Abstract
STUDY QUESTION
Is there an association between sex chromosome disomy and semen concentration, motility and morphology?
SUMMARY ANSWER
Higher rates of XY disomy were associated with a significant increase in abnormal semen parameters, particularly low semen concentration.
WHAT IS KNOWN ALREADY
Although some prior studies have shown associations between sperm chromosomal abnormalities and reduced semen quality, results of others are inconsistent. Definitive findings have been limited by small sample sizes and lack of adjustment for potential confounders.
STUDY DESIGN, SIZE AND DURATION
Cross-sectional study of men from subfertile couples presenting at the Massachusetts General Hospital Fertility Clinic from January 2000 to May 2003.
PARTICIPANTS/MATERIALS, SETTING, METHODS
With a sample of 192 men, multiprobe fluorescence in situ hybridization for chromosomes X, Y and 18 was used to determine XX, YY, XY and total sex chromosome disomy in sperm nuclei. Sperm concentration and motility were measured using computer-assisted sperm analysis; morphology was scored using strict criteria. Logistic regression models were used to evaluate the odds of abnormal semen parameters [as defined by World Health Organization (WHO)] as a function of sperm sex chromosome disomy.
MAIN RESULTS AND THE ROLE OF CHANCE
The median percentage disomy was 0.3 for XX and YY, 0.9 for XY and 1.6 for total sex chromosome disomy. Men who had abnormalities in all three semen parameters had significantly higher median rates of XX, XY and total sex chromosome disomy than controls with normal semen parameters (0.43 versus 0.25%, 1.36 versus 0.87% and 2.37 versus 1.52%, respectively, all P< 0.05). In logistic regression models, each 0.1% increase in XY disomy was associated with a 7% increase (odds ratio: 1.07, 95% confidence interval: 1.02–1.13) in the odds of having below normal semen concentration (<20 million/ml) after adjustment for age, smoking status and abstinence time. Increases in XX, YY and total sex chromosome disomy were not associated with an increase in the odds of a man having abnormal semen parameters. In addition, autosomal chromosome disomy (1818) was not associated with abnormal semen parameters.
LIMITATIONS, REASONS FOR CAUTION
A potential limitation of this study, as well as those currently in the published literature, is that it is cross-sectional. Cross-sectional analyses by nature do not lend themselves to inference about directionality for any observed associations; therefore, we cannot determine which variable is the cause and which one is the effect. Additionally, the use of WHO cutoff criteria for dichotomizing semen parameters may not fully define fertility status; however, in this study, fertility status was not an outcome we were attempting to assess.
WIDER IMPLICATIONS OF THE FINDINGS
This is the largest study to date seeking to understand the association between sperm sex chromosome disomy and semen parameters, and the first to use multivariate modeling to understand this relationship. The findings are similar to those in the published literature and highlight the need for mechanistic studies to better characterize the interrelationships between sex chromosome disomy and standard indices of sperm health.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by grants from NIOSH (T42 OH008416) and NIEHS (R01 ES009718, P30 ES000002 and R01 ES017457). The authors declare no competing interests. At the time this work was conducted and the initial manuscript written, MEM was affiliated with the Environmental Health Department at the Harvard School of Public Health. Currently, MEM is employed by Millennium: The Takeda Oncology Company.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: aneuploidy, sex chromosome, semen parameters, fluorescence in situ hybridization, infertility
Introduction
It has been estimated that infertility affects roughly 15% of couples worldwide and that, in about half of these cases, male infertility is the primary contributing factor (de Kretser, 1997). To fully understand male factor infertility, elucidation of the biological and genetic factors required for normal sperm development and function is necessary. The process of spermatogenesis involves different stages including premeiotic divisions, meiosis and spermiogenesis. During premeiotic divisions and meiosis, successful chromosome segregation is essential, and it is during these phases that aneuploid sperm can arise. After meiosis is complete, sperm gain their characteristic shape, mobility and the ability to fertilize an egg. It is probable that underlying meiotic problems, such as sex chromosome disomy, have direct impacts on semen parameters.
Genetic abnormalities are thought to account for 15–30% of male infertility (Ferlin et al., 2007).
For example, X and Y chromosomes contain genes that have been implicated in a number of spermatogenic disorders (Diemer and Desjardins, 1999); examples of such sex chromosome-linked disorders include X chromosome androgen receptor gene mutations and Y chromosome deletions in azoospermia factor region genes, both of which are associated with decreased semen concentration (Vogt, 1998; O'Brien et al., 2010).
Chromosomal abnormalities, including translocations, have been shown to have variable effects on fertility, ranging from normal spermatogenesis to the inability to produce spermatogonia (Gianaroli et al., 2002; Georgiou et al., 2006), and are 4–10 times more prevalent among men with abnormal semen parameters when compared with those with normal semen parameters (Mak and Jarvi, 1996; Meschede et al., 1998). Abnormalities in the Y chromosome, such as microdeletions, are associated with male infertility, and Y microdeletions are more prevalent in azoospermic (10–15%) and oligozoospermic (concentration <20 million/ml; 5–10%) men (Foresta et al., 2001; Dohle et al., 2002). Aneuploidy, or an incorrect number of chromosomes, is the most common type of chromosomal abnormality, and sex chromosome trisomy is the most common type of aneuploidy at birth (Wyrobek, 1995). Numerous studies have reported a correlation between sperm disomy and male infertility in men with abnormal karyotypes; however, it has only been shown in the last 10 years that men with normal karyotypes but compromised semen parameters have increased levels of sperm aneuploidy (Shi and Martin, 2001), although uncertainty still remains.
Oligozoospermia, having a semen concentration <20 million/ml, has been hypothesized to be the semen abnormality most strongly associated with an increased frequency of sperm chromosome abnormalities, when compared with asthenozoospermia (<50% motile sperm) and teratozoospermia (<4% morphologically normal sperm; Martin et al., 1996; Vegetti et al., 2000; Martin et al., 2003). Men with severe oligozoospermia (concentration <1 million/ml) have increased frequencies of sex chromosome disomy compared with normal controls and men with mild oligozoospermia (1–19 million/ml; Martin et al., 2003; Miharu, 2005). Men with asthenozoospermia have an increased frequency of disomy compared with normal fertile controls (Hristova et al., 2002; Bernardini et al., 2005; Collodel et al., 2007). Lastly, men with teratozoospermia show a small but significant increase in aneuploidy for some chromosomes (including sex chromosomes), when compared with normal controls (Calogero et al., 2001; Harkonen et al., 2001; Templado et al., 2002; Machev et al., 2005; Sun et al., 2006). Most aneuploidy studies to date have identified a significant increase in aneuploidy in men with abnormal semen parameters; however, two early studies reported no association (Miharu et al., 1994; Guttenbach et al., 1997).
For infertile men, it is common to have abnormalities in one or even all three semen parameters. The prevalence of oligoasthenoteratozoospermia (OAT; concentration <20 million/ml, <50% motile, <4% normal morphology) in infertile men was found to be as high as 30% (Hirsh, 2003). Therefore, assessing the independent association of sex chromosome disomy with each specific semen parameter is often difficult because of co-occurrence of abnormal semen parameters. In numerous sperm aneuploidy studies, subjects have been specifically selected, or populations have been restricted, so as to only include men with the abnormal semen parameter(s) of interest. The main limitations of these studies are small sample size (≤30 men), a small number of sperm nuclei scored and an inability to assess all relevant semen parameters. In addition, most of the published literature has employed univariate analyses to make comparisons between mean aneuploidy rates in men with abnormal semen parameters versus normal controls. Few studies have included adequate control for potential confounding variables in adjusted analyses.
The objective of this study was to understand the association of sex chromosome disomy with sperm concentration, motility and morphology, in a large sample of men spanning the continuum of semen parameters, from normal to OAT, with appropriate adjustment for potential confounders.
Materials and Methods
Subjects
We evaluated a subset of participants enrolled in a parent study assessing the impact of environmental exposures on male semen quality. Men aged 20–54 from couples seeking infertility evaluation from the Massachusetts General Hospital (MGH) Fertility Center between January 2000 and May 2003 were eligible for this parent study. Sixty-five percent of eligible men agreed to participate, giving 341 participants. Most men who declined participation cited lack of time on the day of the clinic visit as their reason for non-participation. Men were excluded from the parent study if they were presenting for post-vasectomy semen analysis and/or receiving treatment for infertility (i.e. hormonal treatments). Each participant completed a self-administered questionnaire which requested information on race/ethnicity, medical and fertility history and lifestyle factors. Men were eligible for our substudy if a semen sample was available for fluorescence in situ hybridization (FISH) analysis. Semen sample availability was based on whether an aliquot of sample was available for use from the biorepository, as this cohort has been used for other semen analysis research. Of the 341 eligible men in the parent study, sufficient semen sample was available for 192 (56%) men. There were no other additional eligibility requirements. Information on the female partner's fertility status was not obtained. Both the parent study and this study were approved by the Harvard School of Public Health and MGH Human Subjects Committees. All subjects signed an informed consent form prior to participation.
Semen analysis
All subjects were asked to abstain from ejaculation for 48 h prior to giving a single semen sample by masturbation. Samples were liquefied at 37°C for 20 min before analysis. Analysis of fresh samples took place at the MGH Andrology Laboratory. The volume, pH, color and viscosity were determined for each fresh sample. Sperm counts and percentage motility were first determined manually, then measured by computer-aided sperm analysis (CASA) using the Hamilton–Thorn Motility Analyzer (10HTM-IVO). (Grades of progressive motility were not available for this study population.) A minimum of 200 sperm from four different fields were analyzed for each sample. CASA provides data on rapid and linear motile sperm (%), velocity of average path (μm/s), amplitude of lateral head displacement (μm), linearity and other sperm movement characteristics. Each sample was prepared on two slides for morphological assessment using a Nikon microscope with an oil immersion 100× objective (Nikon Company, Tokyo, Japan). Sperm were scored as normal or abnormal using the Kruger strict criteria (Kruger et al., 1988).
X–Y–18 sperm FISH
A single study investigator (M.E.M.) performed FISH, imaging and nuclei scoring analyses on frozen aliquots of all samples. The investigator was blinded to semen parameters and covariate status while preparing and analyzing all samples. FISH was carried out for chromosomes X, Y and 18 using Vysis probes (Vysis, Downers Grove, IL, USA). Chromosome 18 was used as an autosomal control. These methods have been described in detail (Perry et al., 2011).
Microscopy and semi-automated scoring criteria
Slides were imaged by wide-field fluorescence microscopy using a BD Pathway 855 Bioimager and Atto Vision imaging software (BD Biosciences, San Jose, CA, USA), with a 40×/0.90 air objective (Olympus, Center Valley, PA, USA). From each slide, 384 non-overlapping image fields were acquired, each consisting of one nuclear (4′,6-diamidino-2-phenylindole) and three probe (X, Y and 18) channel images. Image processing, segmentation, classification and scoring were performed using custom image processing and analysis software developed in MATLAB® (The Mathworks, Inc., Natick, MA, USA). Classification and scoring algorithms used were designed based on the scoring criteria described by Baumgartner et al. (1999). The overall hybridization efficiency was 97% and is consistent with the hybridization efficiency reported by other groups (Martin et al., 1996; Johannisson et al., 2002; Tiido et al., 2005). Further details on these methods have been previously described by Perry et al. (2011).
Statistical analysis
We explored the relationship between disomy (1818, XX18, YY18, XY18 and total sex chromosome disomy) and abnormal semen parameters (sperm concentration, motility and morphology). World Health Organization (WHO) cutoff criteria were used to categorize abnormal sperm concentration (≤20 million/ml), motility (≤50% motile) and morphology (≤4% normal; WHO, 1999). The Wilcoxon rank-sum tests were used to compare the distribution of age and BMI across groups with abnormal semen parameters, dichotomized using WHO reference values for sperm concentration (<20 million/ml) and motility (<50% motile sperm) and Kruger strict criteria for morphology (<4% normal sperm). χ2 tests were used to compare categorical variables (abstinence time, smoking status and race) across semen parameter groups. In preliminary analyses, scatterplots and the Spearman correlation coefficients were used to explore the relationships among sex chromosome disomy and semen parameter measures.
In our primary analysis, multivariate logistic regression models were constructed based on abnormal semen parameters as defined above. A logistic regression model was also used to evaluate the increase in the odds of a man having two abnormal semen parameters for each 0.1% increase in sex chromosome disomy. A comparison (control) group was constructed consisting of men who had normal levels of all three semen parameters. In secondary analyses, linear regression models were constructed to evaluate semen parameters on a continuous scale. Because sperm concentration was highly skewed, it was log transformed in linear models. Separate models were constructed for each semen parameter for both multivariate logistic regression models and multiple linear regression models.
Age, abstinence time and smoking status were included in all models based on a priori assumptions about their likely associations with aneuploidy or semen parameters (Blackwell and Zaneveld, 1992; Vine, 1996; Hassan and Killick, 2003). Other potential confounding variables assessed were race and BMI. Inclusion of race and BMI in final models was based on a change of at least 10% in estimated association with sperm sex disomy. In regression models, age was modeled as a continuous variable, abstinence time as an ordinal three-category variable (≤2, 3–4, ≥5 days) and smoking status as a dummy variable (never versus current or former). We conducted two sensitivity analyses to ensure robustness of results; one sensitivity analysis excluded three men with extremely high disomy (>5%) and the other excluded men with <1000 nuclei scored. Two-sided P-values of <0.05 were considered significant. Statistical Analysis Software (SAS) version 9.2 (SAS Institute Inc., Cary, NC, USA) was used for all data analyses.
Results
A majority of the 192 men were Caucasian (85%), with 3% African American and 4% Hispanic. Most men (75%) had never smoked, with 15 (8%) current smokers and 33 (17%) ex-smokers. The 192 participants in this study did not differ with respect to their demographic or semen parameter characteristics compared with those in the full parent study cohort (n = 341; data not shown). Of these 192 men, 21 (11%) had a sperm concentration of < 20million/ml, 84 men (44%) had <50% motile sperm and 35 men (18%) had <4% normally shaped sperm (Table I). Ninety-seven men (51%) had values above the WHO reference for all three semen parameters. The semen parameter categories were not mutually exclusive, in that a man could have any one, two or all three sperm parameter outcomes in the below-reference-value groups. Twelve men (6%) had OAT (all three semen parameters below WHO reference values). Table II highlights the differences in mean and median semen parameter values among men with normal and abnormal semen parameters based on WHO cutoff criteria. Co-occurrence of abnormal semen parameters was high, with 95% of men with abnormal semen concentration, 50% of men with abnormal motility and 74% of men with abnormal morphology having at least one other abnormal semen parameter.
Table I.
Demographic and medical history by semen parameters for men seeking infertility evaluation from January 2000 to May 2003 (N = 192).
| Controls (n = 97)a | Concentration < 20 million/ml (n = 21)b | Motility < 50% (n = 84)b | Morphology < 4% (n = 35)b | |
|---|---|---|---|---|
| Age (years, mean ± SD) | 34.6 ± 5.0 | 37.0 ± 6.5* | 36.0 ± 5.2* | 35.8 ± 4.7 |
| BMI (kg/m2, mean ± SD) | 28.6 ± 5.9 | 28.1 ± 4.8 | 27.6 ± 5.2 | 28.3 ± 4.5 |
| Race [n (%)] | * | * | * | |
| Caucasian | 86 (89) | 18 (86) | 66 (79) | 31 (89) |
| African American | 2 (2) | 0 (0) | 3 (4) | 0 (0) |
| Hispanic | 2 (2) | 2 (10) | 6 (7) | 1 (3) |
| Other | 7 (7) | 1 (5) | 9 (11) | 3 (9) |
| Abstinence time [days; n (%)] | ||||
| ≤2 | 21 (22) | 5 (24) | 17 (20) | 4 (11) |
| 3–4 | 49 (51) | 10 (48) | 39 (46) | 15(43) |
| ≥5 | 26 (27) | 6 (28) | 28 (33) | 16 (46) |
| Smoking status [n (%)]c,* | ||||
| Never smoker | 76 (79) | 10 (48) | 59 (71) | 23 (66) |
| Ever smoker | 20 (21) | 11 (52) | 24 (29) | 12 (34) |
| Current smoker | 5 (5) | 3 (14) | 9 (11) | 3 (9) |
| Ex-smoker | 15 (16) | 8 (38) | 15 (18) | 9 (26) |
Wilcoxon rank-sum tests were used to compare the distribution of age and BMI across semen parameter groups; χ2 tests were used for comparisons of categorical variables across semen parameter groups.
aSubjects with sperm concentration ≥20 million/ml, motility ≥50% motile and morphology ≥4% normal.
bA subject may contribute data to more than one abnormal semen parameter category.
cSmoking status information missing on two men, and abstinence data missing on one.
*P < 0.05 compared with control group.
Table II.
Mean and median semen parameter characteristics by WHO cutoff criteria for men seeking infertility evaluation from January 2000 to May 2003 (N = 192).
| Mean ± SD | Median | |
|---|---|---|
| Concentration (million/ml) | ||
| Normal | 115.10 ± 85.71 | 87.00 |
| Abnormal | 11.44 ± 3.92 | 12.80 |
| Motility (% motile) | ||
| Normal | 69.58 ± 10.54 | 70.46 |
| Abnormal | 29.66 ± 14.30 | 31.91 |
| Morphology (% normal) | ||
| Normal | 8.62 ± 3.62 | 8.00 |
| Abnormal | 1.97 ± 0.92 | 2.00 |
WHO sperm abnormality cutoff criteria: concentration (<20 million/ml), motility (<50% motile sperm) and morphology (<4% normal morphological sperm).
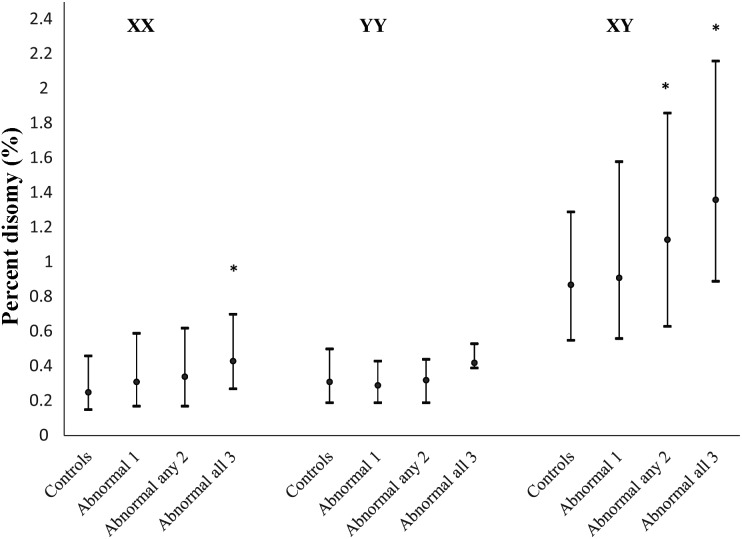
A mean (SD) of 6691 (4644) and median of 5895 sperm nuclei were scored for each subject in this study. Table III provides the median rates of 1818 and sperm sex chromosome disomy in our study population overall and for those with one or more abnormal semen parameters and normal controls. The observed median rates of 1818, XX18, YY18, XY18 and total sex chromosome disomy were 0.04, 0.27, 0.30, 0.89 and 1.56%, respectively. The median percentage XY disomy for men with any two, and with all three, abnormal semen parameters was significantly higher than in controls (1.13 and 1.36%, respectively, versus 0.87%, P<0.05; Table III and Fig. 1). The median percentage of XX disomy was also significantly higher for men with all three semen parameters abnormal compared with controls (0.43 versus 0.25%, P<0.05), and the median percentage of total disomy was significantly higher among men with OAT than in controls (2.37 versus 1.52%, P < 0.05). No differences were observed for median percentage 1818 or YY disomy between controls and men with abnormal semen parameters.
Table III.
Median rate (percentage) of 1818 and sex chromosome disomy by semen concentration, sperm motility and sperm morphology for men seeking infertility evaluation from January 2000 to May 2003 (N = 192).
| 1818 | XX18 | YY18 | XY18 | Total disomya | |
|---|---|---|---|---|---|
| Overall median (n = 192) | 0.04 | 0.27 | 0.30 | 0.89 | 1.56 |
| Controls (n = 97) | 0.03 | 0.25 | 0.31 | 0.87 | 1.52 |
| Concentration <20 million/ml (n = 21)b | 0.06 | 0.37 | 0.38 | 1.64* | 2.76* |
| Motility <50% (n = 84)b | 0.07 | 0.32 | 0.29 | 0.93 | 1.61 |
| Morphology <4% (n = 35)b | 0.06 | 0.36 | 0.39 | 0.80 | 1.63 |
| Abnormal in one parameter (n = 95) | 0.06 | 0.31 | 0.29 | 0.91 | 1.60 |
| Abnormal in any two parameters (n = 33) | 0.07 | 0.34 | 0.32 | 1.13* | 1.98* |
| Abnormal in all three parameters (n = 12) | 0.08 | 0.43* | 0.42 | 1.36* | 2.37* |
Wilcoxon rank-sum tests used to compare across semen parameter groups.
aA subject may contribute data to more than one abnormal semen parameter category.
bTotal disomy = ∑XX18 + YY18 + XY18.
*P < 0.05 compared with the control group.
Figure 1.
Plot of 25th, median and 75th percentile for percentage XX, YY and XY disomy for men with normal semen parameters (controls) and with 1–3 abnormal semen parameters for men seeking infertility evaluation from January 2000 to May 2003 (N = 192) *P ≤ 0.05, a significant difference in median percentage disomy compared with normal controls.
Semen concentration was highly correlated with motility (r = 0.9, P < 0.01), and both semen concentration and motility were moderately correlated with morphology (0.3 ≤ r ≤ 0.4, P < 0.01). XY disomy was weakly, negatively correlated with both semen concentration (r = −0.17, P < 0.05) and motility (r = −0.14, P < 0.05). XX, YY and total sex chromosome disomy were not correlated with any of the semen parameters. Men with abnormal semen concentration were significantly more likely to be ever smokers than controls (P < 0.05). Men with low sperm motility were significantly less likely to be Caucasian than controls, and those with low sperm motility or concentration were significantly older than controls (mean age of 36.0 and 37.0 versus 34.6 years, respectively, Table I).
The odds ratios (ORs) and 95% confidence intervals (CIs) of having semen parameters below the WHO reference for a 0.1% increase in sex chromosome disomy are presented in Table IV. After adjustment for smoking status, age and abstinence time, a 0.1% increase in XY disomy was associated with a 7% increase in the odds of a man having a semen concentration of <20 million/ml compared with normal controls (OR: 1.07, 95% CI: 1.02–1.13). Increases in XX, YY and total sex chromosome disomy were not associated with an increase in the odds of a man having abnormal semen parameters. In the linear regression model (data not shown), a 0.1% increase in XY disomy was associated with a 2% decrease in semen concentration; a similar pattern of findings was observed for XX, YY and total sex chromosome disomy, as in the logistic regression models. Age, smoking status and abstinence time were not significant covariates in logistic or linear regression models; however, they were retained in final models based on a priori assumptions. BMI and race did not meet the criteria of resulting in at least a 10% change in estimated associations and were not retained in either logistic or linear models.
Table IV.
Adjusteda ORs and 95% CIs of having a below reference semen parameter, or having any two semen parameters below the WHO reference compared with normal controls for a 0.1% increase in sperm disomy among men seeking infertility evaluation from January 2000 to May 2003 (N = 192).
| Sex chromosome disomy | Concentration (<20 million/ml) |
Motility (<50% motile) |
Morphology (<4% normal) |
Two semen parameters abnormal |
||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| 1818 | 0.99 | 0.96–1.10 | 0.99 | 0.91–1.11 | 1.00 | 0.93–1.11 | 1.00 | 0.95–1.12 |
| XX18 | 1.06 | 0.97–1.62 | 1.05 | 0.98–1.13 | 0.96 | 0.86–1.06 | 1.03 | 0.94–1.12 |
| YY18 | 0.99 | 0.98–1.14 | 0.96 | 0.88–1.06 | 1.02 | 0.92–1.14 | 0.96 | 0.84–1.10 |
| XY18 | 1.07 | 1.02–1.13 | 1.02 | 0.98–1.05 | 1.00 | 0.95–1.05 | 1.03 | 1.00–1.08 |
| Total | 1.04 | 1.00–1.07 | 1.01 | 0.99–1.03 | 1.00 | 0.96–1.03 | 1.02 | 0.99–1.04 |
Total disomy = ∑XX18 + YY18 + XY18.
aAdjusted for age (continuous), abstinence time (three categories: ≤ 2, 3–4 and ≥5 days) and smoking (never versus either current or former).
We conducted sensitivity analyses after excluding three men with total sex chromosome disomy rates >5%, which far exceeded the current published ranges, to prevent undue statistical influence from these extreme values. In the reanalysis, the results remained essentially unchanged (data not shown). We also conducted a sensitivity analysis after excluding eight men with a total number of nuclei scored <1000, as disomy estimates can be impacted by too few nuclei scored. In this reanalysis, the results also remained qualitatively unchanged (data not shown).
Discussion
To the best of our knowledge, this is the largest epidemiologic study to date which has investigated the association between sex chromosome disomy and semen parameters with appropriate adjustment for potential confounding variables. The results from our study show that an increase in XY disomy was associated with an increase in the odds of a man having abnormal semen concentration (<20 million/ml). Increases in XX, YY and total sex chromosome disomy were not associated with an increase in the odds of a man having abnormal semen parameters. In addition, autosomal chromosome disomy (1818) was not associated with abnormal semen parameters (Table IV).
Several studies have used multiprobe FISH to investigate whether there is an association between sperm sex chromosome disomy and semen concentration and motility and morphology. In a majority of studies, it has been shown that XX disomy is not significantly increased in men with abnormal semen parameters compared with normal controls, as reviewed in Tempest and Griffin (2004). For YY disomy, an equal number of studies have shown there to be an increase (Finkelstein et al., 1998; Pang et al., 1999; Rives et al., 1999; Ushijima et al., 2000; Calogero et al., 2001; Harkonen et al., 2001) or no observed difference (Moosani et al., 1995; Martin et al., 2000; Nishikawa et al., 2000; Ohashi et al., 2001; Hristova et al., 2002; Templado et al., 2002) in men with abnormal semen parameters compared with normal controls. In studies of total sex chromosome disomy, a majority have shown an increase in sex chromosome disomy associated with abnormal semen parameters when compared with normal controls (Aran et al., 1999; Colombero et al., 1999; Carrell et al., 2003). All of these studies assessed disomy in populations of men attending fertility clinics, with small samples sizes of approximately <30 men, using univariate analyses to investigate differences in mean or median disomy frequencies.
Consistent with our findings, most studies have found a statistically significant increase in XY disomy in men with abnormal semen parameters compared with normal controls (Tempest and Griffin, 2004). Martin et al. (2003) found a statistically significant increase in rates of XY disomy in men with decreasing semen concentration [0.25% (10–19 million/ml), 1.04% (1–9 million/ml) and 0.68% (<1 million/ml)] in a population of 30 men attending a fertility clinic and selected for oligozoospermia, without adjustment for potential confounders. Additionally, Ohashi et al. (2001), in a study of 20 candidates for ICSI with normal karyotype, found a significant 2–3-fold increase in frequency of XY sperm in men with severe oligozoospermia (<5 million/ml) compared with normal controls (0.41 compared with 0.18%); however, no difference in XY frequency was observed between oligozoospermia (5–20 million/ml) and normal controls (0.16 compared with 0.18%, respectively). Our findings indicate a roughly 2-fold increase in XY disomy among men with oligozoospermia (1.64 versus 0.87%), compared with normal controls. Additionally, in our multivariate analysis, we observed that a 0.1% level increase of XY disomy was associated with a 7% increase in the odds of having a below normal semen concentration (<20 million/ml). Lastly, a study by Faure et al. (2007) of 31 infertile men found that one-third (n = 9) had an increased rate of XY disomy, ranging from 1.12 to 3.73%, which was three to nine times higher than the mean rate in the control population.
It has been shown that errors during the first meiotic division (meiosis I) result in XY disomy, whereas errors in the second meiotic division (meiosis II) result in XX or YY disomy (Griffin et al., 1995, 1996). Finkelstein et al. (1998) proposed that abnormal chromosome segregation in males with low-quality semen results from chromosome non-disjunction at the first meiotic division. During meiosis I, homologous chromosomes undergo synapsis, in which the chromosomes pair up and a synaptonemal complex (SC) forms between them. Along the SC is where recombination occurs, and numerous studies have shown that aberrant pairing in homologous sequences of nucleotides on the X and Y chromosomes is associated with XY disomy and failure of spermatogenesis, which can result in the loss of germ cells and subsequent infertility (Hassold et al., 1991; Schmid et al., 2003; Tempest et al., 2004; Ferguson et al., 2007). Tempest et al. (2004) suggested that a common mechanism involving alterations in the mechanisms of the synapsis and/or recombination in the XY pairing region may be responsible for increased XY disomy and subsequent oligozoospermia. Another possible explanation for an association of increased XY disomy with decreased semen concentration is the presence of Y chromosome microdeletions. It has been shown that Y chromosome microdeletions are responsible for spermatogenic arrest and are associated with abnormal semen concentration (Balkan et al., 2008). Ferlin et al. (2007) showed that men with increased Y chromosome microdeletions had a higher percentage of sperm with XY disomy, and these authors postulated that Y microdeletions interfere with the meiotic process, thus leading to non-disjunction of the XY tetrad. More research is needed to better understand whether Y chromosome microdeletions adversely impact pairing during synapsis and/or recombination during the first meiotic division.
Many semen parameter studies use WHO cutoff criteria; however, these cutoff criteria may not fully define fertility status. A study by Guzick et al. (2001) determined that subfertile ranges for semen parameters were <13.5 million/ml concentration, <32% motile and <9% normal morphology, whereas fertile ranges were >48 million/ml concentration, >63% motile and >12% normal morphology. Men with semen parameters falling between these ranges were classified as having indeterminate fertility. Interestingly, Guzick et al. (2001) concluded that while these cutoffs helped to distinguish fertile from infertile, they were not powerful discriminators in that there was extensive overlap between fertile and infertile men within the three semen parameters. Although dichotomizing semen parameters based on WHO cutoff criteria may not be appropriate for determining fertility status (an outcome we were not attempting to assess in this research), we found similar results between our multivariate logistic regression models using dichotomous outcomes and multivariate linear regression models with continuous measures of semen quality.
A potential limitation of this study, as well as those currently in the published literature, is that it is cross-sectional. Cross-sectional analyses by nature do not lend themselves to inference about directionality for any observed associations; therefore, we cannot determine which variable is the cause and which one is the effect. We can, however, try to understand the association between variables in a time and cost-efficient manner by making use of data previously collected for other research purposes, which in this study was considered a major advantage.
Comparable associations were not observed among the sex chromosome disomies and semen parameters, i.e. the only significant association observed was between XY disomy and semen concentration. Because sex chromosome disomy is a rare event, it is susceptible to measurement error, which could decrease the precision of the effect estimate but would not bias the results. Importantly, XY disomy was the most common disomy type and showed the most consistent relationship. XX and YY occurred less frequently than XY; less consistent associations with XX or YY may, in part, relate to greater measurement error for these even rarer events. Or, as previously mentioned, this difference might be related to the different underlying mechanism(s) of the action of errors in meiosis I versus meiosis II.
The men in this study spanned the semen parameter continuum. In prior studies of aneuploidy and semen parameters, men with particularly extreme semen parameters (e.g. OAT) were only selected, therefore potentially limiting generalizability of study findings. We believe that our study population, which includes men with more modest semen parameter abnormalities, may increase the generalizability of our study findings; however, replication in other populations is necessary to confirm this. Additionally, because each man in our study presented to a fertility clinic as a partner of a subfertile couple, information on the female partner's fertility status could further inform the male's fertility profile.
A major strength of this research was the use of a validated semi-automated method for counting disomic sperm because it allowed for objective processing of a large number of samples. We believe that there is a high level of internal validity in our study because a single laboratory technician was responsible for processing all samples. Although it is possible that our method inflated our disomy rates, previous validation studies did not find differences between manual scoring and our semi-automated counting method (Perry et al., 2007, 2011). Three other published papers have highlighted the use of automated methods for detecting aneuploidy in sperm. Carrell and Emery (2008) found no significant difference between rates of aneuploidy (chromosomes 13, 18, 21, X and Y) from 10 donors comparing manual and automated methods. Molina et al. (2009) found that roughly 97% of their comparisons between automated and manual methods were concordant. However, some significant differences were observed in mean sex chromosome disomy, with automated methods producing higher sex chromosome disomy frequencies (0.43 manual versus 0.56 automated). Lastly, Tempest et al. (2010) found a statistically significant correlation between manual and automated approaches for scoring sex chromosome sperm disomy (XX18, YY18, XY18, X1818 and Y1818). Continued work in optimizing, validating and replicating automated methods is needed (Levsky and Singer, 2003); however, the current validation studies, some including large samples sizes, using different scoring systems show much promise for automated methods providing comparable aneuploidy frequencies.
In summary, we explored the relationship between human sperm sex chromosome disomy and semen parameters. To the best of our knowledge, this is the largest study to date to evaluate this relationship in multivariate models that include potential confounding variables. The results from our study are consistent with prior findings. For example, results of our analyses unadjusted for potential confounding variables (and thus parallel to most other studies) showed a significant increased median percentage of XY disomy in men abnormal in any two, or all three semen parameters, and an increase in median percentage of XX disomy for men abnormal in all three semen parameters compared with normal controls. In logistic regression models adjusted for potential cofounders, we found that an increase in XY disomy was associated with a small but significant increase in the odds of a man having abnormal semen concentration compared with normal controls; however, this finding may be sensitive to the fact that a majority of the men with abnormal semen concentration also had co-occurrence of other semen parameter abnormalities, an inherent limitation of semen parameter studies. Increases in XX, YY and total sex chromosome disomy were not associated with increased odds of having abnormal semen parameters. Our findings suggest that more research is needed to fully understand the underlying mechanism(s), as well as potential risk factors, associating increased XY disomy with decreased semen concentration, both factors that can be important correlates of impaired male fertility.
Authors' roles
M.E.M collaborated in the design of the study and analysis; performed FISH, imaging and nuclei scoring analyses and led the manuscript writing. P.L.W. collaborated in the design of the study and analysis, provided guidance on statistical programming and collaborated on the interpretation of the results and writing the manuscript. S.A.K. collaborated in the design of the study and analysis and collaborated on the interpretation of the results and writing the manuscript. R.D. performed semen analysis, sample retrieval for FISH and reviewed the manuscript. M.J.P. collaborated in the design of the study and analysis, directed the laboratory that performed FISH and collaborated on the interpretation of the results and writing the manuscript.
Funding
This work was supported by grants from NIOSH (T42 OH008416) and NIEHS (R01 ES009718, P30 ES000002 and R01 ES017457).
Conflict of interest
At the time this work was conducted and the initial manuscript written, M.E.M. was affiliated with the Environmental Health Department at the Harvard School of Public Health. Currently, M.E.M. is employed by Millennium: The Takeda Oncology Company.
Acknowledgements
Thank you to Ana Trisini for assistance with sample retrieval and to Dr. Russ Hauser for instrumental support.
References
- Aran B, Blanco J, Vidal F, Vendrell JM, Egozcue S, Barri PN, Egozcue J, Veiga A. Screening for abnormalities of chromosomes x, y, and 18 and for diploidy in spermatozoa from infertile men participating in an in vitro fertilization-intracytoplasmic sperm injection program. Fertil Steril. 1999;72:696–701. doi: 10.1016/s0015-0282(99)00307-6. [DOI] [PubMed] [Google Scholar]
- Balkan M, Tekes S, Gedik A. Cytogenetic and Y chromosome microdeletion screening studies in infertile males with Oligozoospermia and Azoospermia in Southeast Turkey. J Assist Reprod Gen. 2008;25:559–565. doi: 10.1007/s10815-008-9272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner A, Van Hummelen P, Lowe XR, Adler ID, Wyrobek AJ. Numerical and structural chromosomal abnormalities detected in human sperm with a combination of multicolor fish assays. Environ Mol Mutagen. 1999;33:49–58. doi: 10.1002/(sici)1098-2280(1999)33:1<49::aid-em6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Bernardini LM, Calogero AE, Bottazzi C, Lanteri S, Venturini PL, Burrello N, De Palma A, Conte N, Ragni N. Low total normal motile count values are associated with increased sperm disomy and diploidy rates in infertile patients. Int J Androl. 2005;28:328–336. doi: 10.1111/j.1365-2605.2005.00548.x. [DOI] [PubMed] [Google Scholar]
- Blackwell JM, Zaneveld LJD. Effect of abstinence on sperm acrosin, hypoosmotic swelling, and other semen variables. Fertil Steril. 1992;58:798–802. doi: 10.1016/s0015-0282(16)55330-8. [DOI] [PubMed] [Google Scholar]
- Calogero AE, De Palma A, Grazioso C, Barone N, Romeo R, Rappazzo G, D'Agata R. Aneuploidy rate in spermatozoa of selected men with abnormal semen parameters. Hum Reprod. 2001;16:1172–1179. doi: 10.1093/humrep/16.6.1172. [DOI] [PubMed] [Google Scholar]
- Carrell D, Emery BR. Use of automated imaging and analysis technology for the detection of aneuploidy in human sperm. Fertil Steril. 2008;90:434–437. doi: 10.1016/j.fertnstert.2007.06.095. [DOI] [PubMed] [Google Scholar]
- Carrell DT, Wilcox AL, Lowy L, Peterson CM, Jones KP, Erickson L, Campbell B, Branch DW, Hatasaka HH. Elevated sperm chromosome aneuploidy and apoptosis in patients with unexplained recurrent pregnancy loss. Obstet Gynecol. 2003;101:1229–1235. doi: 10.1016/s0029-7844(03)00339-9. [DOI] [PubMed] [Google Scholar]
- Collodel G, Capitani S, Baccetti B, Pammolli A, Moretti E. Sperm aneuploidies and low progressive motility. Hum Reprod. 2007;22:1893–1898. doi: 10.1093/humrep/dem099. [DOI] [PubMed] [Google Scholar]
- Colombero LT, Hariprashad JJ, Tsai MC, Rosenwaks Z, Palermo GD. Incidence of sperm aneuploidy in relation to semen characteristics and assisted reproductive outcome. Fertil and Steril. 1999;72:90–96. doi: 10.1016/s0015-0282(99)00158-2. [DOI] [PubMed] [Google Scholar]
- de Kretser DM. Male infertility. Lancet. 1997;349:787–790. doi: 10.1016/s0140-6736(96)08341-9. [DOI] [PubMed] [Google Scholar]
- Diemer T, Desjardins C. Developmental and genetic disorders in spermatogenesis. Hum Reprod Update. 1999;5:120–140. doi: 10.1093/humupd/5.2.120. [DOI] [PubMed] [Google Scholar]
- Dohle GR, Halley DJJ, Van Hemel JO, van den Ouwel AMW, Pieters M, Weber RFA, Govaerts LCP. Genetic risk factors in infertile men with severe oligozoospermia and azoospermia. Hum Reprod. 2002;17:13–16. doi: 10.1093/humrep/17.1.13. [DOI] [PubMed] [Google Scholar]
- Faure AK, Aknin-Seifer I, Frerot G, Pelletier R, De Robertis C, Cans C, Levy R, Jimenez C, Lejeune H, Terrier N, et al. Predictive factors for an increased risk of sperm aneuploidies in oligo-astheno-teratozoospermic males. Int J Androl. 2007;30:153–162. doi: 10.1111/j.1365-2605.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- Ferguson KA, Wong EC, Chow V, Nigro M, Ma S. Abnormal meiotic recombination in infertile men and its association with sperm aneuploidy. Hum Mol Genet. 2007;16:2870–2879. doi: 10.1093/hmg/ddm246. [DOI] [PubMed] [Google Scholar]
- Ferlin A, Raicu F, Gatta V, Zuccarello D, Palka G, Foresta C. Male infertility: role of genetic background. Reprod Biomed Online. 2007;14:734–745. doi: 10.1016/s1472-6483(10)60677-3. [DOI] [PubMed] [Google Scholar]
- Finkelstein S, Mukamel E, Yavetz H, Paz G, Avivi L. Increased rate of nondisjunction in sex cells derived from low-quality semen. Hum Genet. 1998;102:129–137. doi: 10.1007/s004390050665. [DOI] [PubMed] [Google Scholar]
- Foresta C, Moro E, Ferlin A. Y chromosome microdeletions and alterations of spermatogenesis. Endocr Rev. 2001;22:226–239. doi: 10.1210/edrv.22.2.0425. [DOI] [PubMed] [Google Scholar]
- Georgiou I, Syrrou M, Pardalidis N, Karakitsios K, Mantzavinos T, Giotitsas N, Loutradis D, Dimitriadis F, Saito M, Miyagawa I, et al. Genetic and epigenetic risks of intracytoplasmic sperm injection method. Asian J Androl. 2006;8:643–673. doi: 10.1111/j.1745-7262.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- Gianaroli L, Magli MC, Ferraretti AP, Munne S, Balicchia B, Escudero T, Crippa A. Possible interchromosomal effect in embryos generated by gametes from translocation carriers. Hum Reprod. 2002;17:3201–3207. doi: 10.1093/humrep/17.12.3201. [DOI] [PubMed] [Google Scholar]
- Griffin DK, Abruzzo MA, Millie EA, Sheean LA, Feingold E, Sherman SL, Hassold TJ. Nondisjunction in human sperm—evidence for an effect of increasing paternal age. Hum Mol Genet. 1995;4:2227–2232. doi: 10.1093/hmg/4.12.2227. [DOI] [PubMed] [Google Scholar]
- Griffin DK, Abruzzo MA, Millie EA, Feingold E, Hassold TJ. Sex ratio in normal and disomic sperm: evidence that the extra chromosome 21 preferentially segregates with the Y chromosome. Am J Hum Genet. 1996;59:1108–1113. [PMC free article] [PubMed] [Google Scholar]
- Guttenbach M, MartinezExposito MJ, Michelmann HW, Engel W, Schmid M. Incidence of diploid and disomic sperm nuclei in 45 infertile men. Hum Reprod. 1997;12:468–473. doi: 10.1093/humrep/12.3.468. [DOI] [PubMed] [Google Scholar]
- Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, Carson SA, Cisneros P, Steinkampf MP, Hill JA, et al. Sperm morphology, motility, and concentration in fertile and infertile men. New Engl J Med. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- Harkonen K, Suominen J, Lahdetie J. Aneuploidy in spermatozoa of infertile men with teratozoospermia. Int J Androl. 2001;24:197–205. doi: 10.1046/j.1365-2605.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- Hassan MAM, Killick SR. Effect of male age on fertility: evidence for the decline in male fertility with increasing age. Fertil Steril. 2003;79:1520–1527. doi: 10.1016/s0015-0282(03)00366-2. [DOI] [PubMed] [Google Scholar]
- Hassold TJ, Sherman SL, Pettay D, Page DC, Jacobs PA. Xy-chromosome nondisjunction in man is associated with diminished recombination in the pseudoautosomal region. Am J Hum Genet. 1991;49:253–260. [PMC free article] [PubMed] [Google Scholar]
- Hirsh A. ABCs of subfertility—male subfertility. Br Med J. 2003;327:669A–673A. [Google Scholar]
- Hristova R, Ko E, Greene C, Rademaker A, Chernos J, Martin R. Chromosome abnormalities in sperm from infertile men with asthenoteratozoospermia. Biol Reprod. 2002;66:1781–1783. doi: 10.1095/biolreprod66.6.1781. [DOI] [PubMed] [Google Scholar]
- Johannisson R, Leuschner E, Huppe M, Hinrichs F, Al-Hasani S, Diedrich K, Schwinger E, Mennicke K. Increased frequency of x-bearing sperm in males from an infertility clinic: analysis by two-color fluorescence in situ hybridization. Cytogenet Genome Res. 2002;98:240–244. doi: 10.1159/000071041. [DOI] [PubMed] [Google Scholar]
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–117. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- Levsky JM, Singer RH. Fluorescence in situ hybridization: past, present and future. J Cell Sci. 2003;116:2833–2838. doi: 10.1242/jcs.00633. [DOI] [PubMed] [Google Scholar]
- Machev N, Gosset P, Viville S. Chromosome abnormalities in sperm from infertile men with normal somatic karyotypes: teratozoospermia. Cytogenet Genome Res. 2005;111:352–357. doi: 10.1159/000086910. [DOI] [PubMed] [Google Scholar]
- Mak V, Jarvi KA. The genetics of male infertility. J Urol. 1996;156:1245–1256. [PubMed] [Google Scholar]
- Martin RH, Spriggs E, Rademaker AW. Multicolor fluorescence in situ hybridization analysis of aneuploidy and diploidy frequencies in 225846 sperm from 10 normal men. Biol Reprod. 1996;54:394–398. doi: 10.1095/biolreprod54.2.394. [DOI] [PubMed] [Google Scholar]
- Martin RH, Greene C, Rademaker A, Barclay L, Ko E, Chernos J. Chromosome analysis of spermatozoa extracted from testes of men with non-obstructive azoospermia. Hum Reprod. 2000;15:1121–1124. doi: 10.1093/humrep/15.5.1121. [DOI] [PubMed] [Google Scholar]
- Martin RH, Rademaker AW, Greene C, Ko E, Hoang T, Barclay L, Chernos J. A comparison of the frequency of sperm chromosome abnormalities in men with mild, moderate, and severe oligozoospermia. Biol Reprod. 2003;69:535–539. doi: 10.1095/biolreprod.102.015149. [DOI] [PubMed] [Google Scholar]
- Meschede D, Lemcke B, Exeler JR, De Geyter C, Behre HM, Nieschlag E, Horst J. Chromosome abnormalities in 447 couples undergoing intracytoplasmic sperm injection—prevalence, types, sex distribution and reproductive relevance. Hum Reprod. 1998;13:576–582. doi: 10.1093/humrep/13.3.576. [DOI] [PubMed] [Google Scholar]
- Miharu N. Chromosome abnormalities in sperm from infertile men with normal somatic karyotypes: oligozoospermia. Cytogenet Genome Res. 2005;111:347–351. doi: 10.1159/000086909. [DOI] [PubMed] [Google Scholar]
- Miharu N, Best RG, Young SR. Numerical chromosome-abnormalities in spermatozoa of fertile and infertile men detected by fluorescence in-situ hybridization. Hum Genet. 1994;93:502–506. doi: 10.1007/BF00202812. [DOI] [PubMed] [Google Scholar]
- Molina O, Sarrate Z, Vidal F, Blanco J. FISH on sperm: spot-counting to stop counting? Not yet. Fertil Steril. 2009;92:1474–1480. doi: 10.1016/j.fertnstert.2008.07.1779. [DOI] [PubMed] [Google Scholar]
- Moosani N, Pattinson HA, Carter MD, Cox DM, Rademaker AW, Martin RH. Chromosomal analysis of sperm from men with idiopathic infertility using sperm karyotyping and fluorescence in-situ hybridization. Fertil Steril. 1995;64:811–817. doi: 10.1016/s0015-0282(16)57859-5. [DOI] [PubMed] [Google Scholar]
- Nishikawa N, Murakami I, Ikuta K, Suzumori K. Sex chromosomal analysis of spermatozoa from infertile men using fluorescence in situ hybridization. J Assist Reprod Gen. 2000;17:97–102. doi: 10.1023/A:1009413916753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien KLO, Varghese AC, Agarwal A. The genetic causes of male factor infertility: a review. Fertil Steril. 2010;93:1–12. doi: 10.1016/j.fertnstert.2009.10.045. [DOI] [PubMed] [Google Scholar]
- Ohashi Y, Miharu N, Honda H, Samura O, Ohama K. High frequency of xy disomy in spermatozoa of severe oligozoospermic men. Hum Reprod. 2001;16:703–708. doi: 10.1093/humrep/16.4.703. [DOI] [PubMed] [Google Scholar]
- Pang MG, Hoegerman SF, Cuticchia AJ, Moon SY, Doncel GF, Acosta AA, Kearns WG. Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9, 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in-situ hybridization in spermatozoa from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection. Hum Reprod. 1999;14:1266–1273. doi: 10.1093/humrep/14.5.1266. [DOI] [PubMed] [Google Scholar]
- Perry MJ, Chen X, Lu X. Automated scoring of multiprobe fish in human spermatozoa. Cytometry A. 2007;71A:968–972. doi: 10.1002/cyto.a.20468. [DOI] [PubMed] [Google Scholar]
- Perry MJ, Xing C, McAuliffe ME, Maity A, Deloid GM. Semi-automated scoring of triple-probe FISH in human sperm: methods and further validation. Cytometry A. 2011;79A:661–666. doi: 10.1002/cyto.a.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rives N, Saint Clair A, Mazurier S, Sibert L, Simeon N, Joly G, Mace B. Relationship between clinical phenotype, semen parameters and aneuploidy frequency in sperm nuclei of 50 infertile males. Hum Genet. 1999;105:266–272. doi: 10.1007/s004390051100. [DOI] [PubMed] [Google Scholar]
- Schmid TE, Kamischke A, Bollwein H, Nieschlag E, Brinkworth MH. Genetic damage in oligozoospermic patients detected by fluorescence in-situ hybridization, inverse restriction site mutation assay, sperm chromatin structure assay and the comet assay. Hum Reprod. 2003;18:1474–1480. doi: 10.1093/humrep/deg259. [DOI] [PubMed] [Google Scholar]
- Shi QH, Martin RH. Aneuploidy in human spermatozoa: fish analysis in men with constitutional chromosomal abnormalities, and in infertile men. Reproduction. 2001;121:655–666. doi: 10.1530/rep.0.1210655. [DOI] [PubMed] [Google Scholar]
- Sun F, Ko E, Martin RH. Is there a relationship between sperm chromosome abnormalities and sperm morphology? Reprod Biol Endocrin. 2006;4:1–5. doi: 10.1186/1477-7827-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest HG, Griffin DK. The relationship between male infertility and increased levels of sperm disomy. Cytogenet Genome Res. 2004;107:83–94. doi: 10.1159/000079575. [DOI] [PubMed] [Google Scholar]
- Tempest HG, Homa ST, Dalakiouridou M, Christopikou D, Wright D, Zhai XP, Griffin DK. The association between male infertility and sperm disomy: evidence for variation in disomy levels among individuals and a correlation between particular semen parameters and disomy of specific chromosome pairs. Reprod Biol Endocrinol. 2004;2:82. doi: 10.1186/1477-7827-2-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest HG, Cheng SY, Gillott DJ, Handyside AH, Thornhill AR, Griffin DK. Scoring of sperm chromosomal abnormalities by manual and automated approaches: qualitative and quantitative comparisons. Asian JAndrol. 2010;12:257–262. doi: 10.1038/aja.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templado C, Hoang T, Greene C, Rademaker A, Chernos J, Martin R. Aneuploid spermatozoa in infertile men: teratozoospermia. Mol Reprod Dev. 2002;61:200–204. doi: 10.1002/mrd.1148. [DOI] [PubMed] [Google Scholar]
- Tiido T, Rignell-Hydbom A, Jonsson B, Giwercman YL, Rylander L, Hagmar L, Giwercman A. Exposure to persistent organochlorine pollutants associates with human sperm y : X chromosome ratio. Hum Reprod. 2005;20:1903–1909. doi: 10.1093/humrep/deh855. [DOI] [PubMed] [Google Scholar]
- Ushijima C, Kumasako Y, Kihaile PE, Hirotsuru K, Utsunomiya T. Analysis of chromosomal abnormalities in human spermatozoa using multi-colour fluorescence in-situ hybridization. Hum Reprod. 2000;15:1107–1111. doi: 10.1093/humrep/15.5.1107. [DOI] [PubMed] [Google Scholar]
- Vegetti W, Van Assche E, Frias A, Verheyen G, Bianchi MM, Bonduelle M, Liebaers I, Van Steirteghem A. Correlation between semen parameters and sperm aneuploidy rates investigated by fluorescence in-situ hybridization in infertile men. Hum Reprod. 2000;15:351–365. doi: 10.1093/humrep/15.2.351. [DOI] [PubMed] [Google Scholar]
- Vine MF. Smoking and male reproduction: a review. Int J Androl. 1996;19:323–337. doi: 10.1111/j.1365-2605.1996.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Vogt PH. Human chromosome deletions in yq11, azf candidate genes and male infertility: history and update. Mol Hum Reprod. 1998;4:739–744. doi: 10.1093/molehr/4.8.739. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th edn. New York: Cambridge University Press; 1999. [Google Scholar]
- Wyrobek AJ. The genetic contribution of sperm: healthy baby or not? Sci Technol Rev. 1995:7–19. https://www.llnl.gov/str/pdfs/11_95.pdf . [Google Scholar]