In the aquatic environment of cyanobacteria, the CO2 concentration often is limiting for photosynthesis, and when combined with high light, this leads to the formation of excess reactive oxygen species (ROS). ROS cause damage to most cellular constituents (membranes, DNA, and proteins), and photosystem II (PSII) is especially vulnerable as it is a primary site of ROS formation that occurs in the chloroplast under high light and low CO2 (reviewed in Bailey and Grossman, 2008; Tikkanen et al., 2011). Zhang et al. (1952–1971) report on a novel type of photoprotection mechanism in cyanobacteria encoded by the flv4-flv2 operon. This operon is present in a number of cyanobacteria and is strongly induced upon low Ci and high light conditions in Synechocystis (Zhang et al., 2009). Three genes found in the operon are arranged in tandem orientation in the same direction of transcription (flv4, Sll0218, and flv2), suggesting an interaction of these proteins. Flv4 and Flv2 encode flavodiiron proteins (FDPs), whereas Sll0218 encodes a hypothetical protein of unknown function. In many strict and facultative anaerobic microorganisms, FDPs are known to function in detoxification of O2 and/or NO (Vicente et al., 2008). Through investigation of the localization of these three proteins in Synechocystis and evaluation of mutant strains, Zhang et al. provide insight into their function in photoprotection of PSII.
The authors found that Flv4 and Flv2 were associated with the plasma membrane at high cation (Ca2+/Mg2+) concentration but were released to the soluble fraction at low cation concentration. Flv4 and Flv2 were found to function as a heterodimer, capable of rapid electron transfer between the flavin mononucleotide moiety of Flv2 and the diiron center of Flv4. This is somewhat unlike the situation in anaerobic microbes, where FDPs function as homodimers. Homology modeling was conducted to study the functional organization of the heterodimeric Flv2/4 complex. The authors speculate that the binding of Flv2/Flv4 to the membrane in vivo might be transient and reversible, since it was dependent on cation concentrations, which typically may be much lower in vivo than the concentrations used in the isolation buffer.
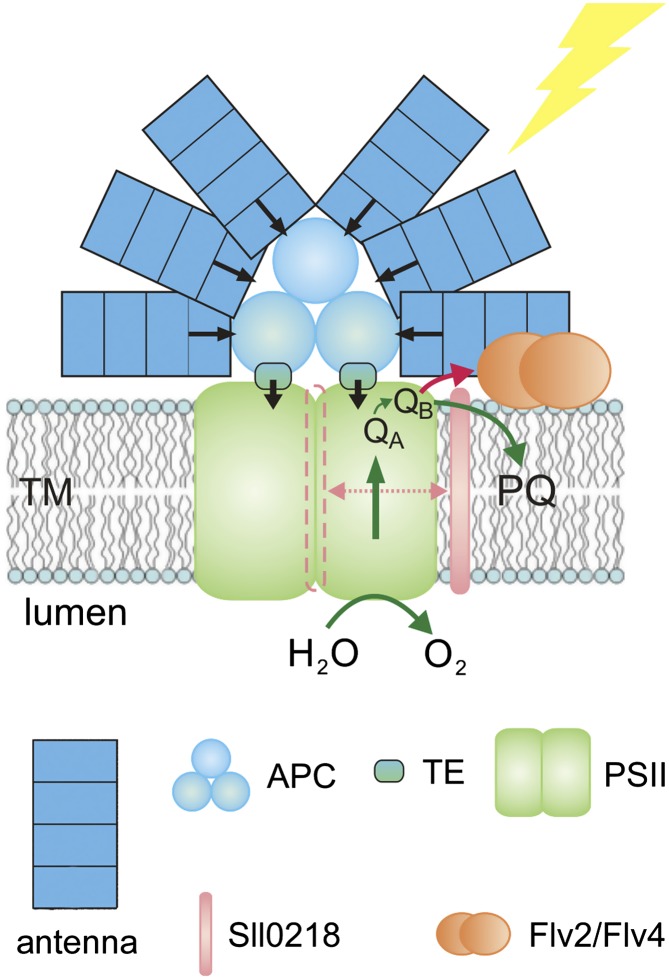
Interestingly, unlike Flv4 and Flv2, Sll0218 was associated with the thylakoid membrane independently of the cation concentration. Isolation in different buffers suggested that Sll0218 is not homogeneous throughout the thylakoid membrane but is located in well-exposed regions of the membrane. Although Sll0218 appears to function in the thylakoid membrane and the Flv2/Flv4 heterodimer in the plasma membrane and/or cytosol, the authors suggest that all three proteins are functionally linked in photoprotection of PSII. Sll0218 was found to operate partially independently of Flv2/Flv4 to stabilize the PSII dimers and open up a novel electron transfer pathway to the Flv2/Flv4 heterodimer from PSII (see figure). Flv2/Flv4 binds to thylakoids in light, mediates electron transfer from PSII, and concomitantly regulates the association of phycobilisomes with PSII. This constitutes a previously unknown type of photoprotection mechanism that evolved in parallel with oxygen-evolving PSII.
Model of the function of Flv2/Flv4 and Sll0218 in energy transfer to PSII and electron transfer from PSII to the Flv2/Flv4 heterodimer at ambient low Ci. Mg2+-induced reversible attachment of the Flv2/Flv4 heterodimer to the thylakoid membrane (TM) coordinates both energy transfer via terminal emitters (TE) from phycobilisomes (represented by allophycocyanin [APC] in Synechocystis) to PSII and the electron transfer to the Flv2/Flv4 heterodimer from PSII centers, which are slightly modified by the Sll0218 protein to facilitate this process. (Figure adapted and reprinted from Zhang et al. [2012], Figure 10B.)
References
- Bailey S., Grossman A. (2008). Photoprotection in cyanobacteria: Regulation of light harvesting. Photochem. Photobiol. 84: 1410–1420 [DOI] [PubMed] [Google Scholar]
- Tikkanen M., Grieco M., Aro E.-M. (2011). Novel insights into plant light-harvesting complex II phosphorylation and ‘state transitions’. Trends Plant Sci. 16: 126–131 [DOI] [PubMed] [Google Scholar]
- Vicente J.B., Carrondo M.A., Teixeira M., Frazão C. (2008). Structural studies on flavodiiron proteins. Methods Enzymol. 437: 3–19 [DOI] [PubMed] [Google Scholar]
- Zhang P., Allahverdiyeva Y., Eisenhut M., Aro E.-M. (2009). Flavodiiron proteins in oxygenic photosynthetic organisms: Photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS ONE 4: e5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Eisenhut M., Brandt A.-M., Carmel D., Silén H.M., Vass I., Allahverdiyeva Y., Salminen T.A., Aro E.-M. (2012). Operon flv4-flv2 provides cyanobacterial photosystem II with flexibility of electron transfer. Plant Cell 24: 1952–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]