Abstract
Chronic pain arising from various pathological conditions such as osteoarthritis, low back or spinal injuries, cancer, and urological chronic pelvic pain syndromes presents significant challenges in diagnosis and treatment. Specifically, since the underlying cause of these pain syndromes is unknown or heterogeneous, physicians diagnose and treat patients based on the symptoms presented. Nerve growth factor (NGF) has been recognized as an important mediator of chronic pain in many pathological conditions, and has been shown to be upregulated in a subset of individuals suffering from such pain syndromes. These findings have led to the development of anti-NGF monoclonal antibodies such as tanezumab as potentially effective therapeutics for chronic pain. Although tanezumab has reached Phase II and III clinical trials, the trials of anti-NGF antibodies were halted due to safety concerns. Some of these trials of anti-NGF treatment have had statistically significant decreases in pain, while others have yielded inconclusive results. These findings are suggestive of, though do not prove, target (NGF) neutralization in chronic pain syndromes. A biomarker-driven anti-NGF clinical study layout is proposed that incorporates NGF measurements in the relevant samples before and after treatment, in addition to collecting the pain scores. This approach might not only confirm the mechanism of tanezumab’s action in these chronic pain patients, but should establish NGF levels as a predictive biomarker for patients who can benefit from anti-NGF treatment, thereby creating a personalized approach to pain treatment.
Keywords: nerve growth factor, chronic pain, anti-NGF antibodies, neurotrophic factor, nociceptor neurons
Introduction
Medically unexplained chronic pain arising from various pathological conditions presents significant challenges in diagnosis and treatment. Such chronic pain conditions include osteoarthritis, low back or spinal injuries, cancer, urological chronic pelvic pain syndromes, and many others. Operationally, clinicians are often dependent on standard diagnostic categories, which might not have captured the full spectrum of heterogeneity of these pain symptoms. Heterogeneity of the underlying signals for chronic pain has been substantiated by the involvement of inflammatory mediators, peripheral neuropathy, post-herpetic reactions, and local production of neurotrophins in several of these pathological conditions.1,2 Current therapies for chronic pain include nonsteroidal anti-inflammatory drugs, antiseizure agents, and opiates, but the results are less than satisfactory in most cases and have often proved toxic with long-term use.1,3,4 More recently, nerve growth factor (NGF) has been recognized as an important mediator of chronic pain syndromes.3 Therefore, different methods to block the NGF signaling at the receptor level or by antibodies against NGF have been tested in preclinical studies as well as in clinical trials. Many humanized anti-NGF monoclonal antibodies have entered clinical trials as potential pain therapies, and tanezumab (an anti-NGF antibody developed by Pfizer, Inc, New York, NY) has advanced beyond the proof-of-concept studies to Phase III trials in osteoarthritis. The role of NGF in pain transduction, antibodies that block NGF signaling, outcomes of previous proof-of-concept studies on anti-NGF antibodies, and potential benefit of personalized treatment based on biomarker assays are the topics of this review.
Nerve growth factor and its receptors in pain sensation
Unexpectedly discovered in tumor cells and salivary glands, NGF is the first neurotrophic factor to be identified, purified, and biochemically characterized.5–7 NGF is a 13 kDa polypeptide secreted as a dimer from target cells of sympathetic and sensory neurons, and is involved in the growth, signaling, and survival of neurons.8,9 In the developing nervous system, the primary role of NGF is in neuronal survival, but this role shifts in adults to a more protective role at the organismal level by mediating pain from noxious stimuli.1,3,10 Afferent nociceptor fibers that conduct pain signals to the central nervous system include the small-diameter, unmyelinated, slow-conducting C fibers and the small-to-medium diameter, lightly myelinated, relatively rapid-conducting Aδ fibers.2 The Aδ fibers mediate acute/sharp pain, whereas the C fibers mediate dull/diffuse pain. These nociceptor fibers that innervate the different parts of the body and head conduct pain signals to the dorsal root ganglia of the spinal cord or the trigeminal ganglia, respectively.2
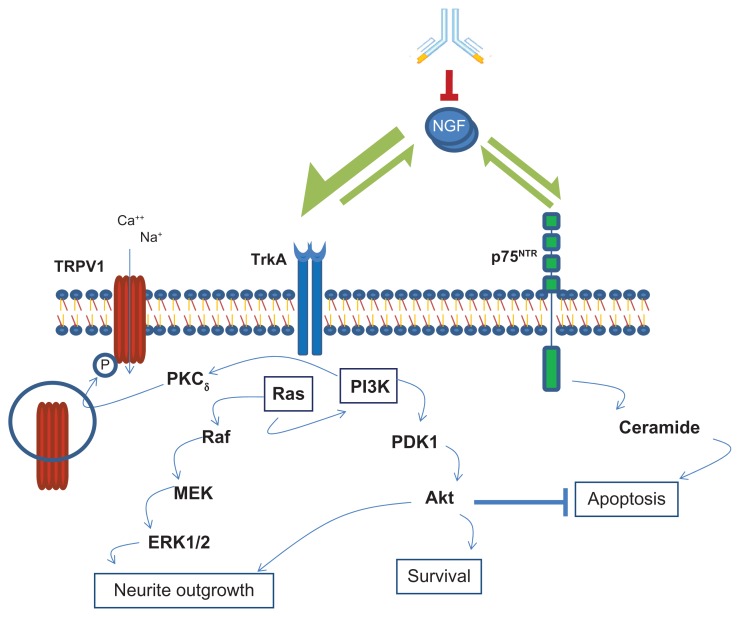
The signaling of NGF in these nociceptor neurons is mediated through two different receptors, the low-affinity 75 kDa neurotrophin receptor (p75NTR), which belongs to the TNF receptor family, as well as the high-affinity tropomyosin-related kinase A (TrkA), a receptor tyrosine kinase.9,11 P75NTR binds neurotrophins such as BDNF, NT3, and NT4, whereas TrkA binds NGF more selectively than the other neurotrophins.11 Signaling through TrkA mediates neurotrophic effects during development, and also mediates the nociceptive functions of the sensory neurons in adult life.10,12 p75NTR signaling in the absence or low expression of TrkA mediates apoptosis, whereas TrkA signaling blocks this apoptotic effect and enhances neuronal survival.13,14 The interesting aspect of NGF/TrkA signaling in the nociceptor neurons is that in addition to its role in neuronal survival by inhibition of apoptosis, the same signaling pathway leads to the generation of action potentials for the neuronal signaling of pain sensation.15,16 Functional nociceptors selectively express the NGF receptor TrkA, the neuropeptides’ substance P and calcitonin gene-related peptide (CGRP), as well as the Transient Receptor Potential Vanilloid 1 (TRPV1), which are important in the nociceptive function.17,18 Immunohistochemical studies on rat dorsal root ganglia have shown that a great majority (>90%) of the TrkA expressing neurons were CGRP-expressing small-diameter sensory neurons of the peptidergic C type sensory fibers.19 Extensive studies using dorsal root ganglia neurons and gene-transferred non-neuronal cells have demonstrated that TRPV1 acts as a non-selective cation channel and plays an important role in the conduction of nociceptive signals.15,16 NGF signaling through TrkA activates the cellular protein kinases of the phosphatidylinositol 3-kinase (PI3K) and Ras signaling pathway, which leads to the phosphorylation and activation of TRPV1 for increased channel activity. This activation might involve the participation of other kinases, particularly protein kinase C (PKC) downstream of PI3K (Figure 1).10,15,18
Figure 1.
NGF signaling pathways in mediating neurotrophic and nociceptor effects.
Notes: NGF signals through the high-affinity TrkA and low p75NTR receptors. TrkA activates both PI3 kinase (PI3K) and Ras pathways, which lead to cell survival and neurite outgrowth. Signaling through p75NTR leads to apoptosis, which is blocked by the TrkA-activated PI3K pathway. PI3K activation also leads to activation of the nonselective cation channel TRPV1 by phosphorylation, thereby generating action potential for nociceptive functions. Moreover, TRPV1 phosphorylation results in increased translocation of TRPV1 to the plasma membrane to enhance channel activity. Antibody-mediated NGF blockade results in the abolition of both neurotrophic and nociceptor functions.
Abbreviation: NGF, nerve growth factor.
Tanezumab does not appreciably cross the blood–brain barrier, as is evident from the preclinical studies of anti-NGF antibodies.8,20 The analgesic effect of anti-NGF treatment is believed to be primarily in the blockade of signaling in the CGRP/TrkA-positive peripheral nociceptive fibers. However, some indirect effect on the central sensitization has also been reported. This is evidenced by the inhibition of c-Fos expression in the deep dorsal horn of the spinal cord in the anti-NGF-treated rat model of cancer-induced pain.21
Several positive feedbacks exist in the NGF signaling loops that potentially enhance the sensation of noxious stimuli. First of all, NGF enhances the expression of its own receptor TrkA, as experimentally demonstrated in basal forebrain neurons from fetal rats.22 Moreover, NGF has been shown to enhance the expression of substance P and CGRP in the neurons of C fibers, thus sensitizing neurons to hyperalgesia. The release of these nociceptive peptides from the neurons in turn stimulate increased production of NGF by the target cells of the nociceptive neurons.22,23 Moreover, TRPV1 phosphorylation resulting from NGF signaling through TrkA leads to increased translocation of TRPV1 channel proteins to the cell surface membrane. These positive feedback loops in the NGF signaling amplify the TRPV1 channel activity leading to NGF-induced hyperalgesia.
The role of NGF in mediating pain in many different pathological conditions now has been well established in experimental and clinical contexts. For example, NGF levels were increased in skin tissues artificially inflamed by treatment with carrageenan, and NGF signaling in this model contributed to the pain sensitization by the afferent nociceptive neurons.24 This is evidenced by the observation that neutralization of NGF by the soluble NGF receptor fusion molecule TrkA-IgG reversed the inflammatory pain sensitization.24,25 Similarly, another TrkA soluble receptor, TrkAd5, has been shown to be effective against pain in experimentally induced osteoarthritis in rats, demonstrating the role of NGF signaling in mediating osteoarthritic pain.26 Genetic evidence for the role of NGF in pain signals comes from a single substitution mutation, R100W, in the NGF gene that resulted in defective processing of the precursor protein and the accompanying changes in the binding affinity to the receptors, leading to severe reduction of pain perception.27 However, kinase domain mutations in the TrkA gene itself that affect NGF signaling resulted in more extensive abnormalities including a human disorder named congenital insensitivity to pain with anhidrosis (CIPA), behavioral defects, and mental retardation.28
There are several clinical situations in which elevated levels of NGF have been implicated in human chronic pain syndromes, such as interstitial cystitis/painful bladder syndrome (IC/PBS), chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), osteoarthritis (OA), diabetic peripheral neuropathy, and psoriasis, and which can serve as biomarkers for the underlying pain mechanism.3,29–32 For example, NGF overexpression in the bladder contributes to the regulation of afferent neural plasticity and reorganization of the micturition pathways in overactive bladder syndrome (OAB) and interstitial cystitis/painful bladder syndrome (IC/PBS).30 In the clinical settings, elevated levels of urinary NGF have been reported to be reliable biomarkers for overactive bladder.33 Similarly, elevated urinary NGF levels were observed in patients with IC/PBS compared to normal controls in a clinical study, and a decrease in urinary NGF was associated with pain reduction in response to different treatments such as hydrodistension, oral pentosan polysulphate, hyaluronic acid instillation, or BoNT-A injections.29 In a small clinical study involving 20 CP/CPPS patients and four control subjects, the expressed prostatic secretion (EPS) contained significantly higher levels of NGF than the control group. There was clear correlation between the NGF levels and pain severity in the patients with CP/CPPS.32 The patients who responded to treatment showed a significant reduction in the NGF levels from the baseline. Therefore, NGF levels in the EPS can serve as a biomarker for the severity of pain and as a measure of treatment response.
Chronic pain associated with advanced malignancies has also been shown to be related to NGF signaling. Prostate and breast cancers, which frequently result in bone metastases, are characterized by severe bone pain. In experimental tumor models in rats, NGF produced by the tumor cells and/or tumor-associated stromal cells has been implicated in the extensive sprouting of sensory neural fibers from the bone tissue and the resulting hyperalgesia.34,35
In streptozotocin-induced diabetes in rats, elevated NGF levels in the skin have been observed along with an increase in the proportion of TrkA-expressing, CGRP-positive neurons, indicating a role for NGF in painful diabetic neuropathy, although the enhanced NGF production might be a natural response to protect the sensory neurons affected by diabetic conditions.36
Therapeutic potential of NGF modulation
The aforementioned studies establish the role of NGF as an important modulator of pain sensation in many different pathological conditions and have led to investigations on the therapeutic strategies for blocking NGF signaling. Studies in a mouse model of bone metastasis of prostate cancer demonstrated that systemic administration of anti-NGF monoclonal antibodies prevented tumor-associated pain behavior and neural sprouting in the vicinity of the tumor, irrespective of the time of starting the therapy relative to the establishment of tumor.37 Most of the sensory nerve fibers that innervate bone are the small-diameter types that express TrkA and CGRP peptide, and therefore blocking these fibers is unlikely to be compensated by alternative nociceptive fibers.21 In another study of autoimmune arthritis in rats, anti-NGF antibodies almost completely eliminated the joint pain, but the underlying inflammatory pathology was not changed.38 Therefore, this highly effective blocking of the sensory signals of joint pain sometimes has the negative effect of encouraging the overuse of an affected bone or joint, as in the case of osteoarthritis discussed later.
Tanezumab: an NGF-targeted approach to treating chronic pain
Tanezumab is a humanized IgG2 monoclonal antibody against NGF developed by Pfizer Inc, based on evidence linking NGF to chronic pain.39,40 Tanezumab was designed to block the NGF-receptor interaction, thus preventing the signaling through the sensory and sympathetic neurons for the perception of pain. Other antibodies against NGF such as REGN 475 (Regeneron, Tarrytown, NY), fulranumab (Johnson & Johnson, New Brunswick, NJ), PG110 (Abbott Laboratories, Abbott Park, IL), and MEDI 578 (AstraZeneca, London, UK) are also in early phases of clinical development.41–43 This review will mainly focus on tanezumab, which is at an advanced stage of development in multiple chronic pain conditions, although the discussion will be generally applicable to other NGF antibodies also. Tanezumab shows tight binding to NGF and the complex has a half-life of >100 hours.39 Pharmacokinetic and toxicological studies in cynomolgus monkeys by weekly intravenous administration for 26 weeks followed by 8 weeks recovery period did not show adverse events (AEs) or any histological abnormalities in brain, spinal cord, nerves, or ganglia.44 To evaluate its clinical efficacy in the treatment of chronic pain syndromes, tanezumab was studied in patients with osteoarthritis, chronic lower back pain, urological chronic pelvic pain syndromes, painful diabetic neuropathy, and cancer pain. Some of the representative studies are described here.
In a Phase II proof-of-concept study, 450 patients with moderate-to-severe osteoarthritis of the knee were randomly assigned to receive two intravenous (iv) administrations of tanezumab at 10, 25, 50, 100, and 200 μg/kg or placebo 8 weeks apart.45 Tanezumab treatment resulted in substantial improvement in the primary efficacy measures of knee pain during walking as well as in global assessment of response to therapy. There was a 45%–62% reduction in knee pain from the baseline in the different dose groups averaged over a 16-week period, compared to 22% reduction in the placebo group. The most common adverse events were mild-to-moderate headache, respiratory infections, and changes in skin sensation (paresthesia).
A similar study was also conducted in Japanese patients having moderate-to-severe OA in the knee, with improvements in knee pain following a single iv administration of different doses of tanezumab up to 200 μg/kg versus placebo, accompanied by mild-to-moderate AEs of peripheral sensation that were transient in nature.46 Tanezumab was also evaluated for long-term effects in another Phase II repeat dose study by administration of 50 μg/kg every 8 weeks for a maximum of eight doses in OA patients.47 While significant improvement in overall knee pain was observed, treatment-related AEs were mild and of low incidence (7.5%), mostly hypoesthesia and paresthesia, most of which resolved before study completion.
Tanezumab was tested for its efficacy for chronic low back pain (LBP) in a Phase II trial of 220 chronic LBP patients who were on analgesic medication for at least 3 months and having an average LBP intensity (aLBPI) score of ≥4 using an 11-point numeric rating scale (NRS).48 The patients received a single iv dose of 200 μg/kg tanezumab (the maximal dose tested in the OA studies), naproxen (a common nonsteroidal anti-inflammatory drug) at 500 mg twice a day for 12 weeks, or placebo in a 2:2:1 ratio and observed over a 12-week period. At week 6, the number of patients showing ≥30% and ≥50% reduction in aLBPI score was much higher in the tanezumab group compared to the naproxen and placebo groups. Tanezumab was superior to both naproxen and placebo at weeks 4 to 12 with a higher proportion of patients showing ≥30% reduction in aLBPI score.
In a similar Phase II study in patients with interstitial cystitis, 34 patients received a single iv dose of tanezumab at 200 μg/kg and 30 patients received placebo.8 At 6 weeks, the tanezumab cohort showed significant reduction in the average pain score on an 11-point NRS versus placebo as the primary end point. Tanezumab safety and efficacy were also evaluated in a Phase II trial of patients with moderate-to-severe CP/CPPS (NIH CPSI of ≥15) with an average daily pain score of ≥4 on a 10-point NRS (ClinicalTrials. gov identifier: NCT00826514).49,50 Sixty-two patients were randomized to receive either a single iv administration of 20 mg tanezumab or placebo and followed up for 16 weeks. The primary efficacy endpoint consisted of changes in the average daily NRS pain score from the baseline to week 6. However, in this case, the patients showed only a marginal improvement in the average daily pain score that was not statistically significant. This result was different from the outcome of other studies of tanezumab in osteoarthritis and low back pain where the tanezumab cohort showed substantial pain reduction over the placebo or active comparator cohorts. One explanation for this observation could be that CP/CPPS is very heterogeneous in the etiology, and selection of patients based on appropriate criteria might improve the response to tanezumab.51
Following successful proof-of-concept studies of tanezumab, several Phase II and III trials in patients with osteoarthritis of the knee or hip have been underway.42 However, a significant number of patients had worsening of osteoarthritis, probably due to overuse of the joints after improvements in pain due to tanezumab therapy.45,51 This led to a clinical hold by the FDA on all trials of osteoarthritis, and later the clinical hold was extended to trials on all indications except cancer pain (ClinicalTrials.gov Identifier: NCT01146561).45,52,53 However, it is possible that tanezumab still holds promise as a highly efficacious therapy for chronic pain arising from different pathological conditions, with only mild-to-moderate drug-related AEs, which are mainly related to peripheral sensation. In some pathological conditions, such as osteoarthritis or diabetic peripheral neuropathy, closer monitoring of the disease condition is essential, because tanezumab mainly reduces the chronic pain associated with the condition, but the underlying cause of the disease might persist in many cases.
Lessons learned from previous tanezumab trials
Adverse events comparable to those seen in the OA trials are yet to be observed in trials involving other pain syndromes, such as urological pelvic pain syndromes, but many studies have been terminated or halted to further assess the safety issues.45,52 Recent clinical studies investigating the efficacy of tanezumab as a treatment for IC/PBS, CP/CPPS, or related pain conditions have shown that tanezumab might provide some symptom improvement compared with placebo.8,30,50 However, the lack of conclusive evidence may be attributed to individual variation in NGF levels in the affected tissues or organs of patients who present similar symptoms (pain, urgency, frequency), which might present a unique challenge in the design of clinical trials.
In such cases, the true effect of tanezumab may not be apparent due to inherent baseline variations in NGF levels, ie, some patients that experience chronic pain may intrinsically have low NGF levels and therefore benefit less from anti-NGF treatment, as may be the case if the pain is due to central sensitization or due to other inflammatory conditions. Therefore, a reasonable hypothesis is that tanezumab might show significant improvements in chronic pain in patients who have a certain threshold level of NGF in the secretions or fluids collected from the affected organs.
A biomarker-guided approach to tanezumab treatment
As a follow-up on the lack of conclusive evidence of tanezumab’s effects in some of the chronic pain syndromes studied in non-selected populations (and the adverse effects revealed in the OA study), future clinical studies might benefit from enriching the enrollment of potential responders in the study based on the baseline NGF levels as a biomarker. Clinical trials in a nonselected population might dilute the true effect of the therapy with the negative results from the non-responders and fail to demonstrate the robustness of clinical benefits in the target population. The key to designing future trials is to unravel the etiology (ie, NGF expression) behind the studied pain syndromes and to examine the interplay with tanezumab, rather than nonselectively treating for the symptom of pain.
In order to establish a mechanistic relation between tanezumab therapy and pain reduction, a clinical study that would incorporate the assessment of NGF as a biomarker is recommended. The study should measure pre-treatment NGF levels as a predictive biomarker for identification of potential responders, and measure posttreatment NGF levels as a pharmacodynamic biomarker for evaluating early signs of treatment efficacy. The correlation between the analgesic efficacy of the treatment and reduction in the NGF levels posttreatment can then be evaluated from pre- and posttreatment standardized pain scores and NGF measurements. A statistically significant correlation between these parameters would be a direct proof of mechanism of the action of the tanezumab therapy in chronic pain disorders, establishing baseline NGF levels as a predictive biomarker for various chronic pain syndromes for response to tanezumab treatment. A lack of correlation between NGF levels and the analgesic effect would indicate that tanezumab action may also be indirectly functioning through other mediators or pathways.
A two-phase optimization of tanezumab study: UCPPS as a paradigm
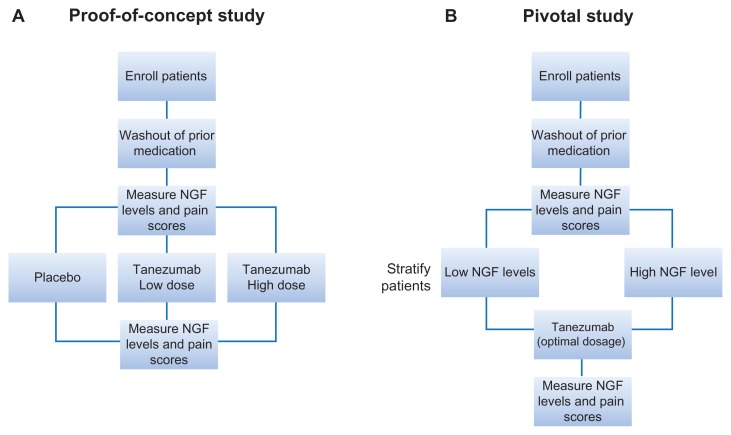
An outline for an optimal clinical investigation of tanezumab is recommended here by using urologic chronic pelvic pain syndrome (UCPPS) as a paradigm. UCPPS includes both CP/CPPS and IC/PBS and should be studied separately to have well-defined study populations. A proof-of-concept study in patients with CP/CPPS is conducted for evaluating the safety, tolerability, and efficacy assessments of tanezumab at two potentially optimal doses based on the results from the previous trials (for example, two tanezumab cohorts of 100 and 200 μg/kg administered twice, 8 weeks apart, and a placebo group) (Figure 2A). This phase will also have the secondary objective of assessing the direct involvement of NGF as a target of the therapy by measuring the NGF levels at pre- and posttreatment time points in the appropriate samples collected (eg, EPS and urine in the case of CP/CPPS). If the pain reduction observed in response to treatment depends on baseline NGF levels that are higher than asymptomatic subjects, the subsequent pivotal studies should include the baseline NGF level (high/low) as a patient stratification criterion (Figure 2B). The low NGF group would serve as a control for qualifying the elevated baseline NGF as a predictive biomarker for response to tanezumab in the pivotal studies. If the pain reduction is accompanied by corresponding reductions in the NGF levels in the posttreatment samples at the evaluated time points, the posttreatment reduction in the NGF levels might serve as early signs of efficacy in the pivotal trial even before the perceived pain reduction.
Figure 2.
Proposed study schema to assess NGF-dependence of tanezumab efficacy. (A) Proof-of-concept study in which NGF is measured in EPS and urine before and after treatment with placebo or tanezumab at two different doses. (B) Pivotal study in which NGF levels are measured at pre- and posttreatment time points to establish NGF levels as a predictive biomarker for anti-NGF therapy.
Abbreviations: EPS, expressed prostatic secretion; NGF, nerve growth factor.
In the case of UCPPS, an acceptable approach to collecting the baseline statistics would be to use the DABBEC phenotyping system, which addresses the underlying pathophysiological mechanism of UCPPS rather than symptoms alone.54,55 In the case of CP/CPPS, prostate fluid and urine, collected via the relatively noninvasive Meares–Stamey four-glass test, can be assayed for NGF by ELISA.32,56,57 In patients having IC/PBS and overactive bladder syndrome, urinary NGF can be measured pre- and posttreatment.29,33 Synovial fluid and serum can serve as the test sample for osteoarthritis studies and diabetic neuropathy, respectively.31
Discussion
Many chronic pain syndromes, including UCPPS, are of heterogeneous etiology, and careful attention should be paid to the underlying mechanism for effective treatment with appropriate therapeutic agents. NGF plays an important role in mediating chronic pain in several pathologic conditions but other inflammatory mediators may also contribute to pain sensation. Previous clinical trials for evaluating the anti-NGF antibodies, particularly tanezumab, as an effective therapy for osteoarthritis and other chronic pain syndromes, have established that the therapy is effective in reducing the pain in most cases. For targeted therapies of pain such as anti-NGF antibodies, best responses are expected if the study population is enriched based on the NGF levels in the affected tissues. A general recommendation for a two-phase clinical study for anti-NGF antibodies such as tanezumab in UCPPS is presented. This general procedure is applicable to the study of anti-NGF on other chronic pain disorders where the etiology for pain is heterogeneous by modifying the sample collection for NGF measurement. UCPPS serves as a paradigm in this example because of the relatively noninvasive and simple techniques used in obtaining prostatic fluid and urine for assaying NGF levels. The advantage of proving a mechanistic function of tanezumab’s effect is to use NGF as a predictive biomarker for tanezumab treatment, allowing for personalized medical care.
The information that is gained from these studies augments existing knowledge regarding the etiology of the pain syndrome and isolates those arising from NGF signaling. This might allow physicians to optimize treatment efficacy by properly selecting patients based on factors such as pretreatment NGF levels and standard pain scores. A potential exploratory addition to the study may be to measure the effect of other proinflammatory factors in causing UCPPS by performing multiplex immunoassays on the prostatic fluid and urine samples. There could be a possibility that only a fraction of the population with a chronic pain disorder may be affected by abnormal NGF levels, or that NGF levels could be just one of the factors in chronic pain. This exploratory study of other proinflammatory factors may further elucidate the complex mechanisms behind chronic pain syndromes.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hefti FF, Rosenthal A, Walicke PA, et al. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci. 2006;27(2):85–91. doi: 10.1016/j.tips.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001 Sep;413(6852):203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 3.Watson JJ, Allen SJ, Dawbarn D. Targeting nerve growth factor in pain: what is the therapeutic potential? Bio Drugs. 2008;22(6):349–359. doi: 10.2165/0063030-200822060-00002. [DOI] [PubMed] [Google Scholar]
- 4.Woodcock J. A difficult balance – pain management, drug safety, and the FDA. N Engl J Med. 2009;361(22):2105–2107. doi: 10.1056/NEJMp0908913. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S. Origins of growth factors: NGF and EGF. Ann N Y Acad Sci. 2004;1038:98–102. doi: 10.1196/annals.1315.017. [DOI] [PubMed] [Google Scholar]
- 6.Tuszynski MH. Nerve growth factor gene delivery: animal models to clinical trials. Dev Neurobiol. 2007;67(9):1204–1215. doi: 10.1002/dneu.20510. [DOI] [PubMed] [Google Scholar]
- 7.Varon S, Conner JM. Nerve growth factor in CNS repair. J Neurotrauma. 1994;11(5):473–486. doi: 10.1089/neu.1994.11.473. [DOI] [PubMed] [Google Scholar]
- 8.Evans RJ, Moldwin RM, Cossons N, Darekar A, Mills IW, Scholfield D. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol. 2011;185(5):1716–1721. doi: 10.1016/j.juro.2010.12.088. [DOI] [PubMed] [Google Scholar]
- 9.Fiore M, Chaldakov GN, Aloe L. Nerve growth factor as a signaling molecule for nerve cells and also for the neuroendocrine-immune systems. Rev Neurosci. 2009;20(2):133–145. doi: 10.1515/revneuro.2009.20.2.133. [DOI] [PubMed] [Google Scholar]
- 10.Zhu W, Galoyan SM, Petruska JC, Oxford GS, Mendell LM. A developmental switch in acute sensitization of small dorsal root ganglion (DRG) neurons to capsaicin or noxious heating by NGF. J Neurophysiol. 2004;92(5):3148–3152. doi: 10.1152/jn.00356.2004. [DOI] [PubMed] [Google Scholar]
- 11.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 12.Holtzman DM, Kilbridge J, Li Y, et al. TrkA expression in the CNS: evidence for the existence of several novel NGF-responsive CNS neurons. J Neurosci. 1995;15(2):1567–1576. doi: 10.1523/JNEUROSCI.15-02-01567.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrett GL. The p75 neurotrophin receptor and neuronal apoptosis. Prog Neurobiol. 2000;61(2):205–229. doi: 10.1016/s0301-0082(99)00056-8. [DOI] [PubMed] [Google Scholar]
- 14.Lad SP, Peterson DA, Bradshaw RA, Neet KE. Individual and combined effects of TrkA and p75NTR nerve growth factor receptors. A role for the high affinity receptor site. J Biol Chem. 2003;278(27):24808–24817. doi: 10.1074/jbc.M212270200. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24(24):4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci. 2007 Apr;34(4):689–700. doi: 10.1016/j.mcn.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMahon SB. NGF as a mediator of inflammatory pain. Philosophical Transactions: Biological Sciences. 1996;351(1338):431–440. doi: 10.1098/rstb.1996.0039. [DOI] [PubMed] [Google Scholar]
- 18.Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551(Pt 2):433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7(7):1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez-Andrade JM, Martin CD, Koewler NJ, et al. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain. 2007;133(1–3):183–196. doi: 10.1016/j.pain.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Sevcik MA, Ghilardi JR, Peters CM, et al. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain. 2005;115(1–2):128–141. doi: 10.1016/j.pain.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Kojima M, Ikeuchi T, Hatanaka H. Role of nerve growth factor in the expression of trkA mRNA in cultured embryonic rat basal forebrain cholinergic neurons. J Neurosci Res. 1995;42(6):775–783. doi: 10.1002/jnr.490420606. [DOI] [PubMed] [Google Scholar]
- 23.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 24.Koltzenburg M, Bennett DL, Shelton DL, McMahon SB. Neutralization of endogenous NGF prevents the sensitization of nociceptors supplying inflamed skin. Eur J Neurosci. 1999;11(5):1698–1704. doi: 10.1046/j.1460-9568.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 25.McMahon SB, Bennett DL, Priestley JV, Shelton DL. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med. 1995;1(8):774–780. doi: 10.1038/nm0895-774. [DOI] [PubMed] [Google Scholar]
- 26.McNamee KE, Burleigh A, Gompels LL, et al. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain. 2010;149(2):386–392. doi: 10.1016/j.pain.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Capsoni S, Covaceuszach S, Marinelli S, et al. Taking pain out of NGF: a “painless” NGF mutant, linked to hereditary sensory autonomic neuropathy type V, with full neurotrophic activity. PLoS One. 2011;6(2):e17321. doi: 10.1371/journal.pone.0017321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Indo Y, Tsuruta M, Hayashida Y, et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet. 1996;13(4):485–488. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- 29.Liu HT, Tyagi P, Chancellor MB, Kuo HC. Urinary nerve growth factor level is increased in patients with interstitial cystitis/bladder pain syndrome and decreased in responders to treatment. BJU Int. 2009;104(10):1476–1481. doi: 10.1111/j.1464-410X.2009.08675.x. [DOI] [PubMed] [Google Scholar]
- 30.Ochodnický P, Cruz CD, Yoshimura N, Michel MC. Nerve growth factor in bladder dysfunction: contributing factor, biomarker, and therapeutic target. Neurourol Urodyn. 2011;30(7):1227–1241. doi: 10.1002/nau.21022. [DOI] [PubMed] [Google Scholar]
- 31.Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470–477. doi: 10.1111/j.1749-6632.2009.04652.x. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe T, Inoue M, Sasaki K, et al. Nerve growth factor level in the prostatic fluid of patients with chronic prostatitis/chronic pelvic pain syndrome is correlated with symptom severity and response to treatment. BJU Int. 2011;108(2):248–251. doi: 10.1111/j.1464-410X.2010.09716.x. [DOI] [PubMed] [Google Scholar]
- 33.Kuo HC, Liu HT, Chancellor MB. Urinary nerve growth factor is a better biomarker than detrusor wall thickness for the assessment of overactive bladder with incontinence. Neurourol Urodyn. 2010;29(3):482–487. doi: 10.1002/nau.20741. [DOI] [PubMed] [Google Scholar]
- 34.Bloom AP, Jimenez-Andrade JM, Taylor RN, et al. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J Pain. 2011;12(6):698–711. doi: 10.1016/j.jpain.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jimenez-Andrade JM, Bloom AP, Stake JI, et al. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci. 2010;30(44):14649–14656. doi: 10.1523/JNEUROSCI.3300-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans L, Andrew D, Robinson P, Boissonade F, Loescher A. Increased cutaneous NGF and CGRP-labelled trkA-positive intra-epidermal nerve fibres in rat diabetic skin. Neurosci Lett. 2012;506(1):59–63. doi: 10.1016/j.neulet.2011.10.049. [DOI] [PubMed] [Google Scholar]
- 37.Jimenez-Andrade JM, Ghilardi JR, Castañeda-Corral G, Kuskowski MA, Mantyh PW. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain. 2011;152(11):2564–2574. doi: 10.1016/j.pain.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shelton DL, Zeller J, Ho WH, Pons J, Rosenthal A. Nerve growth factor mediates hyperalgesia and cachexia in auto-immune arthritis. Pain. 2005;116(1–2):8–16. doi: 10.1016/j.pain.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 39.Abdiche Y, Malashock DS, Pons J. Probing the binding mechanism and affinity of tanezumab, a recombinant humanized anti-NGF monoclonal antibody, using a repertoire of biosensors. Protein Science. 2008;17(8):1326–1335. doi: 10.1110/ps.035402.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantyh P, Tive L, Shelton D. Tanezumab, a humanized anti-nerve growth factor antibody for the treatment of pain. The Journal of Pain: Official Journal of the American Pain Society. 2009;10(4):S44. [Google Scholar]
- 41.Cattaneo A. Tanezumab, a recombinant humanized mAb against nerve growth factor for the treatment of acute and chronic pain. Curr Opin Mol Ther. 2010;12(1):94–106. [PubMed] [Google Scholar]
- 42.Ekman E, Gimbel J, Bello A, et al. Efficacy and safety of intravenous tanezumab in osteoarthritis hip and knee pain: comparison to placebo and naproxen in two phase III studies ( NCT00830063 and NCT00863304) The Journal of Pain: Official Journal of the American Pain Society. 2011;12(4):P55. [Google Scholar]
- 43.Haddley K. Tanezumab. Anti-NGF monoclonal antibody, Treatment of pain, Treatment of osteoarthritis. Drugs of the Future. 2010;35(5):373–378. [Google Scholar]
- 44.Zorbas M, Hurst S, Shelton D, Evans M, Finco D, Butt M. A multiple-dose toxicity study of tanezumab in cynomolgus monkeys. Regul Toxicol Pharmacol. 2011;59(2):334–342. doi: 10.1016/j.yrtph.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363(16):1521–1531. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagashima H, Suzuki M, Araki S, Yamabe T, Muto C. Preliminary assessment of the safety and efficacy of tanezumab in Japanese patients with moderate to severe osteoarthritis of the knee: a randomized, double-blind, dose-escalation, placebo-controlled study. Osteoarthritis and Cartilage/OARS, Osteoarthritis Research Society. 2011;19(12):1405–1412. doi: 10.1016/j.joca.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Schnitzer TJ, Lane NE, Birbara C, Smith MD, Simpson SL, Brown MT. Long-term open-label study of tanezumab for moderate to severe osteoarthritic knee pain. Osteoarthritis Cartilage. 2011;19(6):639–646. doi: 10.1016/j.joca.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Katz N, Borenstein DG, Birbara C, et al. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain. 2011;152(10):2248–2258. doi: 10.1016/j.pain.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Pfizer. ClinicalTrials.gov [website on the Internet] Bethesda, MD: US National Library of Medicine; 2009. [Accessed April 15, 2012]. An efficacy and safety study of tanezumab for the treatment of pain associated with chronic abacterial prostatitis. [updated March 14, 2011]. Available from: http://clinicaltrials.gov/ct2/show/study/NCT00826514?term=NCT00826514&rank=1. NLM identifier: NCT00826514. [Google Scholar]
- 50.Nickel JC, Atkinson G, Krieger J, et al. Tanezumab therapy for chronic prostatitis/chronic pelvic pain syndrome (cp/cpps): preliminary assessment of efficacy and safety in a randomized controlled trial. The Journal of Urology. 2011;185(4):e573–e574. [Google Scholar]
- 51.Strauss AC, Dimitrakov JD. New treatments for chronic prostatitis/chronic pelvic pain syndrome. Nat Rev Urol. 2010;7(3):127–135. doi: 10.1038/nrurol.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wood JN. Nerve growth factor and pain. N Engl J Med. 2010 Oct;363(16):1572–1573. doi: 10.1056/NEJMe1004416. [DOI] [PubMed] [Google Scholar]
- 53.Pfizer. ClinicalTrials.gov [website on the Internet] Bethesda, MD: US National Library of Medicine; 2010. [Accessed April 15, 2012]. Safety and efficacy of tanezumab in patients with chronic pancreatitis. [updated April 13, 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT01146561?term=NCT01146561&rank=1 NLM identifier: NCT01146561. [Google Scholar]
- 54.Allsop SA, Erstad DJ, Brook K, Bhai SF, Cohen JM, Dimitrakoff JD. The DABBEC Phenotyping System: towards a mechanistic understanding of CP/CPPS. Nat Rev Urol. 2011;8(2):107–113. doi: 10.1038/nrurol.2010.227. [DOI] [PubMed] [Google Scholar]
- 55.Mahal BA, Cohen JM, Allsop SA, et al. The role of phenotyping in chronic prostatitis/chronic pelvic pain syndrome. Curr Urol Rep. 2011;12(4):297–303. doi: 10.1007/s11934-011-0196-y. [DOI] [PubMed] [Google Scholar]
- 56.Meares EM, Stamey TA. Bacteriologic localization patterns in bacterial prostatitis and urethritis. Invest Urol. 1968;5(5):492–518. [PubMed] [Google Scholar]
- 57.Stamey TA. Prostatitis. J R Soc Med. 1981;74(1):22–40. doi: 10.1177/014107688107400106. [DOI] [PMC free article] [PubMed] [Google Scholar]