Abstract
Pain is the most common reason patients seek medical attention and pain relief has been put forward as an ethical obligation of clinicians and a fundamental human right. However, pain management is challenging because the pathophysiology of pain is complex and not completely understood. Widely used analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) and paracetamol (acetaminophen) have been associated with adverse events. Adverse event rates are of concern, especially in long-term treatment or at high doses. Paracetamol and NSAIDs are available by prescription, over the counter, and in combination preparations. Patients may be unaware of the risk associated with high dosages or long-term use of paracetamol and NSAIDs. Clinicians should encourage patients to disclose all medications they take in a “do ask, do tell” approach that includes patient education about the risks and benefits of common pain relievers. The ideal pain reliever would have few risks and enhanced analgesic efficacy. Fixed-dose combination analgesics with two or more agents may offer additive or synergistic benefits to treat the multiple mechanisms of pain. Therefore, pain may be effectively treated while toxicity is reduced due to lower doses. One recent fixed-dose combination analgesic product combines tramadol, a centrally acting weak opioid analgesic, with low-dose paracetamol. Evidence-based guidelines recognize the potential value of combination analgesics in specific situations. The current guideline-based paradigm for pain treatment recommends NSAIDs for ongoing use with analgesics such as opioids to manage flares. However, the treatment model should evolve how to use low-dose combination products to manage pain with occasional use of NSAIDs for flares to avoid long-term and high-dose treatment with these analgesics. A next step in pain management guidelines should be targeted therapy when possible, or low-dose combination therapy or both, to achieve maximal efficacy with minimal toxicity.
Keywords: NSAIDs, opioids, combination analgesics, moderate pain, severe pain, analgesics, tramadol/paracetamol
Introduction
Pain is the oldest medical problem and has been a challenge for doctors since the origin of humanity. While scientific and technological breakthroughs have improved care in many areas, eradicating diseases and advancing longevity, pain remains a global public health issue. The World Health Organization (WHO) has promoted and disseminated guidelines on pain management,1 advocated for the use of analgesics, including opioids,2 and encouraged national programs for palliative care and the relief of cancer pain.3,4 Pain relief has been put forward as a fundamental human right.5–8 The third international symposium on the Societal Impact of Pain held in May 2012 in Copenhagen has finalized a position paper, seeking that chronic pain be recognised as a disease by the governments of member states.9 Despite pharmacological advances and numerous guidelines or consensus documents to inform clinicians about the appropriate prescribing of analgesics, pain is often under-treated.10–12 Inadequate analgesia may have roots in social, political, legal, cultural, and religious considerations, as well as the fundamental knowledge, differences in health care systems, and variations in clinical practice.13–16 However, it remains the imperative of medical professionals to relieve pain as much as possible.17–19 Regardless of the social and political factors complicating analgesic therapy, not treating pain is not an option and has been described as a “moral outrage.”20
The European Study of the Epidemiology of Mental Disorders reported from a questionnaire (1659 respondents, all of whom were ≥75 years of age) that pain was the most commonly reported problem in this population (55.2%), far exceeding the rate of depression and anxiety (11.6%).21 In Europe, it is estimated that 19% of the general population suffers from chronic pain.22 A hospital-based survey in Germany reported that over 80% of patients (n = 438) experienced pain in the previous 3 months and pain was the main reason for hospital admission in over 60% of the cases.23 In the USA, chronic pain affects more people every year than diabetes, heart disease, and cancer combined.24,25 Chronic pain can occur in patients of any age, but it is more common among older individuals.26 Inadequately treated persistent pain may be associated with a number of adverse outcomes in older people, including functional impairment, reduced mobility, falls, slower rehabilitation, decreased socialization, inadequate sleep, disturbed appetite, and changes in mood.27 Pain negatively affects quality of life, adversely affects families, may result in lost or diminished productivity for society, and places a large burden on the health care system. In the USA in 2002–2003, over US$4 billion was spent on headache-related care alone, and this did not include over-the-counter medications, self-treatment, and inpatient treatment.28 The total global health care burden related to all types of acute and chronic pain syndromes is difficult to assess.
Although pain management guidelines address specific types of pain, they frequently recommend nonsteroidal anti-inflammatory drugs (NSAIDs) in cases where tissue damage and inflammation are absent. Due to serious gastrointestinal, cardiovascular, and renal side effects, caution is recommended when using high-dose NSAIDs, particularly when taken long-term.27,29 The appropriate use of NSAIDs, paracetamol, opioid analgesics, or combination products in the chronic pain population remains a subject of ongoing research.
Meeting details
A consensus meeting attended by all authors of this publication was held on November 20, 2010 in Paris, France, to discuss the use of high-dose NSAIDs, high-dose paracetamol, or tramadol/paracetamol (as an example of fixed-dose combination analgesics) for the management of moderate to severe pain from different etiologies. Tramadol/Paracetamol is – to our knowledge – the only fixed-dosed combination product where the dual mode of action of tramadol and the analgesic synergy between the two compounds have been proven in both preclinical studies (mouse model)30,31 and companion human studies.32,33 Presentations by five of the authors were followed by a group discussion and review of pain management issues regarding these drug classes and available guidelines/recommendations based on the clinical experiences of the participants. A manuscript was drafted, additional articles were reviewed and incorporated, and a final consensus was adopted by the group.
Pain management and underlying pain mechanisms
Pain management is complex for many reasons. Chronic pain may be broadly classified into nociceptive (pain owing to tissue disease or damage, including inflammatory and visceral pain), neuropathic (pain caused by somatosensory system disease or damage), and mixed syndromes ( coexistence of nociceptive and neuropathic pain).34 However, even the terminology of pain becomes challenging and contentious.35 For example, the International Association for the Study of Pain is currently attempting to distinguish between “nociception” (a sensory process) and “pain” (a subjective phenomenon).36
Multiple mechanisms contribute to painful syndromes, including nociception, peripheral sensitization, central sensitization, phenotypic switches, ectopic excitability, structural reorganization, and compromised inhibitory systems.37–41 Hypersensitivity causes a mild stimulus to provoke pain out of proportion to the stimulus. Hypersensitivity may be categorized academically as allodynia (pain response to nonnociceptive stimuli) or hyperalgesia (increased pain sensitivity in response to nociceptive stimuli),37 although these phenomena may be difficult to distinguish clinically.
The mechanisms may act in different ways. Nociception requires an intact central nervous system; changes in the central nervous system are evident in chronic pain patients.42 Primary afferent or sensory neurons play an important role in nociceptive pain processing, thus involving the peripheral nervous system.42 Inflammation, altered sympathetic and catecholaminergic function, changes in somatosensory processing in spinal cord and brain, pressure, temperature, neuropathic components, along with psychological factors, may also play a role in acute and chronic pain syndromes.43 The transition from acute to chronic pain is not thoroughly understood, but it is likely to involve the interaction among immune, endocrine, and nervous systems44 and, therefore, progressing central and peripheral sensitization.45 Other factors no doubt play a role. A study of trauma patients (n = 290) identified as risk predictors for the transition to chronic pain – that is, pain that persists beyond 3 months: older age, female sex, past alcohol dependence, the amount of morphine equivalents administered on the day of assessment, and attitudes about pain control.46 A two-dimension positron emission tomography scan study of 20 cancer patients found preferential activation of the prefrontal cortex in patients with chronic pain but not in similar patients without pain.47 The prefrontal cortex is associated with emotional response, which may account for the emotional component of chronic pain.
In certain rheumatic pain conditions, selective serotonin reuptake inhibitors, serotonin and noradrenalin reuptake inhibitors, as well as tricyclic antidepressants have been shown to exert an analgesic effect that is distinct from their ability to treat depression, fatigue, and sleep disturbances.48,49 However, the evidence for the efficacy of these drugs in treating common pain syndromes (headache, low back pain, fibromyalgia, postherpetic neuralgia, and others) remains equivocal and, at times, conflicting.50,51 This suggests that these common pain syndromes may involve different pain mechanisms.
The accurate assessment of pain is challenging because pain perception is subjectively reported and may be influenced by the patient’s attitude about health, disease, and personal expectations.52 These differences may be more than just idiosyncratic. For example, men and women not only experience pain differently, they may respond to analgesics differently.53
Pain may be a potentially serious comorbid condition, affecting medical and surgical outcomes.23 Maladaptive chronic pain may even be regarded as a disease in its own right.37 As such, it is crucial to devote our attention to better understanding and superior management of patients dealing with acute and chronic pain. The identification and increased understanding of the multiple mechanisms of pain has been a major advance.
Commonly used agents in the treatment of pain
Since the dawn of medicine, clinicians have treated pain (Table 1). As early as 3000 BC, natural salicylates were applied for the treatment of pain and Hippocrates reported on the analgesic efficacy of opium as early as 400 BC. However, in early medicine, these narcotics enjoyed a dubious reputation because of their potential for misuse, potentially life-threatening side effects, and withdrawal symptoms.54 Chemistry-based anti-inflammatory therapy began in 1897 with the discovery of aspirin, leading to advances in other pharmacological options, including NSAIDs. In 1986, the WHO proposed its well-known “pain ladder,” which calls for the treatment of cancer pain based on level of pain intensity rather than the underlying mechanism, in that it advocates the use of nonopioid agents (such as aspirin, paracetamol, and NSAIDs) for mild pain, weak opioids for moderate pain (tramadol), and strong opioids (morphine) for severe pain.1 The multimechanistic nature of pain is recognized in the WHO ladder insofar as it includes adjuvant medications to treat pain.
Table 1.
Milestones in analgesic agents
| Year | Event |
|---|---|
| 3000 BC | First description of the use of myrtle leaves as systemic pain treatment |
| Approximately 400 BC | Hippocrates reports on the pain-relieving properties of opium in treating internal diseases and diseases of women |
| 1527 | Paracelsus prescribes opium with other agents as an analgesic |
| 1680 | Thomas Sydenham introduces Sydenham’s laudanum (opium mixed with wine and herbs), which becomes a popular home remedy |
| 1803 | Friedrich Sertürner discovers the active ingredient in opium – morphine |
| 1827 | Merck and Company begin first commercial manufacture of morphine |
| 1877 | Synthesis of paracetamol (acetaminophen) at Johns Hopkins University is completed, but the drug would not be used in patients for another 10 years |
| 1890 | Morphine, legal in the USA, is taxed by Congress |
| 1895 | Bayer Company adds acetyls to morphine to reduce side effects to create a drug that would be marketed in 1898 as Heroin (trade name) |
| 1897 | Discovery of aspirin, named for Spiraea (meadowsweet), one of many salicylate sources used to treat pain in the nineteenth century |
| 1905 | USA bans opium (but not opioid drugs) |
| 1910 | Heroin, marketed as a cough suppressant and morphine substitute, is taken off the market when it is found it is more addictive than morphine |
| 1914 | The Harrison Narcotics Act in the USA requires physicians and pharmacists who prescribe or dispense narcotics to register (and pay a tax) |
| 1953 | Paracetamol (acetaminophen) first marketed in the USA by Sterling-Winthrop Company |
| 1955 | McNeil Laboratories first markets Tylenol® brand (paracetamol) in the USA |
| 1956 | Frederick Stearns and Company first markets Panadol in the UK |
| 1963 | Development of nonsteroidal anti-inflammatory drugs (NSAIDs) |
| 1971 | Understanding of the mechanism of action of aspirin |
| 1990–1991 | Discovery of cyclooxygenase-2 (COX-2) |
| 1992 | COX-2 drug development |
| 1998–1999 | Celecoxib and rofecoxib introduced |
| 2004–2006 | Rofecoxib withdrawn from market |
| 2005 | Warning of increased cardiovascular risk must be added to labeling for all NSAIDs in US (FDA requirement) |
| 2006–2010 | Warnings and dose restrictions on NSAIDs |
| 2009 | Dextropropoxyphene withdrawn from market in the European Union |
| 2010 | FDA launches Safe Use Initiative |
| 2010 | Propoxyphene withdrawn from market in the USA |
Abbreviation: FDA, US Food and Drug Administration.
When the WHO ladder was introduced in 1986, oxycodone, hydromorphone, and buprenorphine did not exist. Tramadol was not available worldwide until the 1990s. Transdermal delivery systems for opioids were unknown in 1986. Methadone, not listed on the WHO pain ladder, existed in 1986, but its analgesic benefits in treating cancer pain were unknown. The first guidelines for neuropathic pain management were not published until the first decade of the 21st century55–58 and the neuropathic treatment model differs from the WHO ladder (opioids are adjuvants in neuropathic pain management). Thus, in particular, the pain model should be updated with new pharmacological agents (new opioids, gabapentinoids, etc) according to new insights into adjuvant and multimodal therapies.59 It should also be noted that all treatment options may be combined with nonpharmacological approaches and patients may benefit from these multidisciplinary efforts.
Weighing the risks of treatment with high-dose NSAIDs and paracetamol
Paracetamol or acetaminophen is frequently grouped with NSAIDs, but it is actually an aniline analgesic. The terms “paracetamol” and “acetaminophen” reflect only geographical differences: “acetaminophen” is the term used in the USA, Canada, Hong Kong, Iran, and certain Latin American countries, such as Colombia, while “paracetamol” is used in Europe, Africa, and most of Asia. The drug is sometimes abbreviated to “APAP” in all geographic regions. The mechanism of action of paracetamol is not well understood and several models have been proposed, all of which have certain strengths and limitations.60 Paracetamol is metabolized mainly by conjugation with sulfate and glucuronide, with about 5% to 10% of the drug oxidized by the cytochrome P450 metabolic pathway (mostly CYP2E1 and CYP3A4) to a toxic electrophilic metabolite, N-acetyl-p-benzoquinone imine (NAPQI). NAPQI is subsequently detoxified by glutathione and eliminated in the urine or bile.61 If any residual NAPQI is not detoxified in this manner, it may bind to hepatocytes, where it can lead to cellular necrosis. At appropriate doses in healthy individuals, the small amounts of NAPQI produced by paracetamol metabolism can be effectively eliminated with glutathione. However, at higher doses, paracetamol is associated with serious hepatic toxicity.62 In fact, paracetamol toxicity is the leading indication for liver transplantation in the UK63 and one of the most common causes of poisoning64 and acute liver failure65 in the USA. Paracetamol has also been linked to hypertension,66–68 which is probably caused by the considerable sodium content present in each paracetamol tablet. Thus, there are still unanswered questions about these side effects, including their extent.69–71
NSAIDs encompass a diverse group of drugs that reduce pronociceptive and proinflammatory prostaglandins and other chemical mediators by inhibiting their biotransformation in the arachidonic cascade, a reaction catalyzed by cyclooxygenase (COX) isoenzymes.72 In this way, they are similar to aspirin.73
The safety of many drugs, including pain drugs, has not been studied in as much detail as safety issues of NSAIDs and especially selective COX-2 inhibitors (coxibs). Nonselective NSAIDs block COX, namely COX-1 and COX-2, blocking the synthesis of prostaglandins and consequently shunting arachidonic acid into the lipoxygenase pathway, producing leukotrienes. Leukotrienes are powerful bronchoconstrictors and impair mucociliary clearance, resulting in increased mucus production, mucus filtration, and edema. Obviously, NSAID use has been associated with bronchospasm.74 Coxibs selectively block COX-2 and include such drugs as celecoxib, valdecoxib, and rofecoxib, limiting the COX-1-related inhibition to vital housekeeping functions. All NSAIDs are associated with dose-dependent toxicity, manifesting as gastrointestinal symptoms, including dyspepsia, ulceration, and bleeding, as well as cardio-renal complications including fluid retention, hypertension, and renal dysfunction.75–77 A recent study found even short-term use of NSAIDs was associated with increased risk of death in patients with a history of myocardial infarction (hazard ratio 1.45; 95% confidence interval: 1.29–1.62).78
For such reasons, NSAIDs, including coxibs, should not be prescribed as a panacea for all pains, but restricted to pain related to tissue damage and/or inflammation, in accordance to their mechanism of action.79–81 NSAIDs are to be used cautiously, in patients with or at elevated risk for cardiovascular disease29,78,79,81–84 or gastrointestinal complications.79,81,85
Pharmacological aspects: why combinations might be better than single agents
Rarely does a single known mechanism cause pain. Obviously, no single analgesic agent can fully address multiple mechanisms of pain. Combination analgesic products have been effective because they activate multiple pain-inhibitory pathways and offer a broader spectrum of relief.86 This may include multiple afferents and pathways as well as multiple processes. Combination analgesics might reduce adverse events.86 A given analgesic provides pain relief at a specific dosage and is associated with dose-dependent adverse effects.
Combining analgesics may allow for lower doses of the individual agents, with doses possibly low enough to significantly reduce potential adverse events. While the theory of combination analgesic products holds promise, combination products require rigorous scrutiny and testing since not all combinations are ideal.
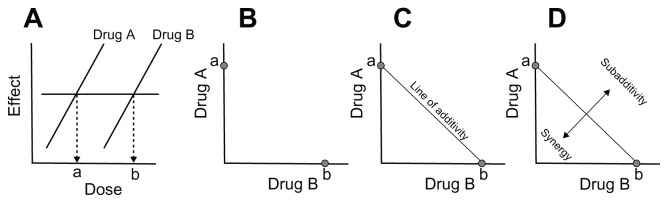
Combining two or more agents may result in an additive or synergistic analgesic effect.86,87 When agents are combined, the combination effect may be greater than, less than, or the same as the predicted magnitude of effect, resulting in synergistic, sub-additive, or additive effects, respectively. Such effects are calculated mathematically based on the concept of dose equivalence, defined as doses of each drug that yield the same magnitude of effect when each is used by itself. These calculations compare actual versus expected effects in graphic representations of dose combinations known as isoboles88–92 (Figure 1). Isobolographic analysis is well accepted and has been used with many drug combinations.93,94 Drugs with a constant potency ratio have linear isoboles of additivity,93–95 but drugs with variable potency ratios can be analyzed as well.96 Receptor saturation of the agents can also be assessed.97
Figure 1.
Representation of isobolographic analysis. Equi-effective doses of two drugs are determined (A) and graphed on Cartesian coordinates (B). The predicted effect of various ratios of combinations of these drugs is simple additivity (C). Actual results on, above, or below the predicted line of additivity (D) are indicative of additive, sub-additive, or supra-additive (synergistic) interaction, respectively.
Combination analgesic products are common and include, but are not limited to, such products as Empirin ® ( paracetamol + codeine), Vicodin® (paracetamol + hydrocodone), Percocet ® (paracetamol + oxycodone), and Zaldiar® or Ultracet® ( paracetamol + tramadol). Table 2 lists selected studies of fixed-dose combinations with paracetamol, all of them having demonstrated good efficacy in several chronic pain conditions.
Table 2.
Selected clinical studies using fixed-dose combination products with paracetamol
| Study | N | Agents | Results | Comments |
|---|---|---|---|---|
| Postoperative pain | ||||
| Dental | ||||
| Fricke et al139 Double-blind, randomized | 200 |
|
|
Removal of ≥2 impacted third molars |
| MacLeod et al140 Double-blind, randomized, parallel-group | 82 |
|
|
Removal of impacted third molars |
| Edwards et al141 Meta-analysis, randomized studies | 5 studies |
|
|
NNH was 5.4 (4.0–8.2) for tramadol/APAP and 5.0 (3.7–7.3) for tramadol |
| Jung et al142 Randomized | 128 |
|
|
Extraction of ≥1 impacted third molar requiring bone removal |
| Litkowski et al143 Double-blind, randomized, placebo-controlled, parallel-group | 249 |
|
|
Removal of 2 or more impacted third molars |
| Daniels et al144 Double-blind, randomized, parallel-group, placebo-controlled | 678 |
|
|
Removal of ≥3 impacted third molars |
| Other procedures | ||||
| White et al145 Double-blind, randomized, parallel-group | 252 |
|
|
Ambulatory arthroscopic or laparoscopic tubal ligation |
| Palangio et al146 Double-blind, randomized, placebo-controlled | 180 |
|
|
Obstetric or gynecological surgery |
| Smith et al132 Double-blind, randomized, placebo-controlled | 305 |
|
|
Orthopedic and abdominal surgery |
| Sniezek et al147 Double-blind, randomized | 210 |
|
|
Mohs micrographic surgery and reconstruction for head and neck skin cancer |
| Rawal et al148 Randomized, double-blind, double-dummy, parallel-group | 261 |
|
|
Ambulatory hand surgery with iv regional anesthesia |
| Musculoskeletal pain | ||||
| Mullican and Lacy131 Double-blind, randomized | 462 |
|
|
Chronic, nonmalignant low back pain and osteoarthritis pain |
| Serrie et al149 Observational, prospective, open-label, in clinical practice (ELZA), mean therapy duration 16.6 days | 5495 |
|
|
Majority of patients had musculoskeletal pain |
| Mejjad et al150 Observational, prospective, open-label, in clinical practice (SALZA), median treatment 30 days | 2663 |
|
|
Patients aged ≥ 65 years, primarily with musculoskeletal pain |
| Osteoarthritis | ||||
| Emkey et al151 Double-blind, randomized, placebo-controlled | 306 |
|
|
Add-on for patients with inadequate pain control by celecoxib or rofecoxib |
| Corsinovi et al152 Randomized, single-blind | 154 |
|
|
Elderly females |
| Pareek et al153 Randomized, open-label | 199 |
|
|
Knee flare-up |
| Pareek et al154 Randomized, double-blind | 220 |
|
|
Knee flare-up |
| Doherty et al155 Double-blind, randomized, parallel-group | 892 |
|
|
≥40 years of age Chronic knee pain, 85% osteoarthritis |
| Conaghan et al156 Open-label, randomized, parallel-group | 220 |
|
|
|
| Low back pain | ||||
| Palangio et al157 Double-blind, randomized, parallel-group | 147 |
|
|
Acute pain |
| Ruoff et al158 Double-blind, randomized | 318 |
|
|
Chronic pain |
| Perrot et al159 Double-blind, randomized, parallel-group | 119 |
|
|
Subacute pain |
| Fibromyalgia | ||||
| Bennett et al160 Double-blind, randomized, placebo-controlled | 315 |
|
|
|
| Rheumatoid arthritis | ||||
| Lee et al161 Double-blind, randomized, placebo-controlled | 277 |
|
|
Add-on for patients with inadequate pain control by conventional NSAIDs and DMARDs |
| Raffaeli et al162 Open-label, case series | 29 |
|
|
Patients under rheumatoid arthritis therapy with biological drugs were excluded |
| Painful diabetic neuropathy | ||||
| Freeman et al130 Double-blind, randomized, placebo-controlled, parallel-group | 313 |
|
|
|
| Ko et al129 Open-label, randomized | 163 |
|
|
Patients with type 2 diabetes aged 25–75 years Dose adjusted to effect, no rescue medication during maintenance phase |
Abbreviations: AEs, adverse events; APAP, paracetamol (acetaminophen); bid, twice daily; DMARD, disease-modifying antirheumatic drug; iv, intravenous; NSAIDs, nonsteroidal anti-inflammatory drugs; NNH, number needed to harm; NNT, number needed to treat; qid, four times per day; QoL, quality of life; tid, three times per day.
As an example of fixed-dose combination, the participants of the meeting discussed tramadol/paracetamol because this product has been more extensively evaluated than other combination products. The theoretical rationale for the combination agents described needs to be backed by clinical evidence because, in some cases, additive benefits do not result in clinically meaningful differences. Tramadol/Paracetamol is – to our knowledge – the only fixed-dose combination where both the dual mechanism of action of tramadol and the analgesic synergy between the two compounds have been demonstrated in both preclinical studies (mouse model) and human companion studies using essentially the same study design.30–33 Table 3 provides an overview of the relevant results. Further study of tramadol/paracetamol combination analgesia in chronic pain syndromes is warranted to better evaluate long-term safety and efficacy.
Table 3.
Companion studies demonstrating mode of action of tramadol/paracetamol fixed-dose combination
| Dual mechanism of action of tramadol | Analgesic synergy between tramadol and paracetamol | |||
|---|---|---|---|---|
|
|
|
|||
| Mouse and rat model30 | Healthy male volunteers32 | Mouse model31 | Healthy volunteers33 | |
| Design | Double-blind, randomized, placebo-controlled, crossover | Double-blind, randomized, placebo-controlled, crossover | ||
| Agents | Tramadol iv |
|
Oral:
|
iv infusions:
|
| Methods |
|
|
|
|
| Results |
|
|
ED50 values:
|
Pain reduction (correction for placebo effects)
|
| Conclusions | The results suggest that tramadol-induced antinociception is mediated by opioid (μ) and nonopioid (inhibition of monoamine uptake) mechanisms | Alpha2-adrenoceptor antagonism reverses tramadol effects, thus pointing to significant role of monoaminergic modulation and synergy with opioid antagonism in tramadol anti-nociception | Supra-additive effects of the combination regarding analgesia and anti-hyperalgesia | |
Abbreviations: APAP, paracetamol (acetaminophen); ED50, the dose of a drug that is pharmacologically effective for 50% of the population exposed to the drug or a 50% response in a biological system that is exposed to the drug; iv, intravenous.
According to these and later studies, the mechanisms of action of tramadol may be described, respectively as: a weak agonist effect at the μ-opioid receptors, inhibition of serotonin reuptake, and inhibition of norepinephrine reuptake.98 In a preclinical model, it has been shown that the nonopioid component in tramadol may enhance its potency ratio relative to morphine in neuropathic pain models.99 Tramadol can increase the risk of convulsions in patients who are taking medicinal products reducing the seizure threshold such as bupropion, serotonin reuptake inhibitor antidepressants, tricyclic antidepressants and neuroleptics. In isolated cases there have been reports of serotonin syndrome in a temporal connection with the therapeutic use of tramadol in combination with other serotoninergic medicines such as selective serotonin reuptake inhibitors.100 The second component in this fixed-dose combination, paracetamol, appears to act at both central and peripheral pathways,101 but its exact mechanism(s) of action has/have yet to be thoroughly elucidated. The maximum recommended adult dose of paracetamol is 4 g/day.102,103 At therapeutic doses, paracetamol is rarely associated with hepatotoxicity.104
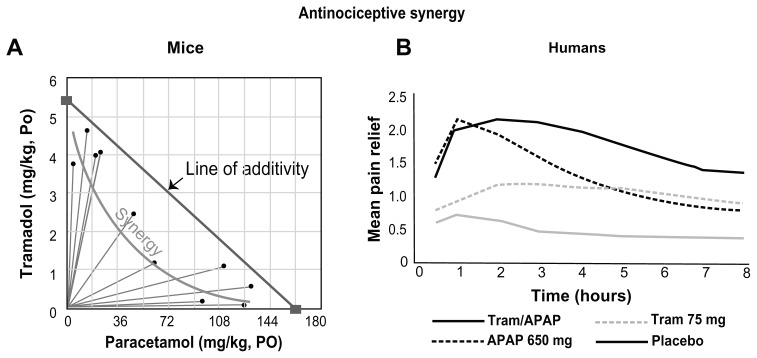
Complementary pharmacokinetics of tramadol/paracetamol in combination enhance the probability of effective pain relief (Figure 2) and supra-additive effects of the combination regarding analgesia and anti-hyperalgesia have been demonstrated in a human pain model.33 Clinical studies have shown good efficacy and safety of this fixed-dose product for a variety of pain conditions.105,106 Details from selected studies can be found in Table 2.
Figure 2.
Mean pain relief with (A) tramadol/paracetamol (Tram/APAP) compared with (B) paracetamol 650 mg alone (APAP 650 mg), tramadol 75 mg alone (Tram 75 mg), and placebo.
Notes: (A) Adapted from Life Sciences, 58(2), Tallarida RJ, Raffa RB, Testing for synergism over a range of fixed ratio drug combinations: replacing the isobologram, PL 23–PL 28,
Copyright (1996), with permission from Elsevier.31 (B) Adapted from an FDA Executive Summary [web page on the Internet; McNeil background package to the Nonprescription Drug Advisory Committee]. 2002.168
Mitigation strategies when prescribing high-dose NSAIDs or high-dose paracetamol
Before high-dose paracetamol or high-dose NSAIDs are considered for patients, mitigation strategies should be undertaken, including the review of patients to verify if they are appropriate candidates for such therapy in light of their comorbidities and co-medications.107 Upper gastrointestinal adverse effects can be mitigated by proton pump inhibitors.108–112 Patients on long-term high-dose paracetamol or NSAID therapy should be educated as to the potential risks of these drugs, the doses, and the fact that these agents may be contained in a variety of prescription and over-the-counter products. In the USA, this has been called a “do ask, do tell” strategy, where clinicians are encouraged to ask patients about their use of concomitant medications, including over-the-counter products and, by the same token, patients are encouraged to fully disclose to their clinicians all of the drugs they take.113 For many patients, it may be appropriate to use a low-dose combination product for maintenance, with occasional NSAIDs to treat breakthrough episodes. An individualized approach to mid- and long-term pain management is required in light of the potential risks and benefits of analgesic agents (Table 4).114
Table 4.
Mitigation strategies that may be useful for patients receiving paracetamol or nonsteroidal anti-inflammatory drugs (NSAIDs) for pain management
| Area of concern | Mitigating strategies |
|---|---|
| Labeling of paracetamol, acetaminophen, and combination products, particularly over-the-counter preparations | Plain language labeling Patient education initiatives about high-dose, long-term, and cumulative doses of paracetamol and NSAIDs “Do ask, do tell” approach |
| High-dose paracetamol seems necessary | Consider lower doses of paracetamol in combination with other pain medication due to risk of hepatotoxicity, hypertension, and gastrointestinal complications |
| High-dose NSAID seems necessary | Consider lower doses used in combination with other pain relievers on account of increased risk for gastrointestinal complications and particularly in light of risk factors (old age, ulcer history, smoking, comorbidities) Add proton pump inhibitor Limit dose |
| NSAID seems necessary in a patient with a cardiovascular risk | Consider the lowest possible dose of NSAIDs or avoid NSAIDs altogether. An alternative might be a low-dose fixed combination product |
The mitigation of adverse events is more than just a matter between clinician and patient. We recommend the use of plain language in labeling over-the-counter products and prescribed medications that contain paracetamol and/or NSAIDs to help patients in monitoring their own daily and cumulative doses. Comprehensive educational efforts are required to alert patients to the dangers of many over-the-counter analgesics and to inform them of appropriate doses and how to calculate them. Many patients consider over-the-counter products “harmless” and may take these agents casually. Patient education should include “do ask, do tell,” such that patients understand the importance of discussing with their clinicians all drugs they take.
Current guidelines and pain management in specific populations
When it comes to pain management, there is no lack of literature, including consensus statements and guidelines. Yet, pain is undertreated. Up to 27% of people with constant or daily musculoskeletal pain never seek treatment and many people with chronic pain seek medical help for the first time only after a year or more of pain.115 It may be inferred that many people feel pain as something they have to live with or that clinicians are unable to treat pain effectively. Between 28% and 54% of patients with musculoskeletal pain under medical care do not take any prescription analgesics.115 Further, patients may have serious concerns about analgesics; for example, 65%–77% of pain patients considering opioid analgesics have fears of tolerance or addiction.115
Many guidelines for the management of pain in specific populations exist.27,29,79,81,116–127 These guidelines are largely evidence-based documents, but at times the absence of evidence is construed as the evidence of absence. Important topics in pain management, such as, but not limited to, the transition from acute to chronic pain, are not addressed by the guidelines. In general, the guidelines tend to stress avoidance of adverse events at the expense of efficacy in the treatment of moderate to severe pain. The American Heart Association scientific statement recommends a stepped-care approach to pharmacological therapy for musculoskeletal pain patients with known cardiovascular disease or at risk for ischemic heart disease that emphasizes avoidance of potential risk at the expense of pain relief.29
Elderly patients
Chronic pain is both common and especially challenging to treat in geriatric patients, who often suffer from comorbidities. Chronic pain adversely affects the quality of life, mobility, and mood, and may limit daily activities and social pursuits in patients of all ages, but younger patients may be more resilient or better able to cope with these limitations than older patients. According to the most recent guidelines issued by The American Geriatrics Society, NSAIDs for the treatment of chronic pain should be avoided in patients aged 75 years or older; NSAIDs should be “considered rarely, and with extreme caution, in highly selected individuals.”27 Paracetamol should be considered as the initial and ongoing therapy of choice except for patients with a known liver disease. The maximum recommended daily dose of paracetamol is 4 g/24 hours and should not be exceeded. This maximum daily intake must include hidden sources in other medications. All patients with moderate to severe pain, pain-related functional impairment, or diminished quality of life due to pain should be considered for opioid therapy.27
Overview on experience with fixed-dose tramadol/paracetamol in the treatment of moderate to severe pain in nonacute conditions: differences to NSAIDs
NSAIDs are frequently prescribed analgesic agents but recent warnings – including a US Food and Drug Administration labeling proposal that all NSAIDs should be prescribed at the lowest possible doses for the shortest possible duration128 – have caused many clinicians to reevaluate these effective painkillers. Recently, new combination analgesic products based on scientifically reasonable design have been introduced to the market to offer effective analgesia with a good risk/benefit ratio. The combination product tramadol/ paracetamol may be an important aid for the treatment of acute and chronic pain syndromes (Table 5).
Table 5.
Comparison of nonsteroidal anti-inflammatory drugs (NSAIDs) with tramadol/paracetamol fixed-dose combination
| Selective and nonselective NSAIDs | Tramadol/paracetamol combination | |
|---|---|---|
| Pain severity | For mild to moderate pain | For moderate to severe pain |
| Clinical application | Wide, including rheumatic disorders, headaches, visceral pain | Wide, indicated for symptomatic relief of moderate to severe pain |
| Acute vs chronic pain | Both | Both |
| Neuropathic pain | No, exclusively for pain related to tissue damage and/or inflammation | Yes129,130 |
| Anti-inflammatory effect | Yes | No |
| Pediatric use | Yes | No |
| Geriatric use | With caution27 | May be appropriate27,150 |
| Use in patients with renal failure | No163 | Not recommended for severe renal insufficiency (creatinine clearance < 10 mL/min) but may be used at reduced dose in patients with moderate renal insufficiency (creatinine clearance between 10 and 30 mL/min) Tramadol is removed only very slowly by hemodialysis or hemofiltration, so post-dialysis administration to maintain analgesia is usually not required |
| Co-medications | Caution with diuretics, anticoagulants, angiotensin-converting-enzyme inhibitors | Caution with other central nervous system depressants, selective serotonin reuptake inhibitors164 |
| Use with concomitant opioids | May be synergistic86 | Overdose considerations |
| Use with anticonvulsants | Not known | Not known |
Pain involving multiple mechanisms, can be safely and effectively treated with combination analgesics, for example, tramadol/paracetamol.129,130 However, there are few direct comparative studies of combination products – for instance, codeine/paracetamol versus tramadol/paracetamol.131–133
Long-term pain management recommendations often feature NSAIDs as a first-line treatment for rheumatic diseases,134,135 with added opioid combination analgesics for flares.136–138 A possible new paradigm would be to treat pain first with opioid combination analgesics then use NSAIDs to manage flares. Table 6 summarizes the strengths and weaknesses of NSAIDs versus tramadol/paracetamol fixed-dose combination products.
Table 6.
Strengths and weakness of tramadol/paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs)
| NSAIDs | Tramadol/paracetamol | |
|---|---|---|
| Strengths |
|
|
| Weaknesses |
|
|
Recent guidelines for pain management and the position of paracetamol, NSAIDs, and fixed-dose combinations such as tramadol/paracetamol are shown in Table 7.
Table 7.
Summary of guidelines and recommendations for paracetamol (APAP), nonsteroidal anti-inflammatory drugs (NSAIDs), and combination products such as tramadol (tram)/paracetamol
| Guideline | APAP | NSAIDs | Combination (tram/APAP) | Comments |
|---|---|---|---|---|
| Osteoarthritis (OA) | ||||
| Management of OA | 1st | (Yes) | Yes | NSAIDS for anti-inflammatory action |
| Altman overview117 | ||||
| Early management of OA | 1st | (2nd) | 3rd | Oral NSAIDs at their lowest effective dose; long-term use should be avoided |
| Altman overview118 | ||||
| NICE OA guideline81 | 1st | 2nd with PPI | – | Oral NSAIDs/COX-2 inhibitors should be used at the lowest effective dose for the shortest possible period of time |
| OARSI guidelines79 | 1st | (2nd) | – | Oral NSAIDs at lowest effective dose; long-term use should be avoided |
| ACR Guidelines165 | ||||
| Hand OA | No | 1st | – | Topical or oral NSAIDs; topical NSAIDs for persons ≥75 years of age recommended |
| Knee OA | 1st | 2nd | – | Health care providers should be aware of the warnings and precautions associated with topical and oral NSAIDs |
| Hip OA | 1st | 2nd | – | Oral NSAIDs; no recommendation on topical NSAIDs |
| Rheumatoid arthritis (RA) | ||||
| NICE RA guideline119 | 1st | (2nd + PPI) | 1st (compound analgesics in general) | Oral NSAIDs/COX-2 inhibitors should be used at the lowest effective dose for the shortest possible period of time |
| BSR guidelines for early RA120 | 2nd (as add-on) | (1st) | 2nd (as add-on) | Long-term use of NSAIDs at lowest effective dose. At present, the use of single or compound analgesics or anti-inflammatory drugs (including coxibs) has to be settled with each individual patient No clear recommendations |
| BSR guidelines for long-term treatment of RA121 | – | 2nd as add-on with PPI | – | No clear recommendations |
| EULAR recommendations early arthritis122 | – | (Yes) | – | NSAIDs after careful evaluation of gastrointestinal, renal, and cardiovascular status |
| Fibromyalgia | ||||
| EULAR recommendations for fibromyalgia123 | Yes | – | – | Tramadol is one of the analgesics of choice |
| APS guidelines for fibromyalgia124 | No | No | 3rd | Tricyclic antidepressants first, serotonin reuptake inhibitors (SSRIs) alone or in combination with tricyclics second. Paracetamol not recommended as monotherapy, only in combination |
| Low back pain | ||||
| European guidelines for chronic nonspecific low back pain125 | – | (Yes) | (Yes) | NSAIDs should only be used for exacerbations or short-term periods (up to 3 months) |
| APS/ACP guidelines126 | 1st | (1st) | – | Oral NSAIDs at their lowest effective dose, for the shortest possible time required |
| NICE. Low back pain guideline127 | 1st | 2nd (+ PPI for patients aged > 45 years) | – | Weak opioids and strong opioids are suggested for more severe pain, but no combinations |
| Musculoskeletal pain | ||||
| Schnitzer, guidelines for chronic musculoskeletal pain116 Osteoarthritis |
1st | No or 2nd | 2nd | NSAIDs not for long-term use or in patients with risk factors; second for short-term use |
| Low back pain | 2nd | 1st | Young, healthy individuals could receive NSAIDs alone or at a reduced dose combined with paracetamol/tramadol | |
| following injury | 2nd as add-on | (1st) | 3rd | |
| Rehabilitation | 1st | 1st for pain in motion and for inflammation | 2nd as add-on | |
| Specific patient populations | ||||
| AGS geriatric guidelines27 | 1st | (2nd) + PPI or misoprostol | (2nd) | For paracetamol, maximum daily recommended dosages of 4 g per 24 hours should not be exceeded and must include “hidden sources” Nonselective NSAIDs and COX-2 selective inhibitors may be considered rarely, and with extreme caution, in highly selected individuals All patients with moderate to severe pain, pain-related functional impairment, or diminished quality of life due to pain should be considered for opioid therapy Maximal safe doses of paracetamol or NSAIDs should not be exceeded when using fixed-dose opioid combination agents |
| AHA guidelines29 | 1st | (3rd) | – | NSAIDs at their lowest effective dose + ASA 81 mg and PPI for patients at increased risk of thrombotic events |
| Neuropathic pain | ||||
| Dworkin et al166 | – | – | – | Tramadol is recommended as second-line treatment Standard treatments such as NSAIDs and paracetamol have no proven efficacy against neuropathic pain although they are frequently prescribed for patients with neuropathic pain167 |
Notes: –, not mentioned in guideline; 1st, first-line therapy; 2nd, second-line therapy; 3rd, third-line therapy; NO, not recommended; Yes, recommended but not first-, second-, or third-line recommendation; (Yes), recommended with caution.
Abbreviations: ACR, American College of Rheumatology; ACP, American College of Physicians; AGS, The American Geriatrics Society; AHA, American Heart Association; APS, American Pain Society; ASA, acetylsalicylic acid; BSR, British Society for Rheumatology; COX, cyclooxygenase; coxibs, selective COX-2 inhibitors; EULAR, European League Against Rheumatism; NICE, National Institute for Health and Excellence; PPI, proton pump inhibitor.
Consensus statements
The group arrived at several consensus statements. These follow, grouped by topic.
Pain management
There are many reasons why pain management is complex, including the classification of pain, mechanisms, knowledge, individualization, lack of universally accepted guidelines, social and psychological factors, as well as various influences from the health care system itself. Nevertheless, not treating pain is not an option.
Individualization of treatment in patients suffering from moderate to severe pain should be the ultimate goal of the health care team.
Pain management guidelines must take into consideration the type of pain, its intensity, the particular patient characteristics, and expected duration of treatment. This requires a multidimensional approach, which creates difficulty in making generalized recommendations.
Many evidence-based guidelines for pain management are available, but none is universally accepted by all health care providers. These guidelines may benefit by addressing topics such as the chronicity of pain, barriers to treatment, patient preferences influencing pain therapy, and practical clinical considerations. Current guidelines mostly contain strong evidence for pharmacological approaches; however, they would benefit from the addition of considerations related to the evidence or absence of evidence of risks of drugs and inclusion of nonpharmacological treatment options.
The use of NSAIDs and paracetamol in chronic pain management
NSAIDs and paracetamol are commonly used and commonly recommended agents for the management of pain and are helpful for many patients. However, they are not without potential risks, especially in the elderly and in patients with renal, gastrointestinal, or cardiovascular disease. High doses and long-term use of NSAIDs to manage moderate to severe pain have been associated with tolerability issues, including serious adverse events.
Fixed-dose combinations provide a multi-mechanistic analgesic approach. Clinical studies have demonstrated effective management of various types of moderate to severe pain with mostly good tolerability.
A new approach to managing arthritis-related pain is to consider the long-term use of low-dose combination products for moderate to severe pain, and reserving NSAID use for acute flares related to inflammation.
The role of fixed-dose combinations in chronic pain management using tramadol/paracetamol as an example
Tramadol/Paracetamol may offer distinct advantages in certain patient populations and for certain types of pain, compared with high doses of NSAIDs or paracetamol or when NSAIDs or paracetamol are expected to be used for long durations. However, long-term studies of fixed-dose combinations are required.
Potential advantages of a fixed-dose tramadol/paracetamol analgesic product include a broader analgesic spectrum, a complementary pharmacokinetic profile, potentially synergistic analgesic effect, greater convenience (possibly resulting in better compliance, thus, improved therapy), and an improved ratio of efficacy to adverse effects.
Conclusion
Pain management is a global challenge to clinicians and, despite the plethora of evidence-based guidelines, all analgesic options must be individually assessed and weighed for specific risks and benefits in a given patient. Many effective analgesics exist but are associated with adverse events. NSAIDs and paracetamol are effective pain relievers, but recent studies have raised safety concerns, particularly when these agents are used at high doses, long-term, or in special patient populations. Opioid analgesics are effective but are associated with adverse events as well as concerns over tolerance and addiction. Finding an analgesic product that offers both effective pain relief and a good safety profile has led to increasing interest in combination products.
Combination agents may offer analgesic synergy that allows them to provide effective analgesia at reduced doses. However, careful study of combination agents is warranted, as such combination products might also exacerbate side effects. New fixed-dose combination products may offer an improved method of treating the newly recognized multi-mechanistic nature of pain. Studies of fixed-dose combinations such as tramadol/paracetamol for the treatment of chronic pain syndromes are promising, showing safe and effective pain relief with good tolerability and safety profiles.
A new practice paradigm may be to use low-dose paracetamol or fixed-dose combination products, and NSAIDs to manage acute flares. However, further studies are warranted to establish the long-term efficacy and safety of these products.
Acknowledgments
The meeting was supported by Grünenthal GmbH, Aachen, Germany. Thanks go to Jo Ann LeQuang (LeQ Medical, USA), Birgit Brett (Brett Medical Writing, Germany), and Elke Grosselindemann (Brett Medical Writing, Australia) for editorial assistance and publication coordination. All costs associated with the publication of the manuscript were met by Grünenthal GmbH, Aachen, Germany.
Footnotes
Disclosure
JV Pergolizzi received consultancy honoraria from Grünenthal GmbH, Baxter, Endo Pharmaceuticals, Purdue Pharma, Janssen, and Hospira. M van de Laar received consultancy honoraria from Merck Netherlands, Pfizer Europa, and Grünenthal GmbH, and speaker honoraria from Pfizer Europa. R Langford has received honoraria and traveling expenses for speaking engagements and consultancy activities from Grünenthal, Janssen-Cilag, Napp/Mundipharma/Purdue, Novartis, Johnson and Johnson/ Ortho-McNeil USA, Pfizer, Bristol Myers Squibb, Javelin Pharmaceuticals, and AstraZeneca. HU Mellinghoff received consultancy honoraria from Grünenthal GmbH. I Moron Merchante received consultancy honoraria from Boehringer Ingelheim, Grünenthal GmbH, Merck Sharp and Dohme Corporation, and Takeda Pharmaceuticals Europe and has received lecture fees from Almirall, Astra-Zeneca, Boehringer Ingelheim, Bristol Myers Squibb, Esteve, Grünenthal GmbH, Eli Lilly and Company, Merck Sharp and Dohme Corporation, Novartis, and Sanofi-Aventis. S Nalamachu has received consultancy honoraria or research grants from the following companies in the past 5 years: Grünenthal GmbH, Johnson and Johnson, Endo Pharmaceuticals, Cephalon, Alphapharma, King Pharmaceuticals, Allergan, ProStakan, and Covidien. J O’Brien received consultancy honoraria from Grünenthal GmbH. S Perrot received consultancy honoraria from Grünenthal GmbH. RB Raffa is a speaker, consultant, and/or basic science investigator for several pharmaceutical companies involved in analgesic research but receives no royalty (cash or otherwise) from the sale of any product; he received consultancy honoraria from Grünenthal GmbH.
List of conference participants
Participants in the meeting were (authors are indicated with an asterisk): Joseph Pergolizzi* (Johns Hopkins University, Baltimore, Maryland, USA and the Association of Chronic Pain Patients, Houston, Texas, USA); Mart van de Laar* (Arthritis Center Twente, Enschede, The Netherlands); Richard Langford* (Anaesthetics Laboratory, St Bartholomew’s Hospital, London, UK); Hans-Ulrich Mellinghoff* (Department of Endocrinology, Diabetology and Osteology, Kantonsspital St Gallen, Switzerland); Ignacio Morón Merchante* (Centro de Salud Universitario Goya, Madrid, Spain); Srinivas Nalamachu* (Kansas University Medical Center, Kansas City, Missouri and International Clinic Research, Leawood, Kansas, USA); Joanne O’Brien* (Beaumont Hospital, Dublin, Ireland); Serge Perrot* (Internal Medicine and Therapeutics Department, Hôtel Dieu Hospital, Paris Descartes University, France); Robert B Raffa* (Department of Pharmaceutical Sciences, Temple University School of Pharmacy, Philadelphia, USA); Birgit Brett (Brett Medical Writing, Pulheim, Germany); Karla Schwenke (Medical Affairs, Grünenthal GmbH, Aachen Germany); and Detlef von Zabern (Medical Affairs, Grünenthal GmbH, Aachen, Germany).
References
- 1.World Health Organization (WHO) WHO’s pain ladder [web page on the Internet] Geneva: WHO; 2012. [Accessed May 12, 2011]. Available from: http://www.who.int/cancer/palliative/painladder/en/ [Google Scholar]
- 2.WHO. Achieving Balance in National Opioids Control Policy: Guidelines for Assessment. Geneva: WHO; 2000. [Accessed May 13, 2011]. Available from: whqlibdoc.who.int/hq/2000/who_edm_qsm_20004.pdf. [Google Scholar]
- 3.WHO. National Cancer Control Programmes: Policies and Managerial Guidelines. 2nd ed. Geneva: WHO; 2002. [Accessed May 13, 2010]. Available from: http://www.who.int/cancer/media/en/408.pdf. [Google Scholar]
- 4.WHO. Cancer Pain Relief with a Guide to Opioid Availability. 2nd ed. Geneva: WHO; 1996. [Accessed May 12, 2011]. Available from: whqlibdoc.who.int/publications/9241544821.pdf. [Google Scholar]
- 5.Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg. 2007;105(1):205–221. doi: 10.1213/01.ane.0000268145.52345.55. [DOI] [PubMed] [Google Scholar]
- 6.Hall JK, Boswell MV. Ethics, law, and pain management as a patient right. Pain Physician. 2009;12(3):499–506. [PubMed] [Google Scholar]
- 7.Gwyther L, Brennan F, Harding R. Advancing palliative care as a human right. J Pain Symptom Manage. 2009;38(5):767–774. doi: 10.1016/j.jpainsymman.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Fishman SM. Recognizing pain management as a human right: a first step. Anesth Analg. 2007;105(1):8–9. doi: 10.1213/01.ane.0000267526.37663.41. [DOI] [PubMed] [Google Scholar]
- 9.The Societal Impact of Pain. European positioning statement: chronic pain fundamental for European health. International multi-stakeholder platform acknowledges chronic pain as disease in its own-European policy-makers challenged to respond. 2012. [Accessed on June 27, 2012]. Accessed from http://www.sip-platform.eu/sip-2012.html.
- 10.Varrassi G, Müller-Schwefe G, Pergolizzi J, et al. Pharmacological treatment of chronic pain – the need for CHANGE. Curr Med Res Opin. 2010;26(5):1231–1245. doi: 10.1185/03007991003689175. [DOI] [PubMed] [Google Scholar]
- 11.Claxton RN, Blackhall L, Weisbord SD, Holley JL. Undertreatment of symptoms in patients on maintenance hemodialysis. J Pain Symptom Manage. 2010;39(2):211–218. doi: 10.1016/j.jpainsymman.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Ott BB. Progress in ethical decision making in the care of the dying. Dimens Crit Care Nurs. 2010;29(2):73–80. doi: 10.1097/DCC.0b013e3181c9301a. [DOI] [PubMed] [Google Scholar]
- 13.Fontana JS. The social and political forces affecting prescribing practices for chronic pain. J Prof Nurs. 2008;24(1):30–35. doi: 10.1016/j.profnurs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Nathan JI. Chronic pain treatment: a high moral imperative with offsetting personal risks for the physician – a medical student’s perspective. Pain Pract. 2010;9(2):155–163. doi: 10.1111/j.1533-2500.2008.00257.x. [DOI] [PubMed] [Google Scholar]
- 15.Rejeh N, Vaismoradi M. Perspectives and experiences of elective surgery patients regarding pain management. Nurs Health Sci. 2010;12(1):67–73. doi: 10.1111/j.1442-2018.2009.00488.x. [DOI] [PubMed] [Google Scholar]
- 16.Benyamin RM, Datta S, Falco FJ. A perfect storm in interventional pain management: regulated, but unbalanced. Pain Physician. 2010;13(2):109–116. [PubMed] [Google Scholar]
- 17.Ferrell B. Ethical perspectives on pain and suffering. Pain Manage Nur. 2005;63(3):83–90. doi: 10.1016/j.pmn.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Rejeh N, Ahmadi F, Mohamadi E, Anoosheh M, Kazemnejad A. Ethical challenges in pain management post-surgery. Nurs Ethics. 2009;16(2):161–171. doi: 10.1177/0969733008100077. [DOI] [PubMed] [Google Scholar]
- 19.Macpherson C. Undertreating pain violates ethical principles. J Med Ethics. 2009;35(10):603–606. doi: 10.1136/jme.2008.026443. [DOI] [PubMed] [Google Scholar]
- 20.Ferrell BR. The role of ethics committees in responding to the moral outrage of unrelieved pain. Bioethics Forum. 1997;13(3):11–16. [PubMed] [Google Scholar]
- 21.König H, Heider D, Lehnert T, et al. ESEMeD/MHEDEA 2000 investigators. Health status of the advanced elderly in six European countries: results from a representative survey using EQ-5D and SF-12. Health Qual Life Outcomes. 2010;8:143. doi: 10.1186/1477-7525-8-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Gerbershagen K, Gerbershagen H, Lutz J, et al. Pain prevalence and risk distribution among inpatients in a German teaching hospital. Clin J Pain. 2009;25(5):431–437. doi: 10.1097/AJP.0b013e31819ff515. [DOI] [PubMed] [Google Scholar]
- 24.Vlajkovic G, Sindjelic R, Stefanovic I. Ketorolac as a pre-emptive analgesic in retinal detachment surgery: a prospective, randomized clinical trial. Int J Clin Pharmacol Ther. 2007 May;45(5):259–263. doi: 10.5414/cpp45259. [DOI] [PubMed] [Google Scholar]
- 25.Weiner K. Pain Issues: Pain is an Epidemic. Sonora, CA: American Academy of Pain Management; 2001. [Accessed May 13, 2011]. Available from: http://www.aapainmanage.org/literature/Articles/PainAnEpidemic.pdf. [Google Scholar]
- 26.Christo PJ, Li S, Gibson SJ, Fine P, Hameed H. Effective treatments for pain in the older patient. Curr Pain Headache Rep. 2011;15(1):22–34. doi: 10.1007/s11916-010-0164-0. [DOI] [PubMed] [Google Scholar]
- 27.American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. [Accessed December 6, 2010];J Am Geriatr Soc. 2009 57(8):1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. http://www.cdc.gov/nchs/data/hus/hus06.pdf. [DOI] [PubMed] [Google Scholar]
- 28.National Center for Health Statistics. Health, United States, 2006: with Chartbook on Trends in the Health of Americans. Hyattsville, MD: National Center for Health Statistics; 2006. [Accessed December 6, 2010]. Available from: http://www.cdc.gov/nchs/data/hus/hus06.pdf. [Google Scholar]
- 29.Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA American Heart Association. Use of nonsteroidal anti-inflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634–1642. doi: 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]
- 30.Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992;260(1):275–285. [PubMed] [Google Scholar]
- 31.Tallarida RJ, Raffa RB. Testing for synergism over a range of fixed ratio drug combinations: replacing the isobologram. Life Sci. 1996;58(2):PL 23–PL 28. doi: 10.1016/0024-3205(95)02271-6. [DOI] [PubMed] [Google Scholar]
- 32.Desmeules JA, Piguet V, Collart L, Dayer P. Contribution of mono-aminergic modulation to the analgesic effect of tramadol. Br J Clin Pharmacol. 1996;41(1):7–12. doi: 10.1111/j.1365-2125.1996.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 33.Filitz J, Ihmsen H, Günther W, et al. Supra-additive effects of tramadol and acetaminophen in a human pain model. Pain. 2008;136(3):262–270. doi: 10.1016/j.pain.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 34.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9(8):807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 35.Loeser JD, Treede RD. The Kyoto protocol of IASP basic pain terminology. Pain. 2008;137(3):473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 36.International Association for the Study of Pain (IASP) IASP taxonomy [web page on the Internet] Seattle, WA: IASP; nd. [Accessed June 10, 2012]. [updated May 22, 2012]. http://www.iasp-pain.org/Content/NavigationMenu/GeneralResourceLinks/PainDefinitions/default.htm. [Google Scholar]
- 37.Woolf CJ American College of Physicians; American Physiological Society. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441–451. doi: 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- 38.Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci. 2002;5(Suppl):1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- 39.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 40.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 41.Mogil JS, Yu L, Basbaum AI. Pain genes?: natural variation and transgenic mutants. Annu Rev Neurosci. 2000;23:777–811. doi: 10.1146/annurev.neuro.23.1.777. [DOI] [PubMed] [Google Scholar]
- 42.Gold MS, Gebhart GF. Nociceptor sensitisation in pain pathogenesis. Nat Med. 2010;16(11):1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruehl S. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology. 2010;113(3):713–725. doi: 10.1097/ALN.0b013e3181e3db38. [DOI] [PubMed] [Google Scholar]
- 44.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16(11):1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graven-Nielsen T, Arednt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol. 2010;6(10):599–606. doi: 10.1038/nrrheum.2010.107. [DOI] [PubMed] [Google Scholar]
- 46.Holmes A, Williamson O, Hogg M, et al. Predictors of pain severity 3 months after serious injury. Pain Med. 2010;11(7):990–1000. doi: 10.1111/j.1526-4637.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 47.Buvanendran A, Ali A, Stoub TR, Kroin JS, Tuman KJ. Brain activity associated with chronic cancer pain. Pain Phys. 2010;13(5):E337–E342. [PubMed] [Google Scholar]
- 48.Perrot S, Javier RM, Marty M, Le Jeunne C, Laroche F. CEDR (Cercle d’Etude de la Douleur en Rhumatologie France), French Rheumatological Society, Pain Study Section Is there any evidence to support the use of anti-depressants in painful rheumatological conditions? Systematic review of pharmacological and clinical studies. Rheumatology (Oxford) 2008;47(8):1117–1123. doi: 10.1093/rheumatology/ken110. [DOI] [PubMed] [Google Scholar]
- 49.Chappell AS, Ossanna MJ, Liu-Seifert H, et al. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain. 2009;146(3):253–260. doi: 10.1016/j.pain.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 50.Jung AC, Staigler T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. J Gen Intern Med. 1997;12(6):384–389. doi: 10.1046/j.1525-1497.1997.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mease PJ. Further strategies for treating fibromyalgia: the role of serotonin and norepinephrine reuptake inhibitors. Am J Med. 2009;122(12 Suppl):S44–S55. doi: 10.1016/j.amjmed.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Perrot S, Allaert FA, Concas V, Laroche F. “When will I recover?” A national survey on patients’ and physicians’ expectations concerning the recovery time for acute back pain. Eur Spine J. 2009;18(3):419–429. doi: 10.1007/s00586-008-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Javier RM, Perrot S. Do men and women experience pain differently? What are the implications for the rheumatologist? Joint Bone Spine. 2010;77(3):198–200. doi: 10.1016/j.jbspin.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Booth M. Opium: A History. New York, NY: Simon and Schuster; 1996. [Google Scholar]
- 55.Moulin DE, Clark AJ, Gilron I, et al. Canadian Pain Society. Pharmacological management of chronic neuropathic pain – consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13–21. doi: 10.1155/2007/730785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Attal N, Cruccu G, Haanpää M, et al. EFNS Task Force. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13(11):1153–1169. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 57.Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 58.Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 59.Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Can Fam Physician. 2010;56(6):514–517. [PMC free article] [PubMed] [Google Scholar]
- 60.Smith HS. Potential analgesic mechanisms of acetaminophen. Pain Physician. 2009;12(1):269–280. [PubMed] [Google Scholar]
- 61.American Academy of Pediatrics. Committee on Drugs. Acetaminophen toxicity in children. Pediatrics. 2001;108(4):1020–1024. doi: 10.1542/peds.108.4.1020. [DOI] [PubMed] [Google Scholar]
- 62.James LP, Letzig L, Simpson PM, et al. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos. 2009;37(8):1779–1784. doi: 10.1124/dmd.108.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryder SD, Beckingham IJ. ABC of diseases of liver, pancreas and biliary system. Other causes of parenchymal liver disease. BMJ. 2001;322(7281):290–292. doi: 10.1136/bmj.322.7281.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Griffin SL. 2008 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 26th Annual Report. Clin Toxicol. 2009;47(10):911–1084. doi: 10.3109/15563650903438566. [DOI] [PubMed] [Google Scholar]
- 65.Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007;102(11):2459–2463. doi: 10.1111/j.1572-0241.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- 66.Wilson SL, Poulter NR. The effect of non-steroidal anti-inflammatory drugs and other commonly used non-narcotic analgesics on blood pressure level in adults. J Hypertens. 2006;24(8):1457–1469. doi: 10.1097/01.hjh.0000239278.82196.a5. [DOI] [PubMed] [Google Scholar]
- 67.Forman JP, Rimm EB, Curhan GC. Frequency of analgesic use and risk of hypertension among men. Arch Intern Med. 2007;167(4):394–399. doi: 10.1001/archinte.167.4.394. [DOI] [PubMed] [Google Scholar]
- 68.Sudano I, Flammer AJ, Périat D, et al. Acetaminophen increases blood pressure in patients with coronary artery disease. Circulation. 2010;122(18):1789–1796. doi: 10.1161/CIRCULATIONAHA.110.956490. [DOI] [PubMed] [Google Scholar]
- 69.Montgomery B. Does paracetamol cause hypertension? BMJ. 2008;336(7654):1190–1191. doi: 10.1136/bmj.39526.654016.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Montgomery BD. Analgesic use and risk of hypertension: concern about bias. Arch Intern Med. 2007;167(21):2368–2369. doi: 10.1001/archinte.167.21.2368-b. [DOI] [PubMed] [Google Scholar]
- 71.White WB, Campbell P. Blood pressure destabilization on nonsteroidal anti-inflammatory agents: acetaminophen exposed? Circulation. 2010;122(18):1779–1781. doi: 10.1161/CIRCULATIONAHA.110.984054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rao P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci. 2008;11(2):81s–110s. doi: 10.18433/j3t886. [DOI] [PubMed] [Google Scholar]
- 73.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110(5–6):255–258. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 74.Leuppi JD, Schnyder P, Hartmann K, Reinhart WH, Kuhn M. Drug-induced bronchospasm: analysis of 187 spontaneously reported cases. Respiration. 2001;68(4):345–351. doi: 10.1159/000050525. [DOI] [PubMed] [Google Scholar]
- 75.van der Linden MW, van der Bij S, Welsing P, Kupiers EJ, Herings RM. The balance between severe cardiovascular and gastrointestinal events among users of selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis. 2009;68(5):668–673. doi: 10.1136/ard.2007.087254. [DOI] [PubMed] [Google Scholar]
- 76.Ng SC, Chan FK. NSAID-induced gastrointestinal and cardiovascular injury. Curr Opin Gastroenterol. 2010;26(6):611–617. doi: 10.1097/MOG.0b013e32833e91eb. [DOI] [PubMed] [Google Scholar]
- 77.Lanas A, Perez-Aisa MA, Feu F, et al. Investigators of the Asociación Española de Gastroenterología (AEG) A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal anti-inflammatory drug use. Am J Gastroenterol. 2005;100(8):1685–1693. doi: 10.1111/j.1572-0241.2005.41833.x. [DOI] [PubMed] [Google Scholar]
- 78.Schjerning Olsen AM, Fosbøl EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: a nationwide cohort study. Circulation. 2011;123:2226–2235. doi: 10.1161/CIRCULATIONAHA.110.004671. [DOI] [PubMed] [Google Scholar]
- 79.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 80.American College of Rheumatology Ad Hoc Group on Use of Selective and Nonselective Nonsteroidal Anti-inflammatory Drugs. Recommendations for use of selective and nonselective nonsteroidal anti-inflammatory drugs: an American College of Rheumatology white paper. Arthritis Rheum. 2008;59(8):1058–1073. doi: 10.1002/art.23929. [DOI] [PubMed] [Google Scholar]
- 81.National Institute for Health and Excellence (NICE) Osteoarthritis: The Care and Management of Osteoarthritis in Adults. London: NICE; 2008. [Accessed December 15, 2010]. (NICE clinical guideline 59). Available from: http://www.nice.org.uk/nicemedia/pdf/CG59NICEguideline.pdf. [Google Scholar]
- 82.Lanza FL, Chan FK, Quigley EM Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104(3):728–738. doi: 10.1038/ajg.2009.115. [DOI] [PubMed] [Google Scholar]
- 83.Bhatt DL, Scheiman J, Abraham NS, et al. ACCF Task Force ACCF/ACG/AHA. 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52(18):1502–1517. doi: 10.1016/j.jacc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 84.Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Desai JC, Sanyal SM, Goo T, et al. Primary prevention of adverse gastroduodenal effects from short-term use of non-steroidal anti-inflammatory drugs by omeprazole 20 mg in healthy subjects: a randomized, double-blind, placebo-controlled study. Dig Dis Sci. 2008;53(8):2059–2065. doi: 10.1007/s10620-007-0127-4. [DOI] [PubMed] [Google Scholar]
- 86.Raffa RB. Pharmacology of oral combination analgesics: rational therapy for pain. J Clin Pharm Ther. 2001;26(4):257–264. doi: 10.1046/j.1365-2710.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 87.Raffa RB, Pergolizzi JV, Segarnick DJ, Tallarida RJ. Oxycodone combinations for pain relief. Drugs Today (Barc) 2010;46(6):379–398. doi: 10.1358/dot.2010.46.6.1470106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tallarida RJ, Cowan A, Raffa RB. Antinociceptive synergy, additivity, and subaddivity with combinations of oral glucosamine plus nonopioid analgesics in mice. J Pharmacol Exp Ther. 2003;307(2):699–704. doi: 10.1124/jpet.103.054320. [DOI] [PubMed] [Google Scholar]
- 89.Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298(3):865–872. [PubMed] [Google Scholar]
- 90.Tallarida RJ, Stone DJ, Jr, Raffa RB. Efficient designs for studying synergistic drug combinations. Life Sci. 1997;61(26):PL 417–PL 425. doi: 10.1016/s0024-3205(97)01030-8. [DOI] [PubMed] [Google Scholar]
- 91.Tallarida RJ. Interactions between drugs and occupied receptors. Pharmacol Ther. 2007;113(1):197–209. doi: 10.1016/j.pharmthera.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tallarida RJ. An overview of drug combination analysis with isobolograms. Perspectives in pharmacology. J Pharmacol Exp Ther. 2006;319(1):1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- 93.Loewe L. Antagonisms and antagonists. Pharmacol Rev. 1957;9(2):237–242. [PubMed] [Google Scholar]
- 94.Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3(6):285–290. [PubMed] [Google Scholar]
- 95.Tallarida RJ. Drug Synergism and Dose-Effect Data Analysis. Boca Raton, FL: CRC/Chapman-Hall; 2000. [Google Scholar]
- 96.Grabovsky Y, Tallardia RJ. Isobologrphic analysis for combinations of full and partial agonists: curved isoboles. J Pharmacol Exp Ther. 2004;310(3):981–986. doi: 10.1124/jpet.104.067264. [DOI] [PubMed] [Google Scholar]
- 97.Braverman AS, Tallarida RJ, Ruggieri MR., Sr The use of occupation isoboles for analysis of a response mediated by two receptors: M2 and M3 muscarinic receptor subtype-induced mouse stomach contraction. J Pharmacol Exp Ther. 2008;325(3):954–960. doi: 10.1124/jpet.108.137018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ide S, Minami M, Ishihara K, Uhl GR, Sora I, Ikeda K. Mu opioid receptor-dependent and independent components in effects of tramadol. Neuropharmacology. 2006;51(3):651–658. doi: 10.1016/j.neuropharm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 99.Christoph T, Kögel B, Strassburger W, Schug SA. Tramadol has a better potency ratio relative to morphine in neuropathic than in nociceptive pain models. Drugs R D. 2007;8(1):51–57. doi: 10.2165/00126839-200708010-00005. [DOI] [PubMed] [Google Scholar]
- 100.Zaldiar® product monograph. Aachen: Gruenenthal GmbH; 2002. [Accessed June 27, 2012]. Available from: http://www.zaldiar.com/zal/en_EN/pdf/zaldiar_product_monograph.pdf. [Google Scholar]
- 101.Bonnefont J, Courade JP, Alloui A, Eschalier A. Antinociceptive mechanism of action of paracetamol. Drugs. 2003;63(Spec No 2):1–4. French. [PubMed] [Google Scholar]
- 102.Hawton K, Ware C, Mistry H, et al. Paracetamol self-poisoning: characteristics, prevention and harm reduction. Br J Psychiatry. 1996;168(1):43–48. doi: 10.1192/bjp.168.1.43. [DOI] [PubMed] [Google Scholar]
- 103.Kuffner EK, Dart RC, Bogdan GM, Hill RE, Casper E, Darton L. Effect of maximal daily doses of acetaminophen on the liver of alcoholic patients: a randomized, double-blind, placebo-controlled trial. Arch Intern Med. 2001;161(18):2247–2252. doi: 10.1001/archinte.161.18.2247. [DOI] [PubMed] [Google Scholar]
- 104.Gregoire N, Hovsepian L, Gualano V, Evene E, Dufour G, Gendron A. Safety and pharmacokinetics of paracetamol following intravenous administration of 5 g during the first 24 h with a 2-g starting dose. Clin Pharmacol Ther. 2007;81(3):401–405. doi: 10.1038/sj.clpt.6100064. [DOI] [PubMed] [Google Scholar]
- 105.McClellan K, Scott LJ. Tramadol/paracetamol. Drugs. 2003;63(11):1079–1086. doi: 10.2165/00003495-200363110-00007. [DOI] [PubMed] [Google Scholar]
- 106.Dhillon S. Tramadol/paracetamol fixed-dose combination: a review of its use in the management of moderate to severe pain. Clin Drug Investig. 2010;30(10):711–738. doi: 10.2165/11205830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 107.Scheiman JM. Balancing risks and benefits of cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs. Gastroenterol Clin N Am. 2009;38(2):305–314. doi: 10.1016/j.gtc.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 108.van der Linden MW, Gaugris S, Kuipers EJ, et al. COX-2 inhibitors: complex association with lower risk of hospitalization for gastrointestinal events compared to traditional NSAIDs plus proton pump inhibitors. Pharmacoepidemiol Drug Saf. 2009;18(10):880–890. doi: 10.1002/pds.1782. [DOI] [PubMed] [Google Scholar]
- 109.Rahme E, Barkun AN, Toubouti Y, Scalera A, Rochon S, Lelorier J. Do proton-pump inhibitors confer additional gastrointesntinal protection in patients given celecoxib? Arthritis Rheum. 2007;57(5):748–755. doi: 10.1002/art.22764. [DOI] [PubMed] [Google Scholar]
- 110.Laine L. Proton pump inhibitor co-therapy with nonsteroidal anti-inflammatory drugs – nice or necessary? Rev Gastroenterol Disord. 2004;4(Suppl 4):S33–S41. [PubMed] [Google Scholar]
- 111.Chan FK, Wong VW, Suen BY, et al. Combination of a cyclo-oxygenase-2 inhibitor and a proton-pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: a double-blind, randomised trial. Lancet. 2007;369(9573):1621–1626. doi: 10.1016/S0140-6736(07)60749-1. [DOI] [PubMed] [Google Scholar]
- 112.Vonkeman HE, Fernandes RW, van der Palen J, van Roon EN, van de Laar MA. Proton-pump inhibitors are associated with a reduced risk for bleeding and perforated gastroduodenal ulcers attributable to non-steroidal anti-inflammatory drugs: a nested case-control study. Arthritis Res Ther. 2007;9(3):R52. doi: 10.1186/ar2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.US Food and Drug Administration (FDA) Safe Use Initiative: collaborating to reduce preventable harm from medications [web page on the Internet] Silver Spring, MD: FDA; nd. [Accessed May 13, 2011]. [updated April 26, 2012]. Available from: http://www.fda.gov/Drugs/DrugSafety/SafeUseInitiative/default.htm. [Google Scholar]
- 114.Wong VW, Chan FK. Cycolooxygenase-2 inhibitors in patients with high gastrointestinal risk: are we there yet? J Gastroenterol. 2009;44(Suppl 19):53–56. doi: 10.1007/s00535-008-2260-z. [DOI] [PubMed] [Google Scholar]
- 115.Woolf AD, Zeidler H, Haglund U, et al. Musculoskeletal pain in Europe: its impact and a comparison of population and medical perceptions of treatment in eight European countries. Ann Rheum Dis. 2004;63(4):342–347. doi: 10.1136/ard.2003.010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schnitzer TJ. Update on guidelines for the treatment of chronic musculoskeletal pain. Clin Rheumatol. 2006;25(Suppl 1):S22–S29. doi: 10.1007/s10067-006-0203-8. [DOI] [PubMed] [Google Scholar]
- 117.Altman RD. Practical considerations for the pharmacologic management of osteoarthritis. Am J Manag Care. 2009;15(8 Suppl):S236–S243. [PubMed] [Google Scholar]
- 118.Altman RD. Early management of osteoarthritis. Am J Manag Care. 2010;16(Suppl Management):S41–47. [PubMed] [Google Scholar]
- 119.NICE. Rheumatoid Arthritis: The Management of Rheumatoid Arthritis in Adults. London: NICE; 2009. [Accessed December 18, 2010]. (NICE clinical guideline 79). Available from: http://www.nice.org.uk/nicemedia/live/12131/43327/43327.pdf. [Google Scholar]
- 120.Luqmani R, Hennell S, Estrach C, et al. British Society for Rheumatology; British Health Professionals in Rheumatology Standards, Guidelines and Audit Working Group. British Society for Rheumatology and british health professionals in Rheumatology guideline for the management of rheumatoid arthritis (the first two years) Rheumatology (Oxford) 2006;45(9):1167–1169. doi: 10.1093/rheumatology/kel215a. [DOI] [PubMed] [Google Scholar]
- 121.Luqmani R, Hennell S, Estrach C, et al. British Society for Rheumatology; British Health Professionals in Rheumatology Standards, Guidelines and Audit Working Group. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of rheumatoid arthritis (after the first 2 years) Rheumatology (Oxford) 2009;48(4):436–439. doi: 10.1093/rheumatology/ken450a. [DOI] [PubMed] [Google Scholar]
- 122.Combe B, Landewe R, Lukas C, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2007;66(1):34–45. doi: 10.1136/ard.2005.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carville SF, Arendt-Nielsen S, Bliddal H EULAR. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67(4):536–541. doi: 10.1136/ard.2007.071522. [DOI] [PubMed] [Google Scholar]
- 124.Burckhardt C, Goldenberg D, Crofford L, et al. Guideline for the Management of Fibromyalgia Syndrome Pain in Adults and Children. 2005. [Accessed December 18, 2010]. (Clinical practice guideline 4). Available from: http://www.ampainsoc.org/library/pt_fibromyalgia.htm.
- 125.Airaksinen O, Brox JI, Cedraschi C, et al. COST B13 Working Group on Guidelines for Chronic Low Back Pain. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;15(Suppl 2):S192–300. doi: 10.1007/s00586-006-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chou R, Hoyt-Huffman L American Pain Society; American College of Physicians. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):505–514. doi: 10.7326/0003-4819-147-7-200710020-00008. [DOI] [PubMed] [Google Scholar]
- 127.NICE. Low Back Pain: Early Management of Persistent Non-Specific Low Back Pain. London: NICE; 2009. [Accessed December 18, 2010]. (NICE clinical guideline 88). Available from: http://www.nice.org.uk/nicemedia/live/11887/44343/44343.pdf. [Google Scholar]
- 128.FDA. Proposed NSAID package insert labeling template 1. Silver Spring, MD: FDA; nd. [Accessed December 18, 2010]. Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm106230.pdf. [Google Scholar]
- 129.Ko SH, Kwon HS, Yu JM, et al. Comparison of the efficacy and safety of tramadol/acetaminophen combination therapy and gabapentin in the treatment of painful diabetic neuropathy. Diabet Med. 2010;27(9):1033–1040. doi: 10.1111/j.1464-5491.2010.03054.x. [DOI] [PubMed] [Google Scholar]
- 130.Freeman R, Raskin P, Hewitt DJ, et al. CAPSS-237 Study Group. Randomized study of tramadol/acetaminophen versus placebo in painful diabetic peripheral neuropathy. Curr Med Res Opin. 2007;23(1):147–161. doi: 10.1185/030079906X162674. [DOI] [PubMed] [Google Scholar]
- 131.Mullican WS, Lacy JR TRAMAP-ANAG-006 Study Group. Tramadol/acetaminophen combination tablets and codeine/ acetaminophen combination capsules for the management of chronic pain: a comparative trial. Clin Ther. 2001;23(9):1429–1445. doi: 10.1016/s0149-2918(01)80118-1. [DOI] [PubMed] [Google Scholar]
- 132.Smith AB, Ravikumar TS, Kamin M, Jordan D, Xiang J, Rosenthal N CAPSS-115 Study Group. Combination tramadol plus acetaminophen for postsurgical pain. Am J Surg. 2004;187(4):521–527. doi: 10.1016/j.amjsurg.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 133.Pickering G, Estrade M, Dubray C. Comparative trial of tramadol/ paracetamol and codeine/paracetamol combination tablets on the vigilance of healthy volunteers. Fundam Clin Pharmacol. 2005;19(6):707–711. doi: 10.1111/j.1472-8206.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 134.Richmond J, Hunter D, Irrgang J, et al. American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of osteoarthritis (OA) of the knee. J Bone Joint Surg Am. 2010;92(4):990–993. doi: 10.2106/JBJS.I.00982. [DOI] [PubMed] [Google Scholar]
- 135.Cibulka MT, White DM, Woehrle J, et al. Hip pain and mobility deficits – hip osteoarthritis: clinical practice guidelines linked to the international classification of functioning, disability, and health from the orthopaedic section of the American Physical Therapy Association. J Orthop Sports Phys Ther. 2009 Apr;39(4):A1–A25. doi: 10.2519/jospt.2009.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schnitzer TJ. American College of Rheumatology Update of ACR guidelines for osteoarthritis: role of the coxibs. J Pain Symptom Manage. 2002;23(4 Suppl):S24–30. doi: 10.1016/s0885-3924(02)00372-x. discussion S31–34. [DOI] [PubMed] [Google Scholar]
- 137.Conaghan PG, Dickson J, Grant RL. Care and management of osteoarthritis in adults: summary of NICE guidance. BMJ. 2008;336(7642):502–503. doi: 10.1136/bmj.39490.608009.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang W, Doherty M, Arden N, et al. EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2005;64(5):669–681. doi: 10.1136/ard.2004.028886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fricke JR, Jr, Karim R, Jordan D, Rosenthal N. A double-blind, single-dose comparison of the analgesic efficacy of tramadol/acetaminophen combination tablets, hydrocodone/acetaminophen combination tablets, and placebo after oral surgery. Clin Ther. 2002;24(6):953–968. doi: 10.1016/s0149-2918(02)80010-8. [DOI] [PubMed] [Google Scholar]
- 140.Macleod AG, Ashford B, Voltz M, et al. Paracetamol versus paracetamol-codeine in the treatment of post-operative dental pain: a randomized, double-blind, prospective trial. Aust Den J. 2002;47(2):147–151. doi: 10.1111/j.1834-7819.2002.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 141.Edwards JE, McQuay HJ, Moore RA. Combination analgesic efficacy: individual patient data meta-analysis of single-dose oral tramadol plus acetaminophen in acute postoperative pain. J Pain Symptom Manage. 2002;23(2):121–130. doi: 10.1016/s0885-3924(01)00404-3. [DOI] [PubMed] [Google Scholar]
- 142.Jung YS, Kim DK, Kim MK, Kim HJ, Cha IH, Lee EW. Onset of analgesia and analgesic efficacy of tramadol/acetaminophen and codeine/acetaminophen/ibuprofen in acute postoperative pain: a single-center, single-dose, randomized, active-controlled, parallel-group study in a dental surgery pain model. Clin Ther. 2004;26(7):1037–1045. doi: 10.1016/s0149-2918(04)90175-0. [DOI] [PubMed] [Google Scholar]
- 143.Litkowski LJ, Christensen SE, Adamson DN, Van Dyke T, Han SH, Newman KB. Analgesic efficacy and tolerability of oxycodone 5 mg/ibuprofen 400 mg compared with those of oxycodone 5 mg/ acetaminophen 325 mg and hydrocodone 7.5 mg/acetaminophen 500 mg in patients with moderate to severe postoperative pain: a randomized, double-blind, placebo-controlled, single-dose, parallel-group study in a dental pain model. Clin Ther. 2005;27(4):418–429. doi: 10.1016/j.clinthera.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 144.Daniels SE, Goulder MA, Aspley S, Reader S. A randomised, five-parallel-group, placebo-controlled trial comparing the efficacy and tolerability of analgesic combinations including a novel single-tablet combination of ibuprofen/paracetamol for postoperative dental pain. Pain. 2011;152(3):632–642. doi: 10.1016/j.pain.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 145.White PF, Joshi GP, Carpenter RL, Fragen RJ. A comparison of oral ketorolac and hydrocodone-acetaminophen for analgesia after ambulatory surgery: arhroscopy versus laparoscopic tubal ligation. Anesth Analg. 1997;85(1):37–43. doi: 10.1097/00000539-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 146.Palangio M, Damask MJ, Morris E, et al. Combination hydrocodone and ibuprofen versus combination codeine and acetaminophen for the treatment of chronic pain. Clin Ther. 2000;22(7):879–892. doi: 10.1016/S0149-2918(00)80060-0. [DOI] [PubMed] [Google Scholar]
- 147.Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37(7):1007–1013. doi: 10.1111/j.1524-4725.2011.02022.x. [DOI] [PubMed] [Google Scholar]
- 148.Rawal N, Macquaire V, Catalá E, Berti M, Costa R, Wietlisbach M. Tramadol/paracetamol combination tablet for postoperative pain following ambulatory hand surgery: a double-blind, double-dummy, randomized, parallel-group trial. J Pain Res. 2011;4:103–110. doi: 10.2147/JPR.S16760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Serrie A, Ganry H, Creuzé A, Schatz B. Epidemiological data, efficacy and safety on a fixed combination of paracetamol (325 mg) and tramadol (37.5 mg) in the treatment of moderate to severe pain, in general practice (ELZA survey: Efficacité et ToLerance de ZAldier®) Journal of Applied Therapeutic Research. 2011;8(1):3–5. [Google Scholar]
- 150.Mejjad O, Serrie A, Ganry H. Epidemiological data, efficacy and safety of a paracetamol–tramadol fixed combination in the treatment of moderate-to-severe pain. SALZA: a post-marketing study in general practice. Curr Med Res Opin. 2011;27(5):1013–1020. doi: 10.1185/03007995.2011.565045. [DOI] [PubMed] [Google Scholar]
- 151.Emkey R, Rosenthal N, Wu SC, Jordan D, Kamin M CAPSS-114 Study Group. Efficacy and safety of tramadol/acetaminophen tablets (Ultracet) as add-on therapy for osteoarthritis pain in subjects receiving a COX-2 nonsteroidal anti-inflammatory drug: a multicenter, randomized, double-blind, placebo-controlled trial. J Rheumatol. 2004;31(1):150–156. [PubMed] [Google Scholar]
- 152.Corsinovi L, Martinelli E, Fonte G, et al. Efficacy of oxycodone/ acetaminophen and codeine/acetaminophen vs conventional therapy in elderly women with persistent, moderate to severe osteoarthritis-related pain. Arch Gerontol Geriatr. 2009;49(3):387–382. doi: 10.1016/j.archger.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 153.Pareek A, Chandurkar N, Sharma VD, Desai M, Kini S, Bartakke G. A randomized, multicentric, comparative evaluation of aceclofenac-paracetamol combination with aceclofenac alone in Indian patients with osteoarthritis flare-up. Expert Opin Pharmacother. 2009;10(5):727–735. doi: 10.1517/14656560902781931. [DOI] [PubMed] [Google Scholar]
- 154.Pareek A, Chandurkar N, Ambade R, Chandanwale A, Bartakke G. Efficacy and safety of etodolac-paracetamol fixed dose combination in patients with knee osteoarthritis flare-up: a randomized, double-blind comparative evaluation. Clin J Pain. 2010;26(7):561–566. doi: 10.1097/AJP.0b013e3181e15bba. [DOI] [PubMed] [Google Scholar]
- 155.Doherty M, Hawkey C, Goulder M, et al. A randomised controlled trial of ibuprofen, paracetamol or a combination tablet of ibuprofen/ paracetamol in community-derived people with knee pain. Ann Rheum Dis. 2011;70(9):1534–1541. doi: 10.1136/ard.2011.154047. [DOI] [PubMed] [Google Scholar]
- 156.Conaghan PG, O’Brien CM, Wilson M, Schofield JP. Transdermal buprenorphine plus oral paracetamol vs an oral codeine-paracetamol combination for osteoarthritis of hip and/or knee: a randomised trial. Osteoarthritis Cartilage. 2011;19(8):930–938. doi: 10.1016/j.joca.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 157.Palangio M, Morris E, Doyle RT, Jr, Dornseif BE, Valente TJ. Combination hydrocodone and ibuprofen versus combination oxycodone and acetaminophen in the treatment of moderate or severe acute low back pain. Clin Ther. 2002;24(1):87–99. doi: 10.1016/s0149-2918(02)85007-x. [DOI] [PubMed] [Google Scholar]
- 158.Ruoff GE, Rosenthal N, Jordan D, Karim R, Kamin M Protocol CAPSS-112 Study Group. Tramadol/acetaminophen combination tablets for the treatment of chronic lower back pain: a multicenter, randomized, double-blind, placebo-controlled outpatient study. Clin Ther. 2003;25(4):1123–1141. doi: 10.1016/s0149-2918(03)80071-1. [DOI] [PubMed] [Google Scholar]
- 159.Perrot S, Krause D, Crozes P, Naïm C GRTF-ZAL-1 Study Group. Efficacy and tolerability of paracetamol/tramadol (325 mg/37.5 mg) monotherapy in patients with subacute low back pain: a multicenter, randomized, double-blind, parallel-group, 10-day treatment study. Clin Ther. 2006;28(10):1592–1606. doi: 10.1016/j.clinthera.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 160.Bennett RM, Kamin M, Karim R, Rosenthal N. Tramadol and acetaminophen combination tablets in the treatment of fiobromyalgia pain: a double-blind, randomized, placebo-controlled study. Am J Med. 2003;114(7):537–545. doi: 10.1016/s0002-9343(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 161.Lee EY, Lee EB, Park BJ, et al. Tramadol 37.5-mg/acetaminophen 325-mg combination tablets added to regular therapy for rheumatoid arthritis pain: a 1-week, randomized, double-blind, placebo-controlled trial. Clin Ther. 2006;28(12):2052–2060. doi: 10.1016/j.clinthera.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 162.Raffaeli W, Pari C, Corvetta A, et al. Oxycodone/acetaminophen at low dosage: an alternative pain treatment for patients with rheumatoid arthritis. J Opioid Manag. 2010;6(1):40–46. doi: 10.5055/jom.2010.0003. [DOI] [PubMed] [Google Scholar]
- 163.Graham GG, Graham RI, Day RO. Comparative analgesia, cardiovascular, and renal effects of celecoxib, rofecoxib and acetaminophen (paracetamol) Curr Pharm Des. 2002;8(12):1063–1075. doi: 10.2174/1381612023394917. [DOI] [PubMed] [Google Scholar]
- 164.Hersh EV, Pinto A, Moore PA. Adverse drug interactions involving common prescription and over-the-counter analgesic agents. Clin Ther. 2007;29(Suppl):2477–2497. doi: 10.1016/j.clinthera.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 165.Hochberg MC, Altman RD, Toupin April K, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care and Research. 2012;64(4):465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 166.Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3–S14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Haanpää M, Backonja MM, Bennett M, et al. Assessment of neuropathic pain in primary care. Am J Med. 2009;122(10 Suppl):S13–S21. doi: 10.1016/j.amjmed.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 168.FDA Executive summary [web page on the Internet; McNeil background package to the Nonprescription Drug Advisory Committee] Silver Spring, MD: FDA; 2002. [Accessed May 13, 2011]. Available from: http://www.fda.gov/ohrms/dockets/ac/02/briefing/3882B1_13_McNeil-Acetaminophen.htm#_Toc18717551. [Google Scholar]