Abstract
Background
Pain following thoracotomy is common. The objective of this study was to assess whether pain 3 months post-thoracotomy negatively impacts quality of life.
Methods
One hundred ten patients were prospectively assessed using the Short Form-36 (SF-36) Health Survey before and 3 months after elective thoracotomy. Pain and medication use were evaluated by questionnaire. Patients experiencing pain at 3 months were compared with patients who did not have post-thoracotomy pain.
Results
Seventy-five patients (68%) had pain 3 months post-thoracotomy; 12 patients (11%) rated their average pain greater than 3 (out of 10). Eighteen (16%) patients required opioid analgesics. The pain group reported lower SF-36 scores in: physical functioning (P=0.049), bodily pain (P=0.0002), and vitality (P=0.044). There were no other significant differences in any SF-36 scale between the pain and non-pain groups.
Conclusion
Pain is commonly reported at 3 months following elective thoracotomy, but is generally mild, shows improvement over with time, and does not usually require opioid analgesics. Patients who experience post-thoracotomy pain at 3 months are at risk for significantly decreased physical functioning and vitality, but are not at risk for significantly decreased social, emotional, or mental health functioning compared with patients who do not experience post-thoracotomy pain at 3 months.
Keywords: Lung cancer surgery, Outcomes
Introduction
We recently reported a randomized, double-blinded, active placebo-controlled study of patients undergoing thoracotomy that evaluated the effect of preoperative gabapentin treatment on postoperative pain [1]. A single 600 mg oral dose of gabapentin preoperatively did not impact postoperative pain scores or opioid consumption during the first 3 days following thoracotomy. Patients received standard care by a dedicated inpatient pain service including a thoracic epidural infusion of bupivacaine and hydromorphone, intravenous patient-controlled fentanyl, intravenous ketorolac, and oral acetaminophen, and had only mild to moderate pain postoperatively. Despite aggressive acute pain management, the frequency of pain at 3 months was 68%.
The purpose of this follow-up study was to determine the effects of persistent postoperative pain on health-related measures of quality of life among patients 3-months following thoracotomy. Previous studies have highlighted the overall declines in quality of health in patients post-thoracotomy [2,3,4], but have not examined the specific impact of persistent pain on health-related quality of life. We also sought to describe quantitatively and qualitatively patients’ post-thoracotomy pain symptoms and analgesic medication use.
Material and Methods
Data were obtained as part of a randomized, controlled, double-blinded trial that was designed to evaluate the effect of preoperative gabapentin on acute post-thoracotomy pain and opioid consumption [1]. Patients aged 45 to 75 years undergoing elective thoracotomy via either serratus-sparing posterolateral thoracotomy or limited thoracotomy were included in the study. Patients with pre-existing pain syndromes or daily opioid therapy in excess of 20 mg of oral morphine equivalents were excluded. Preoperatively, a thoracic epidural catheter at the T4-8 level was placed in all patients, bolused with 5 ml of 0.25% bupivacaine and 0.5 mg hydromorphone, and an epidural infusion of 0.075% bupivacaine and 10 mcg/ml hydromorphone was delivered at 6 ml/hr intraoperatively and postoperatively. General anesthesia was based on inhaled agents after intravenous induction, and intravenous opioids were administered according to standardized protocol. Postoperatively, intravenous fentanyl patient-conrolled analgesia (10 micrograms every 10 minutes with a 200 microgram 4 hour lockout) was provided, as were intravenous ketorolac (15 mg every 6 hours as needed) and oral acetaminophen (650 mg every 6 hours as needed) per protocol. The study was approved by the Mayo Clinic Institutional Review Board and was registered in ClinicalTrials.gov (NCT00588159). All patients provided written informed consent.
Health-related quality of life was assessed with the Medical Outcome Study 36-Item Short Form (SF-36) questionnaire. The SF-36 is a widely used instrument that is standardized and validated [5]. The SF-36 assesses eight scales: physical functioning, role functioning-physical, bodily pain, general health, energy, social functioning, role functioning-emotional, and mental health. Higher scores indicate better health. All patients completed the baseline SF-36 preoperatively. At 3 months postoperatively patients completed the SF-36 questionnaire, after being contacted by mail or by telephone. Patients were asked pain questions from the validated Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) [6] and from a previous study investigating the presence of neuropathic post-thoracotomy pain [7]. (Appendix 1: http://mayoresearch.mayo.edu/mayo/research/chronic-post-thoracotomy-pain/upload/appendix-a-ats-paper.pdf)
Statistical Analysis
Study data were collected using REDCap (Research Electronic Data Capture) [8]. Data were summarized and compared for the gabapentin vs. placebo treatment groups, for patients reporting pain vs. not at 3 months postoperatively, and for patients who had both a preoperative (baseline) and 3-month post-thoracotomy SF-36 survey. Categorical variables were compared using the chi-square test or Fisher’s exact test. Continuous variables were compared using the two-sample t-test (or rank sum test). All analyses were two-tailed with a significance level of P<0.05 and were performed using SAS version 9.1 (SAS Institute Inc.; Cary, NC). The magnitude of change in SF-36 scores was analyzed using Cohen’s d [9], which is a standardized measure of effect size [10]. Available benchmarks describe effect size values of 0.20–0.49 as small, 0.50–0.79 as moderate, and effect size values ≥0.80 as large [9,10]. Cohen’s d was calculated as the difference between the baseline and the 3 month SF-36 scale score, divided by the standard deviation of the baseline score.
Results
Two hundred twenty-seven patients were evaluated for enrollment. All patients survived the operation and were discharged from the hospital. Baseline patient and procedural characteristics did not differ across groups (Table 1). Lobectomy was the most frequently performed surgical procedure. Of the 120 thoracotomy patients, 100 (83%) underwent surgery for neoplasm. A serratus-sparing posterolateral thoracotomy was performed in 109 patients (91%) and a limited thoracotomy was performed in 11 patients (9%). Ten patients were lost to follow-up at 3 months; thus, 3-month follow-up data were analyzed for 110 patients: 57 were randomized to active placebo (diphenhydramine; 12.5 mg) and 53 were randomized to a single preoperative dose of gabapentin (600 mg). No differences were evident between the 110 patients analyzed at 3 months and the original cohort of 120 patients.
Table 1.
Patient and Procedural Characteristics
| Gabapentin (N=57) | Placebo (N=63) | Overall (N=120) | 3-month responders (N=110) | |
|---|---|---|---|---|
| Age | 64.4 ± 7.4 | 64.3 ± 6.7 | 64.3 ± 7.0 | 64.5 ± 6.9 |
| BMI | 28.1 ± 4.8 | 28.4 ± 4.3 | 28.3 ± 4.5 | 28.3 ± 4.5 |
| Gender | ||||
| • Male | 32 (56%) | 30 (48%) | 62 (52%) | 56 (51%) |
| • Female | 25 (44%) | 33 (52%) | 58 (48%) | 54 (49%) |
| Cancer | 50 (88%) | 50 (79%) | 100 (83%) | 91 (83%) |
| Type of surgery | ||||
| • Lobectomy | 32 (56%) | 37 (59%) | 69 (58%) | 63 (57%) |
| • Bilobectomy | 4 (7%) | 2 (3%) | 6 (5%) | 4 (4%) |
| • Wedge resection | 8 (14%) | 16 (25%) | 24 (20%) | 23 (21%) |
| • Segmentectomy | 4 (7%) | 3 (5%) | 7 (6%) | 7 (6%) |
| • Pneumonectomy | 2 (4%) | 3 (5%) | 5 (4%) | 5 (5%) |
| • Other | 7 (12%) | 2 (3%) | 9 (8%) | 8 (7%) |
| Chest wall resection | 1 (2%) | 3 (5%) | 4 (3%) | 4 (4%) |
Table 2 describes pain and medication use 3 months post-thoracotomy. There was no significant difference between treatment groups in terms of presence of post-thoracotomy pain at 3 months (68% overall). The average pain score was less than 4 (out of 10) for 89% of all patients, with no difference between treatment groups. Overall, 44% of patients were taking some form of pain medication at 3 months post-thoracotomy, with no significant difference between the gabapentin (53%) and placebo (35%) groups. The only significant difference in analgesic use at 3 months post-thoracotomy related to acetaminophen consumption. More patients in the gabapentin group were taking acetaminophen at 3 months than the placebo group (28% vs. 12%, respectively; P=0.036). Overall, 16% of patients were taking opioids and 19% were taking NSAIDs at 3 months post-thoracotomy, with no significant difference between treatment groups.
Table 2.
Pain and medication use 3 months post-thoracotomy.
| Gabapentin (N=53) | Placebo (N=57) | Overall (N=110) | |
|---|---|---|---|
| Do you have pain as a result of your chest surgery? | |||
| • Yes | 37 (70%) | 38 (67%) | 75 (68%) |
| Average pain score of post-thoracotomy pain in the last week | |||
| • 0 | 17 (32%) | 24 (42%) | 41 (37%) |
| • 1 to 3 | 31 (58%) | 26 (46%) | 57 (52%) |
| • 4 or more | 5 (9%) | 7 (12%) | 12 (11%) |
| Highest pain score of post-thoracotomy pain in the last week | |||
| • 0 | 16 (30%) | 20 (35%) | 36 (33%) |
| • 1 to 3 | 16 (30%) | 15 (26%) | 31 (28%) |
| • 4 or more | 21 (40%) | 22 (39%) | 43 (39%) |
| Are you using opioids for post-thoracotomy pain? | |||
| • Yes | 11 (21%) | 7 (12%) | 18 (16%) |
| Are you using acetaminophen for post-thoracotomy pain? | |||
| • Yes | 15 (28%) | 7 (12%) | 22 (20%) |
| Are you using NSAIDS for post-thoracotomy pain? | |||
| • Yes | 11 (21%) | 10 (18%) | 21 (19%) |
Pain symptoms at 3 months post-thoracotomy in the 75 patients who had post-thoracotomy pain in the week prior to completing the questionnaire were evaluated. There were no significant differences in pain symptoms between the placebo and gabapentin treatment groups. Overall, 56 (75%) patients stated that “pain makes the affected skin abnormally sensitive to touch,” and 31 (42%) patients noted that their pain comes on suddenly and in bursts for no apparent reason when they are still. Most (77%) patients reported their pain improved with time.
Analysis of SF-36 data was performed for those 99 patients with complete values for all scales, including 50 placebo and 49 gabapentin group patients. There were no significant differences between treatment groups. Overall, there were significant declines in physical functioning, role-physical, bodily pain, vitality, and social functioning (P<0.001) at 3 months post-thoracotomy compared with preoperative values (Table 3). The effect size (Cohen’s d) was large for role physical (0.9) and bodily pain (0.8), moderate for vitality (0.5) and social functioning (0.5), and small for physical functioning (0.3). There was no change in these findings with exclusion of the four unplanned chest wall resection patients.
Table 3.
SF-36 components
| Effect size (Cohen’s d)a | Overall (N=99) | |
|---|---|---|
| Physical functioning | ||
| • Pre-op | 72.9 ± 25.8 | |
| • 3 month | 0.3 | 64.2 ± 24.2* |
| Role-physical | ||
| • Pre-op | 67.6 ± 40.6 | |
| • 3 month | 0.9 | 31.7 ± 38.4* |
| Pain index | ||
| • Pre-op | 81.2 ± 21.8 | |
| • 3 month | 0.8 | 62.4 ± 23.0* |
| General health perceptions | ||
| • Pre-op | 66.7 ± 18.7 | |
| • 3 month | 0.1 | 63.9 ± 18.8 |
| Vitality | ||
| • Pre-op | 57.7 ± 19.9 | |
| • 3 month | 0.5 | 48.0 ± 22.2* |
| Social functioning | ||
| • Pre-op | 83.9 ± 19.9 | |
| • 3 month | 0.5 | 71.4 ± 26.5* |
| Role-emotional | ||
| • Pre-op | 81.8 ± 32.5 | |
| • 3 month | 0.1 | 77.2 ± 36.8 |
| Mental health index | ||
| • Pre-op | 75.2 ± 17.9 | |
| • 3 month | 0.1 | 76.8 ± 18.4 |
The magnitude of change in SF-36 scores was analyzed using Cohen’s d (see Statistical Analysis). Available benchmarks describe effect size values of 0.20–0.49 as small, 0.50–0.79 as moderate, and effect size values ≥0.80 as large.
Paired t-test p <0.001.
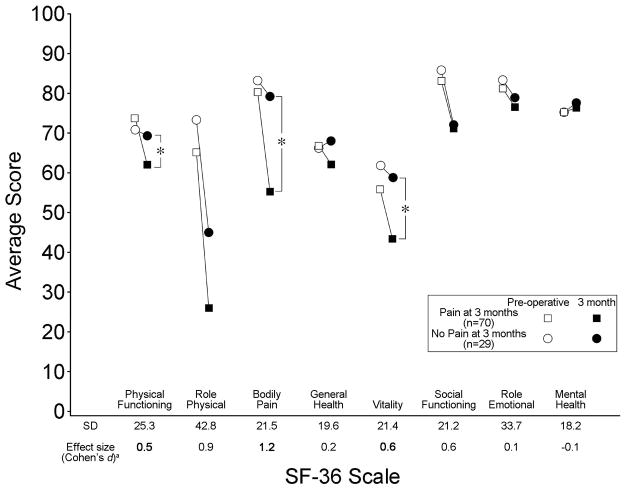
There were no significant group differences in mean age, gender or diagnosis of cancer between patients who had post-thoracotomy pain at 3 months (N=75) and those who had no pain (N=35). Perioperative pain did not differ between patients reporting pain at 3 months versus those who had no pain. Specifically, there were no differences in preoperative pain ratings: 0.0±0.2 for the group without pain at three months; 0.5±1.4 for the group with pain at three months (p=0.08), postoperative opioid analgesic use (in oral morphine equivalents) [11] or pain scores at rest or with coughing on the first or second morning following surgery. Pain at 3 months following surgery did not differ between surgical sides [chi square P=0.222; right side = 40 (63.5%), left side = 35 (74.5%)]. In comparing the SF-36 scales between patients who had post-thoracotomy pain at 3 months versus those who did not (Figure 1), there were 3 significant differences with the pain group reporting poorer measures of physical functioning (P=0.049), bodily pain (P=0.0002), and vitality (P=0.044). Cohen’s d effect size was large for bodily pain (1.2), and moderate for both physical functioning (0.5) and vitality (0.6). There were no significant differences in general health, role physical or role emotional functioning, social functioning, or the mental health scale.
Figure 1.
Mean pre-operative and 3-month SF-36 scale scores in patients with and without post-thoracotomy at 3 months. a In patients with post-thoracotomy pain at 3 months, the SD for the preoperative scores in each SF-36 scale and the magnitude of change in SF-36 scores is presented (effect size was analyzed using Cohen’s d - see Statistical Analysis). Available benchmarks describe effect size values of 0.20–0.49 as small, 0.50–0.79 as moderate, and effect size values ≥0.80 as large. *Paired t-test p<0.05.
Comment
The present report is one of the largest prospective studies of post-thoracotomy patients with an assessment of health-related quality of life based on the presence of pain three months following surgery. Previous studies have highlighted the overall declines in quality of health in patients post-thoracotomy, [2,3,4] but did not examine the impact of persistent pain on health-related quality of life. By evaluating SF-36 scores in a large patient cohort we were able to show that chronic pain 3 months post-thoracotomy is associated with significantly decreased physical functioning and vitality scores, despite the fact that most patients reported only mild pain. Importantly, all patients were aggressively treated perioperatively using state-of-the-art, multimodal analgesia regimens that included thoracic epidural analgesia, intravenous opioids, oral acetaminophen, and intravenous ketorolac, and management by a dedicated, inpatient pain service. Perioperative analgesia during the hospital course resulted in low pain scores that were comparable between the group of patients that subsequently reported persistent pain and those that did not. Unfortunately, post-thoracotomy pain is common. It remains to be determined whether alternate pain regimens or surgical techniques will result in a reduced frequency of post-thoracotomy pain and improved quality of life for these patients.
The current study found a 68% incidence of chronic post-thoracotomy pain at three months, and this is similar to other prospective studies [12, 13, 14]. Post-thoracotomy pain at 3 months was generally mild, often improved with time, and few patients took opioid analgesic medications for pain. For patients with post-thoracotomy pain at three months, the clinically important differences in SF-36 scores would be regarded as moderate-to-large for bodily pain, moderate for vitality, and small for physical function compared to patients who did not have post-thoracotomy pain at three months. Clinically important differences in SF-36 scores have been reported previously in selected patient populations, including patients with chronic obstructive pulmonary disease, asthma, heart disease [15,16] and rheumatoid arthritis [17]. The reported change in SF-36 scores for the vitality scale, for instance, would be described as “moderately worse” to a “good deal worse” and the clinically important difference in bodily pain would be regarded as “a great deal worse” to “a very great deal worse” [16].
Our patients’ baseline SF-36 component scores were similar to normative data derived from large cross-sectional studies in the general population with chronic medical and psychiatric conditions [5]. In addition, the observed changes in our SF-36 scales at three months are similar to the SF-36 scores at one month post-thoracotomy in a study by Mangione et al. [3] They assessed the responsiveness of the SF-36 to changes in health status over time in 123 patients who underwent thoracic surgery for lung cancer [3]. Physical function, role-physical, bodily pain, social function, and vitality were significantly decreased in the present study at 3 months as well as in the study by Mangione et al. [3] 1-month post-thoracotomy.. The changes in SF-36 scores found in the present study represent a large clinically important difference in role-physical scale, a moderate clinically important difference in the bodily pain scale, and small clinically important differences in physical functioning, vitality, and social functioning. Follow-up beyond 3 months post-thoracotomy was not performed in this study, and might have been valuable in observing a possible return to preoperative quality of life. Of note, the study by Mangione et al. demonstrated that thoracic surgery patients had greater decrements in physical function, role-physical, bodily pain, and vitality at one month, six months and 12 months compared to patients who had undergone total hip arthroplasty or abdominal aortic aneurysm repair [3]. Although, not significantly different between patients with pain and those without pain at 3 months post-thoracotomy, the decline in role-functioning-physical would be described as “a very great deal worse” or “a great deal worse” [16].
Other studies examining quality of life post-thoracotomy have also shown the impact of surgery on bodily pain and physical functioning. Brunelli et al. [2] prospectively assessed 156 patients who were undergoing major lung resection for lung cancer, and assessed serial quality of life scales through 3 months postoperatively. The physical functioning scale of the SF-36 remained below preoperative values at 3 months, as was also demonstrated in our current study. Mangione et al. [3] also reported increased pain and significant declines in physical function, role limitations due to physical limitations, and bodily pain in 123 patients who underwent thoracic surgery for lung cancer. Schulte et al. [4] prospectively evaluated 159 patients and found that physical function was lowest at 3 months post-thoracotomy and pain scores peaked at that time. Handy et al. [18] compared SF-36 scales in 139 patients through 6 months postoperatively following an assortment of thoracic surgeries (62.6% of patients underwent thoracotomy, 36.7% underwent median sternotomy, and 0.7% underwent video-assisted thoracic surgery). Six-month postoperative scores demonstrated significant decline in physical functioning, role-functioning-physical, social functioning, mental health, and bodily pain. Postoperative visual analog pain scores were significantly worse (2.20 ± 2.58) at 6 months, and the authors suggested this could have accounted for most of the functional health status and quality of life issues identified. Although patients with post-thoracotomy pain and decreased physical functioning, bodily pain, and physical role scores would also be expected to have significant decreases in emotional and social functioning, this was not observed in the current study.
Eighty-three percent of patients in this series presented with cancer, and there are multiple other issues that can impact quality of life after thoracotomy, separate from post-thoracotomy pain. These would include patients who are actively undergoing adjuvant therapy for their lung cancer, patients who experience dyspnea after anatomical resection, or people who are found to have advanced disease that would impact their prognosis. For patients with lung cancer, more specific quality of life assessments are available [19] and might have demonstrated additional quality of life issues in this population. Barlesi et al. [20] evaluated 110 patients undergoing thoracic surgery for non-small cell lung cancer with the EORTC QLQ-C30 and LC-13 module questionnaire [19], and found a median chest pain score of 33 (0–100) at one month postoperatively. Win et al. [21] evaluated 110 patients undergoing lung cancer surgery with the EORTC QLQ-C30 and LC-13 module. Thoracotomy was associated with short-term negative effects on quality of life, but symptoms returned to preoperative values at 6 months. In general agreement, Mangione et al. [3] noted that at 1 month post-thoracotomy, the mental health, role limitations due to mental health, and health perception did not decline in patients who underwent thoracic surgery for lung cancer; all of which are consistent with the findings in our present study. Of note, the mental health index was higher than preoperative baseline up to 12 months post-thoracotomy [3]. We found this to be the case in our study at three months post-thoracotomy as well. In contrast, Handy et al. reported that the mental health index in patients undergoing lung cancer resection remained significantly below both the preoperative mean and the age-matched healthy normal mean at 6 months [18].
Although usually mild, the significant incidence of post-thoracotomy pain argues for finding preventative options. Aggressive multimodal pain relief in our current study did not decrease the incidence of chronic post-thoracotomy pain below previously reported levels. A variety of factors may be related to the development of post-thoracotomy pain, including surgical and anesthetic techniques [4, 22–28]. Win et al. [21] reported an association between preoperative pain scale and 6 month global health status. In the surgical population included in the present study, preoperative pain scores were minimal [1]. The role of surgical technique is controversial in its contribution to chronic post-thoracotomy pain [12]. Minimally invasive thoracoscopic surgery is gaining wider acceptance, with the reduction of post-thoracotomy pain as one of the leading objectives [29, 30]. Nevertheless, a large number of patients still undergo a conventional thoracotomy as their surgical approach. Indeed, the 2007 data from the Society of Thoracic Surgeons database reported that 71% of pulmonary lobectomies were performed via thoracotomy emphasizing the continued importance of managing post-thoracotomy pain [31]. The present study used limited, nerve-sparing techniques in contrast to previous studies. Although this study did not directly compare surgical techniques across the 6 thoracic surgeons, the conduct of thoracotomy is fairly uniform at our institution.
Post-thoracotomy pain at 3 months is unfortunately common, but is generally mild. Most patients reported that pain improved with time, and few were taking opioid analgesic medications for pain. Patients who experience post-thoracotomy pain at 3 months experience significantly decreased physical functioning and vitality, but are not at risk for significantly decreased social, emotional, or mental health functioning compared with patients who do not experience pain at 3 months.
Acknowledgments
The author would like to acknowledge Mayo Clinic’s Department of Anesthesiology, Anesthesia Clinical Research Unit (ACRU), Division of Thoracic Surgery, Department of Nursing, and secretarial assistance by Pamela J. Fenske.
Funding for this study was provided by the Mayo Foundation, and the project described was supported by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kinney MAO, Mantilla CB, Carns PE, et al. Preoperative gabapentin for acute post-thoracotomy analgesia: a randomized, double-blinded, active placebo-controlled study. Pain Pract. 2011 doi: 10.1111/j.1533–2500.2011.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunelli A, Socci L, Refai M, Salati M, Xiume F, Sabbatini A. Quality of life before and after major lung resection for lung cancer: a prospective follow-up analysis. Ann Thorac Surg. 2007;84:410–416. doi: 10.1016/j.athoracsur.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Mangione CM, Goldman L, Orav EJ, et al. Health-related quality of life after elective surgery: measurement of longitudinal changes. J Gen Intern Med. 1997;12:686–697. doi: 10.1046/j.1525-1497.1997.07142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulte T, Schniewind B, Dohrmann P, Kuchler T, Kurdow R. The extent of lung parenchyma resection significantly impacts long-term quality of life in patients with non-small cell lung cancer. Chest. 2009;135:322–329. doi: 10.1378/chest.08-1114. [DOI] [PubMed] [Google Scholar]
- 5.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-ltem Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92:147–157. doi: 10.1016/s0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 7.Maguire MF, Ravenscroft A, Beggs D, Duffy JP. A questionnaire study investigating the prevalence of the neuropathic component of chronic pain after thoracic surgery. Eur J Cardiothorac Surg. 2006;29:800–805. doi: 10.1016/j.ejcts.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen J. Statistical Power Analysis for Social and Behavioural Sciences. 2. Hillsdale: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 10.Busija L, Osborne RH, Nilsdotter A, Buchbinder R, Roos EM. Magnitude and meaningfulness of change in SF-36 scores in four types of orthopedic surgery. Health Qual Life Outcomes. 2008;6:55. doi: 10.1186/1477-7525-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanks GW, Conno Fd, Cherny N, et al. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer. 2001;84:587–593. doi: 10.1054/bjoc.2001.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochroch EA, Gottschalk A, Augoustides JG, Aukburg SJ, Kaiser LR, Shrager JB. Pain and physical function are similar following axillary, muscle-sparing vs posterolateral thoracotomy. Chest. 2005;128:2664–2670. doi: 10.1378/chest.128.4.2664. [DOI] [PubMed] [Google Scholar]
- 13.Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: a follow-up study. Acta Anaesthesiol Scand. 1999;43:563–567. doi: 10.1034/j.1399-6576.1999.430513.x. [DOI] [PubMed] [Google Scholar]
- 14.Guastella V, Mick G, Soriano C, et al. A prospective study of neuropathic pain induced by thoracotomy: incidence, clinical description, and diagnosis. Pain. 2011;152:74–81. doi: 10.1016/j.pain.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Wyrwich KW, Tierney WM, Babu AN, Kroenke K, Wolinsky FD. A comparison of clinically important differences in health-related quality of life for pateints with chronic lung disease, asthma, or heart disease. Health Serv Res. 2005;40:577–591. doi: 10.1111/j.1475-6773.2005.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyrwich KW, Metz SM, Kroenke K, Tierney WM, Babu AN, Wolinsky FD. J Gen Intern Med. 2007;22:161–170. doi: 10.1007/s11606-006-0063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosinski M, Zhao SZ, Dedhiya S, Osterhaus JT, Ware JE., Jr Determining minimally important changes in generic and disease-specific health-related qualilty of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis Rheum. 2000;43:1478–1487. doi: 10.1002/1529-0131(200007)43:7<1478::AID-ANR10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 18.Handy JR, Jr, Asaph JW, Skokan L, et al. What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. Chest. 2002;122:21–30. doi: 10.1378/chest.122.1.21. [DOI] [PubMed] [Google Scholar]
- 19.Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer. 1994;30A:635–642. doi: 10.1016/0959-8049(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 20.Barlesi F, Doddoli C, Loundou A, Pillet E, Thomas P, Auquier P. Preoperative psychological global well being index (PGWBI) predicts postoperative quality of life for patients with non-small cell lung cancer managed with thoracic surgery. Eur J Cardiothorac Surg. 2006;30:548–553. doi: 10.1016/j.ejcts.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 21.Win T, Sharples L, Wells FC, Ritchie AJ, Munday H, Laroche CM. Effect of lung cancer surgery on quality of life. Thorax. 2005;60:234–238. doi: 10.1136/thx.2004.031872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochroch EA, Gottschalk A, Augostides J, et al. Long-term pain and activity during recovery from major thoracotomy using thoracic epidural analgesia. Anesthesiology. 2002;97:1234–1244. doi: 10.1097/00000542-200211000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Ali M, Winter DC, Hanly AM, O’Hagan C, Keaveny J, Broe P. Prospective, randomized, controlled trial of thoracic epidural or patient-controlled opiate analgesia on perioperative quality of life. Br J Anaesth. 2010;104:292–297. doi: 10.1093/bja/aeq006. [DOI] [PubMed] [Google Scholar]
- 24.Senturk M, Ozcan PE, Talu GK, et al. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesth Analg. 2002;94:11–5. doi: 10.1213/00000539-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Tiippana E, Nilsson E, Kalso E. Post-thoracotomy pain after thoracic epidural analgesia: a prospective follow-up study. Acta Anaesthesiol Scand. 2003;47:433–438. doi: 10.1034/j.1399-6576.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 26.Akcali Y, Demir H, Tezcan B. The effect of standard posterolateral versus muscle-sparing thoracotomy on multiple parameters. Ann Thorac Surg. 2003;76:1050–1054. doi: 10.1016/s0003-4975(03)00565-4. [DOI] [PubMed] [Google Scholar]
- 27.Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg. 2009;36:170–180. doi: 10.1016/j.ejcts.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Gotoda Y, Kambara N, Sakai T, Kishi Y, Kodama K, Koyama T. The morbidity, time course and predictive factors for persistent post-thoracotomy pain. Eur J Pain. 2001;5:89–96. doi: 10.1053/eujp.2001.0225. [DOI] [PubMed] [Google Scholar]
- 29.Tomaszek SC, Cassivi SD, Shen KR, et al. Clinical outcomes of video-assisted thoracoscopic lobectomy. Mayo Clin Proc. 2009;84:509–513. doi: 10.4065/84.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg. 2006;244:420–425. doi: 10.1097/01.sla.0000234892.79056.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139:366–378. doi: 10.1016/j.jtcvs.2009.08.026. [DOI] [PubMed] [Google Scholar]