels of islet COX-1 mRNA. Levels of COX-1 mRNA were similar under basal and IL-1β-stimulated conditions, whereas COX-2 mRNA levels after stimulation with IL-1β were higher than their basal levels (n = 7 pairs, p < 0.01). cPGES mRNA levels under basal and IL-1β-stimulated conditions were similar, whereas mPGES-1 mRNA levels after stimulation with IL-1β were higher than basal levels (n = 7 pairs, p < 0.02).
COXs and PGESs Protein Levels
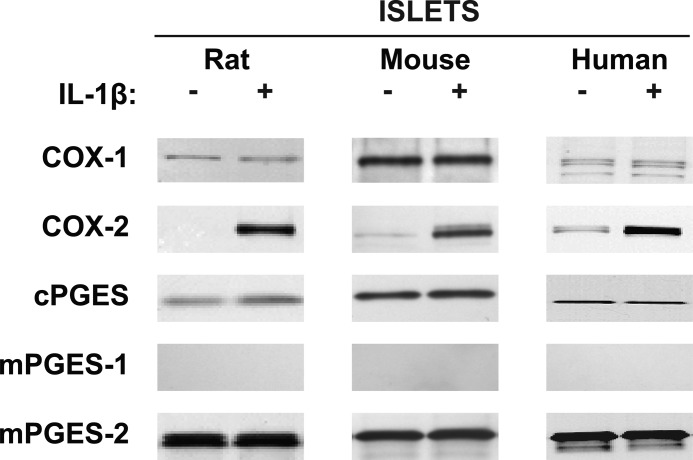
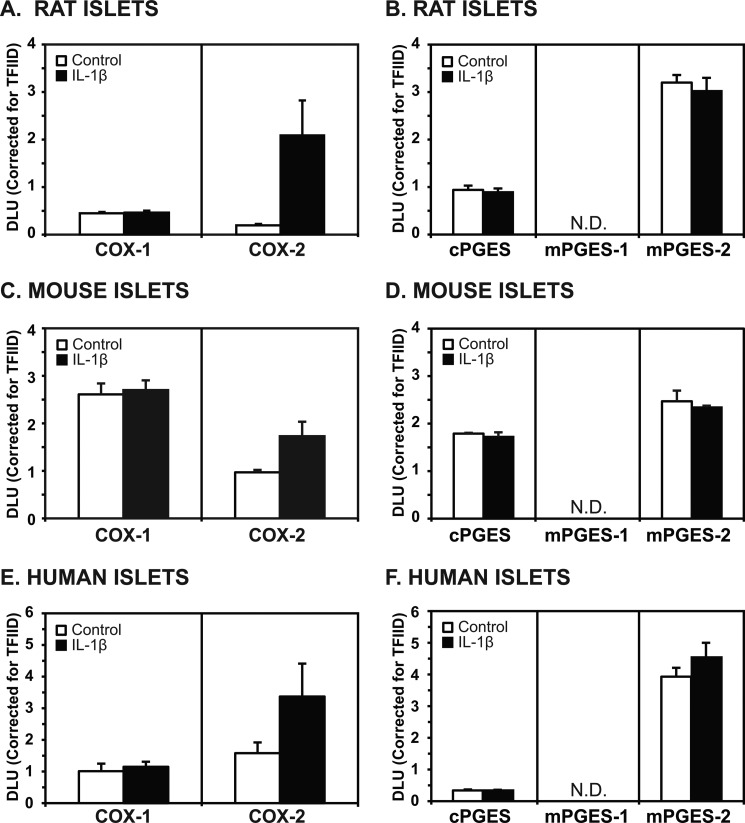
Western analysis of rat, mice, and human islets (25 μg of protein) revealed basal expression of COX-1 that was not increased by exposure to IL-1β (Fig. 3). COX-2 protein levels were low under unstimulated conditions in all three species, but they were greatly increased by IL-1β. Basal levels of cPGES protein were observable, but no increases were seen with IL-1β exposure. Unlike mPGES-1 mRNA, no detectable protein levels of mPGES-1 were observed in whole cell extracts either under basal conditions or after IL-1β stimulation in rat, mouse, or human islets. On the other hand, basal mPGES-2 protein levels were readily detectable in all three species but did not increase after exposure to IL-1β. Summaries of these results using multiple samples from different rats (n = 3 in triplicate), mice (n = 3 in triplicate), and humans (n = 3 in triplicate) are shown in Fig. 4.
FIGURE 3.
Western analysis using 25 μg of protein of whole cell extracts of rat, mouse, and human islets for basal and IL-1β-stimulated levels of COXs and PGESs. COX-1 protein levels did not increase with IL-1β, whereas COX-2 protein levels did. cPGES and mPGES-2 protein levels were present in the nonstimulated state, and neither was stimulated by IL-1β. mPGES-1 was not detectable with or without stimulation by IL-1β.
FIGURE 4.
Summary of results from Western analysis of islets using whole cell extracts (25 μg of protein). A and B, islets from three different rats are shown. A, COX-1 levels did not increase with IL-1β, whereas COX-2 levels did (p < 0.05). B, levels of cPGES and mPGES-2 proteins were readily detectable but did not increase with IL-1β stimulation. C and D, islets from three different mice are shown. C, COX-1 levels did not increase with IL-1β, whereas COX-2 levels did (p < 0.05). D, levels of cPGES and mPGES-2 proteins were detectable but did not increase with IL-1β stimulation. E and F, human islets from three different donors are shown. E, constitutive levels of COX-1 and COX-2 were detectable, but only COX-2 levels were higher after exposure to IL-1β (p < 0.057). F, cPGES levels were not prominent in either the nonstimulated or stimulated state. Levels of mPGES-2 protein were prominently detected in the nonstimulated state but did not increase with IL-1β stimulation (N.D., nondetectable).
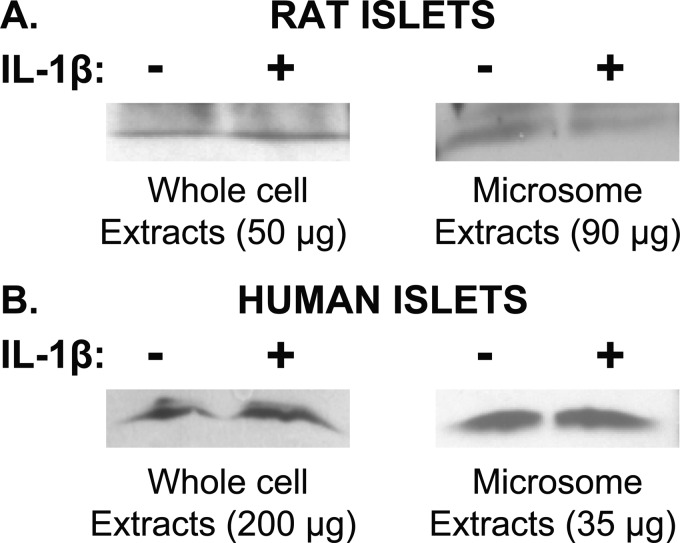
Because of the surprising absence of mPGES-1 protein, Western analyses were repeated using 50 μg rather than 25 μg of protein of whole cell extracts and 90 μg of protein of microsomal extracts (Fig. 5, upper panel). Peripheral blood mononuclear cells (with and without LPS treatment) were used as methodological controls for the whole cell extracts; and spleen, kidney, and A549 cells (with and without IL-1β treatment) were used as controls for microsomal extracts (data not shown). Use of greater amounts of islet rat protein for whole cell extracts and microsomal extracts revealed only trace amounts of mPGES-1, but no stimulation was observed after IL-1β treatment in islets. In human islets, use of whole cell extracts with 200 μg of protein and microsomal extracts with 35 μg of protein allowed visualization of mPGES-1, but no stimulation by IL-1β was observed in either case (Fig. 5, lower panel). Therefore, unlike reports examining cell lines and tumor tissues, mPGES-1 protein levels in islets were low and not stimulated by IL-1β.
FIGURE 5.
Western analyses for mPGES-1 using microsomal versus whole cell extracts. Top panel, rat islets are shown. Whole cell extracts and microsomal extracts using greater amounts of protein than were used in Fig. 3 failed to reveal stimulation of mPGES-1 levels by IL-1β. Bottom panel, human islets are shown. mPGES-1 was detectable in whole cell extracts using greater amounts of protein than were used in Fig. 3 and revealed mPGES-1, but these levels were not increased when islets were treated with IL-1β.
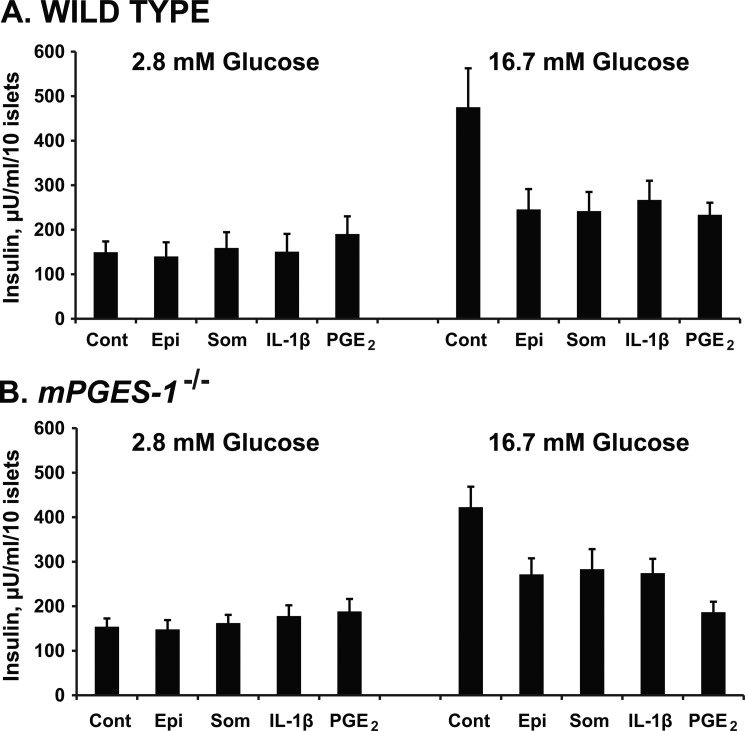
Effects of IL-1β and PGE2 on Glucose-induced Insulin Secretion from Wild Type and mPGES-1-deficient Mice
Although minimal mPGES-1 levels were observed with no regulation by IL-1β treatment, this did not rule out the possibility that some level of mPGES-1 activity is required for IL-1β-induced PGE2 production. To address this issue, islets from wild type (WT) and mPGES-1-deficient (mPGES-1−/−) mice were isolated and tested for their ability to release insulin in the presence of known inhibitors. Insulin secretion was normally stimulated by 16.7 mm glucose in both the WT and mPGES-1−/− islets (Fig. 6). This physiologic response was inhibited to the same magnitude by optimal concentrations of epinephrine, somatostatin, and PGE2, indicating that insulin secretory pathways were physiologically comparable with wild type islets. IL-1β also inhibited GSIS to the same degree as the other inhibitors in islets from wild type mice, and this inhibitory effect was preserved in isolated islets from the mPGES-1-deficient mice. These results confirm that mPGES-1 is not required for IL-1β-induced inhibition of insulin secretion.
FIGURE 6.
Inhibitory effects of IL-1β on glucose-induced insulin secretion from isolated islets of wild type and mPGES-1-deficient mice. Insulin secretion from wild type islets incubated in buffer containing 2.8 mm glucose was unaffected by IL-1β. In contrast, when a stimulatory concentration of glucose (16.7 mm) was used, IL-1β significantly inhibited insulin secretion (p < 0.05–0.01). Identical findings were observed when islets from mPGES-1-deficient mice were used. Experiments with epinephrine (epi), somatostatin (som), and PGE2 are also shown for both wild type and mPGES-1 KO mice as comparators for the efficacy of IL-1β as an inhibitor of insulin secretion.
DISCUSSION
Gene expressions of COX-1 and cPGES have often been reported to be constitutive and coordinately expressed but not stimulated by cytokines, whereas basal mRNA levels of COX-2 and mPGES-1 have been usually reported to be minimal but coordinately stimulated by cytokines. This has led to the generalization that COX-1 and cPGES are housekeeping genes whereas COX-2 and mPGES-1 are coordinately stimulated by cytokines (11–25).
Our studies were designed to determine whether these generalizations hold true for pancreatic islets of three species, i.e. mouse, rat, and human. Using rodent and human islets, we first demonstrated that IL-1β increased production of PGE2, an effect blocked by the specific COX-2 inhibitor, NS-398. These data are consistent with our previous findings using the β-cell lines, HIT-T15 and βHC-3 (35). Heitmeier et al. reported anecdotally (data not shown) that IL-1β alone does not increase PGE2 synthesis in human islets and that a mixture of three cytokines was required (36). A possible explanation is that these authors used much smaller concentrations of IL-1β than we did.
A very different picture emerged when we examined tissues by Western analysis. Protein levels of basal COX-2 were not readily visible. In all three species, mPGES-1 protein levels were absent/low, and basal mPGES-2 levels were the most evident of all enzymes. Therefore, whereas our findings in islets support the concept of coordinated constitutive expression of COX-1 and cPGES mRNAs and proteins, they do not support the concept (11–25) of coordinated regulation of COX-2 and mPGES-1 protein levels by IL-1β. Rather, in islets our findings indicate that only COX-2 protein is up-regulated by IL-1β. Thus, we conclude that in islets COX-2, not mPGES-1, is the dominant regulator of PGE2 synthesis stimulated by IL-1β. Our findings that IL-1β increases PGE2 production by the islet and that exogenous IL-1β inhibits GSIS in both wild type and mPGES-1 knock-out islets are consistent with our overall concept that PGE2 is a negative regulator of glucose-induced insulin secretion and that the inhibitory effects of IL-1β on insulin secretion are mediated by endogenous PGE2 (35).
In retrospect, it is notable that most previous studies suggesting that mPGES-1 is paired with COX-2 to provide regulated synthesis of PGE2 (11–16, 19, 22) were based chiefly on measurements of mRNAs and not proteins. Such differences between mRNA and protein levels may reflect important differences in efficiency/regulation of translation or differences in the half-life of the mRNA versus protein under nonstimulated conditions, as reported previously (37). In any event, these conflicting phenomena raise the caution that measurements of gene expression without corroboration by protein levels can lead to erroneous conclusions about regulation of PGE2 synthesis by IL-1β. An important exception is the work of Båge et al. (38) using human gingival fibroblasts that successfully demonstrated regulation by IL-1β of mPGES-1 protein. Importantly, however, in that study mPGES-1 siRNA down-regulated mPGES-1 protein levels but did not affect cytokine-stimulated PGE2 production. This surprising result supports our conclusion that mPGES-1 is not an important regulator of PGE2 synthesis during inflammation in islets; rather, it is more likely to be COX-2.
Murakami et al. identified a second mPGES enzyme, mPGES-2, and showed that it was not increased appreciably by tissue inflammation or damage, but that it is considerably elevated in human colorectal cancer tissues (19). We observed that mPGES-2 protein is readily detectable in islets. Because Western analysis has only been reported rarely in studies of PGESs, it seems possible that other normal tissues may also express mPGES-2 protein. In the current studies we also noted a discrepancy between gene expression and protein levels of islet COX-2. We observed high constitutive mRNA levels for COX-2 relative to COX-1, but Western analysis failed to detect COX-2 protein levels under nonstimulated conditions in which COX-1 was clearly present. Although we reported previously basal levels of COX-2 protein in HIT-T15 cells (7), our current study using isolated islets from mice, rat, and human does not confirm this observation made in a β-cell line.
Our observation that COX-2 protein is the only one of the five COX and PGES enzymes that increases in response to IL-1β in the islet has pathophysiologic implications for type 2 diabetes. Many groups have published that PGE2 inhibits insulin secretion (see Ref. 34 for a review). The effect of PGE2 to inhibit insulin secretion is specific for first phase insulin release in response to glucose (PGE2 does not affect first phase insulin responses to other nonglucose agonists (39)). We have identified previously its mechanism as a PGE2 receptor-mediated, pertussis toxin-sensitive-coupled inactivation of adenylate cyclase (40, 41). Inhibition of COXs by nonsteroidal drugs increases insulin secretion in vitro (31). Sodium salicylate partially restores first phase insulin secretion in humans with type 2 diabetes (33).
However, not all reports have confirmed the inhibitory effect of PGE2 on β-cell function. The reasons for this inconsistency have not always been apparent, although important differences in study design have likely played important roles. Some of these differences include use of static incubations for 40 h (36) and exclusion of isobutylmethylxanthine from perifusions (42, 43). These are relevant concerns, because although PGE2 inhibits both first and second phase glucose-induced insulin secretion, it primarily inhibits first phase, which occurs within minutes of glucose stimulation. Use of 40-h static incubations is not likely to reflect first phase insulin secretion. Because perifusion commonly employs isobutylmethylxanthine to inhibit phosphodiesterase to augment endogenous levels of cAMP to support glucose-stimulated insulin release, PGE2 action via EP3 receptors to decrease cAMP levels would be expected to neutralize the effects of isobutylmethylxanthine and to reduce insulin secretion. Assessment of the relative endogenous intrinsic activities of EP2 and EP3 receptors may be required in study preparations for a more accurate analysis of this issue, because both are activated by PGE2 but they have opposite effects on cAMP levels.
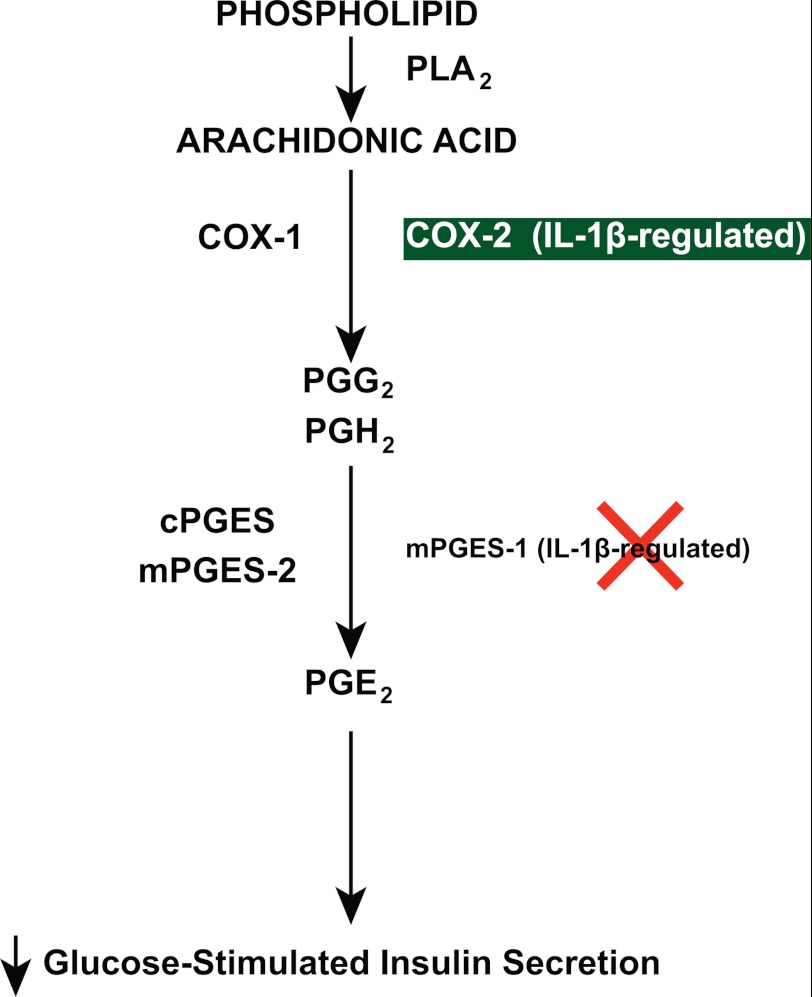
Recently, Oshima et al. reported that β-cell-specific overexpression of COX-2 and mPGES-1 in mice caused an increase in islet PGE2, a decrease in β-cell mass, a decrease in GSIS, and hyperglycemia (44). More recently, Meng et al. reported that PGE2 inhibits insulin secretion via a JNK-dependent pathway, which leads to dephosphorylation of Akt and FOXO1, causing nuclear localization and activation of FOXO1 as well as nuclear exclusion of PDX-1 (45), a protein that is an important stimulus for insulin gene expression. Our findings suggest that when type 2 diabetes involves excessive islet levels of IL-1β (27, 28), this cytokine increases COX-2 activity and PGE2, which in turn inhibits GSIS and contributes to β-cell dysfunction (Fig. 7).
FIGURE 7.
General scheme illustrating the arachidonic acid pathway through which IL-1β regulates PGE2 production. Arachidonic acid is cleaved from phospholipid by phospholipase A2 (PLA2), followed by synthesis of endoperoxides (PGG2, PGH2) via COX-1 and COX-2. Activation of COX-2 by IL-1β increases intracellular PGE2 levels. This scheme emphasizes that islets differ importantly from other cell lines and tumor tissues, because they contain little to no mPGES-1 in mice and rats, and although detectable in humans, this protein is not increased by IL-1β, whereas COX-2 is. Therefore, COX-2, and not mPGES-1, in islets is the mechanism whereby IL-1β increases PGE2 production, which in turn inhibits glucose-induced insulin secretion.
This work was funded by National Institutes of Health Grant NIDDK R01 38325 (to R. P. R.).
- COX
- cyclooxygenase
- PGE2
- prostaglandin E2
- PGES
- prostaglandin E synthase
- GSIS
- glucose-stimulated insulin secretion.
REFERENCES
- 1. Rosen G. D., Birkenmeier T. M., Raz A., Holtzman M. J. (1989) Identification of a cyclooxygenase-related gene and its potential role in prostaglandin formation. Biochem. Biophys. Res. Commun. 164, 1358–1365 [DOI] [PubMed] [Google Scholar]
- 2. Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. (1990) The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J. Biol. Chem. 265, 16737–16740 [PubMed] [Google Scholar]
- 3. Herschman H. R. (1996) Prostaglandin synthase 2. Biochim. Biophys. Acta 1299, 125–140 [DOI] [PubMed] [Google Scholar]
- 4. Wu K. K., Sanduja R., Tsai A. L., Ferhanoglu B., Loose-Mitchell D. S. (1991) Aspirin inhibits interleukin 1-induced prostaglandin H synthase expression in cultured endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 88, 2384–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crofford L. J., Wilder R. L., Ristimäki A. P., Sano H., Remmers E. F., Epps H. R., Hla T. (1994) Cyclooxygenase-1 and -2 expression in rheumatoid synovial tissues: effects of interleukin-1β, phorbol ester, and corticosteroids. J. Clin. Invest. 93, 1095–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robertson R. P. (1998) Dominance of cyclooxygenase-2 in the regulation of pancreatic islet prostaglandin synthesis. Diabetes 47, 1379–1383 [DOI] [PubMed] [Google Scholar]
- 7. Sorli C. H., Zhang H. J., Armstrong M. B., Rajotte R. V., Maclouf J., Robertson R. P. (1998) Basal expression of cyclooxygenase-2 and nuclear factor-interleukin 6 are dominant and coordinately regulated by interleukin 1 in the pancreatic islet. Proc. Natl. Acad. Sci. U.S.A. 95, 1788–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamagata K., Andreasson K. I., Kaufmann W. E., Barnes C. A., Worley P. F. (1993) Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron 11, 371–386 [DOI] [PubMed] [Google Scholar]
- 9. Harris R. C., McKanna J. A., Akai Y., Jacobson H. R., Dubois R. N., Breyer M. D. (1994) Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J. Clin. Invest. 94, 2504–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Asano K., Lilly C. M., Drazen J. M. (1996) Prostaglandin G/H synthase-2 is the constitutive and dominant isoform in cultured human lung epithelial cells. Am. J. Physiol. 271, L126–131 [DOI] [PubMed] [Google Scholar]
- 11. Jakobsson P. J., Thorén S., Morgenstern R., Samuelsson B. (1999) Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. U.S.A. 96, 7220–7225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watanabe K., Kurihara K., Suzuki T. (1999) Purification and characterization of membrane-bound prostaglandin E synthase from bovine heart. Biochim. Biophys. Acta 1439, 406–414 [DOI] [PubMed] [Google Scholar]
- 13. Tanikawa N., Ohmiya Y., Ohkubo H., Hashimoto K., Kangawa K., Kojima M., Ito S., Watanabe K. (2002) Identification and characterization of a novel type of membrane-associated prostaglandin E synthase. Biochem. Biophys. Res. Commun. 291, 884–889 [DOI] [PubMed] [Google Scholar]
- 14. Thorén S., Weinander R., Saha S., Jegerschöld C., Pettersson P. L., Samuelsson B., Hebert H., Hamberg M., Morgenstern R., Jakobsson P. J. (2003) Human microsomal prostaglandin E synthase-1: purification, functional characterization, and projection structure determination. J. Biol. Chem. 278, 22199–22209 [DOI] [PubMed] [Google Scholar]
- 15. Guan Y., Zhang Y., Schneider A., Riendeau D., Mancini J. A., Davis L., Kömhoff M., Breyer R. M., Breyer M. D. (2001) Urogenital distribution of a mouse membrane-associated prostaglandin E2 synthase. Am. J. Physiol. Renal Physiol. 281, F1173–1177 [DOI] [PubMed] [Google Scholar]
- 16. Han R., Smith T. J. (2002) Cytoplasmic prostaglandin E2 synthase is dominantly expressed in cultured KAT-50 thyrocytes, cells that express constitutive prostaglandin-endoperoxide H synthase-2: basis for low protaglandin E2 production. J. Biol. Chem. 277, 36897–36903 [DOI] [PubMed] [Google Scholar]
- 17. Tanioka T., Nakatani Y., Semmyo N., Murakami M., Kudo I. (2000) Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J. Biol. Chem. 275, 32775–32782 [DOI] [PubMed] [Google Scholar]
- 18. Murakami M., Naraba H., Tanioka T., Semmyo N., Nakatani Y., Kojima F., Ikeda T., Fueki M., Ueno A., Oh S., Kudo I. (2000) Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 275, 32783–32792 [DOI] [PubMed] [Google Scholar]
- 19. Murakami M., Nakashima K., Kamei D., Masuda S., Ishikawa Y., Ishii T., Ohmiya Y., Watanabe K., Kudo I. (2003) Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. J. Biol. Chem. 278, 37937–37947 [DOI] [PubMed] [Google Scholar]
- 20. Trebino C. E., Stock J. L., Gibbons C. P., Naiman B. M., Wachtmann T. S., Umland J. P., Pandher K., Lapointe J. M., Saha S., Roach M. L., Carter D., Thomas N. A., Durtschi B. A., McNeish J. D., Hambor J. E., Jakobsson P. J., Carty T. J., Perez J. R., Audoly L. P. (2003) Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc. Natl. Acad. Sci. U.S.A. 100, 9044–9049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakatani Y., Hokonohara Y., Kakuta S., Sudo K., Iwakura Y., Kudo I. (2007) Knockout mice lacking cPGES/p23, a constitutively expressed PGE2 synthetic enzyme, are perinatally lethal. Biochem. Biophys. Res. Commun. 362, 387–392 [DOI] [PubMed] [Google Scholar]
- 22. Sweeney F. J., Wachtmann T. S., Eskra J. D., Verdries K. A., Lambalot R. H., Carty T. J., Perez J. R., Audoly L. P. (2003) Inhibition of IL-1β-dependent prostaglandin E2 release by antisense microsomal prostaglandin E synthase 1 oligonucleotides in A549 cells. Mol. Cell. Endocrinol. 205, 151–157 [DOI] [PubMed] [Google Scholar]
- 23. Subbaramaiah K., Yoshimatsu K., Scherl E., Das K. M., Glazier K. D., Golijanin D., Soslow R. A., Tanabe T., Naraba H., Dannenberg A. J. (2004) Microsomal prostaglandin E synthase-1 is overexpressed in inflammatory bowel disease: evidence for involvement of the transcription factor Egr-1. J. Biol. Chem. 279, 12647–12658 [DOI] [PubMed] [Google Scholar]
- 24. Hanaka H., Pawelzik S. C., Johnsen J. I., Rakonjac M., Terawaki K., Rasmuson A., Sveinbjörnsson B., Schumacher M. C., Hamberg M., Samuelsson B., Jakobsson P. J., Kogner P., Rådmark O. (2009) Microsomal prostaglandin E synthase 1 determines tumor growth in vivo of prostate and lung cancer cells. Proc. Natl. Acad. Sci. U.S.A. 106, 18757–18762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Radilova H., Libra A., Holasova S., Safarova M., Viskova A., Kunc F., Buncek M. (2009) COX-1 is coupled with mPGES-1 and ABCC4 in human cervix cancer cells. Mol. Cell. Biochem. 330, 131–140 [DOI] [PubMed] [Google Scholar]
- 26. Palmer J. P., Helqvist S., Spinas G. A., Mølvig J., Mandrup-Poulsen T., Andersen H. U., Nerup J. (1989) Interaction of β-cell activity and IL-1 concentration and exposure time in isolated rat islets of Langerhans. Diabetes 38, 1211–1216 [DOI] [PubMed] [Google Scholar]
- 27. Maedler K., Sergeev P., Ris F., Oberholzer J., Joller-Jemelka H. I., Spinas G. A., Kaiser N., Halban P. A., Donath M. Y. (2002) Glucose-induced β-cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J. Clin. Invest. 110, 851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Larsen C. M., Faulenbach M., Vaag A., Vølund A., Ehses J. A., Seifert B., Mandrup-Poulsen T., Donath M. Y. (2007) Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 [DOI] [PubMed] [Google Scholar]
- 29. Owyang A. M., Maedler K., Gross L., Yin J., Esposito L., Shu L., Jadhav J., Domsgen E., Bergemann J., Lee S., Kantak S. (2010) XOMA 052, an anti-IL-1β monoclonal antibody, improves glucose control and β-cell function in the diet-induced obesity mouse model. Endocrinology 151, 2515–2527 [DOI] [PubMed] [Google Scholar]
- 30. Robertson R. P, Gavareski D. J., Porte D., Jr., Bierman E. L. (1974) Inhibition of in vivo insulin secretion by prostaglandin E1. J. Clin. Invest. 54, 310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Metz S. A., Robertson R. P., Fujimoto W. Y. (1981) Inhibition of prostaglandin E synthesis augments glucose-induced insulin secretion is cultured pancreas. Diabetes 30, 551–557 [DOI] [PubMed] [Google Scholar]
- 32. Tran P. O., Gleason C. E., Robertson R. P. (2002) Inhibition of IL-1-induced COX-2 and EP3 gene expression by sodium salicylate enhances pancreatic islet β-cell function. Diabetes 51, 1772–1778 [DOI] [PubMed] [Google Scholar]
- 33. Robertson R. P, Chen M. (1977) A role for prostaglandin E in defective insulin secretion and carbohydrate intolerance in diabetes mellitus. J. Clin. Invest. 60, 747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uematsu S., Matsumoto M., Takeda K., Akira S. (2002) Lipopolysaccharide-dependent prostaglandin E2 production is regulated by the glutathione-dependent prostaglandin E2 synthase gene induced by the Toll-like receptor 4/MyD88/NF-IL6 pathway. J. Immunol. 168, 5811–5816 [DOI] [PubMed] [Google Scholar]
- 35. Tran P. O., Gleason C. E., Poitout V., Robertson R. P. (1999) Prostaglandin E2 mediates inhibition of insulin secretion by interleukin-1β. J. Biol. Chem. 274, 31245–31248 [DOI] [PubMed] [Google Scholar]
- 36. Heitmeier M. R., Kelly C. B., Ensor N. J., Gibson K. A., Mullis K. G., Corbett J. A., Maziasz T. J. (2004) Role of cyclooxygenase-2 in cytokine-induced β-cell dysfunction and damage by isolated rat and human islets. J. Biol. Chem. 279, 53145–53151 [DOI] [PubMed] [Google Scholar]
- 37. Burke S. J., Collier J. J. (2011) The gene encoding cyclooxygenase-2 is regulated by IL-1β and prostaglandins in 832/13 rat insulinoma cells. Cell. Immunol. 271, 379–384 [DOI] [PubMed] [Google Scholar]
- 38. Båge T., Modéer T., Kawakami T., Quezada H. C., Yucel-Lindberg T. (2007) Regulation of prostaglandin E synthases: effects of siRNA-mediated inhibition of microsomal prostaglandin E synthase-1. Biochim. Biophys. Acta 1773, 1589–1598 [DOI] [PubMed] [Google Scholar]
- 39. Robertson R. P. (1986) Arachidonic acid metabolite regulation of insulin secretion. Diabetes Metab. Rev. 2, 261–296 [DOI] [PubMed] [Google Scholar]
- 40. Seaquist E. R., Walseth T. F., Nelson D. M., Robertson R. P. (1989) Pertussis toxin-sensitive G protein mediation of PGE2 inhibition of cAMP metabolism and phasic glucose-induced insulin secretion in HIT cells. Diabetes 38, 1439–1445 [DOI] [PubMed] [Google Scholar]
- 41. Robertson R. P., Tsai P., Little S. A., Zhang H. J., Walseth T. F. (1987) Receptor-mediated adenylate cyclase-coupled mechanism for PGE2 inhibition of insulin secretion in HIT cells. Diabetes 36, 1047–1053 [DOI] [PubMed] [Google Scholar]
- 42. Zawalich W. S., Zawalich K. C., Yamazaki H. (2007) Divergent effects of epinephrine and prostaglandin E2 on glucose-induced insulin secretion from perifused rat islets. Metab. Clin. Exp. 56, 12–18 [DOI] [PubMed] [Google Scholar]
- 43. Persaud S. J., Muller D., Belin V. D., Kitsou-Mylona I., Asare-Anane H., Papadimitriou A., Burns C. J., Huang G. C., Amiel S. A., Jones P. M. (2007) The role of arachidonic acid and its metabolites in insulin secretion from human islets of Langerhans. Diabetes 56, 197–203 [DOI] [PubMed] [Google Scholar]
- 44. Oshima H., Taketo M. M., Oshima M. (2006) Destruction of pancreatic β-cells by transgenic induction of prostaglandin E2 in the islets. J. Biol. Chem. 281, 29330–29336 [DOI] [PubMed] [Google Scholar]
- 45. Meng Z., Lv J., Luo Y., Lin Y., Zhu Y., Nie J., Yang T., Sun Y., Han X. (2009) Forkhead box O1/pancreatic and duodenal homeobox 1 intracellular translocation is regulated by c-Jun N-terminal kinase and involved in prostaglandin E2-induced pancreatic β-cell dysfunction. Endocrinology 150, 5284–5293 [DOI] [PubMed] [Google Scholar]