Abstract
Background: Small peak-to-trough drug levels have been suggested to be related to improved tolerability. The aim of this study is to review the steady-state, peak-to-trough, plasma-concentration fluctuation of long-acting injectable antipsychotics and equivalent oral formulations.
Methods: A review of published literature and clinical study reports identified references that reported, depicted, or permitted derivation of the steady-state, peak-to-trough, plasma-concentration fluctuation of antipsychotics (the ratio of maximum concentration to minimum concentration following administration according to the recommended dosing interval) over the dosing interval. Suitable references were identified for haloperidol decanoate, olanzapine pamoate, paliperidone palmitate, risperidone long-acting injectable, and zuclopenthixol decanoate and their oral equivalents except zuclopenthixol. The single-dose time to maximum plasma concentration (Tmax) and half-life (t1/2) were also identified.
Results: The steady-state, peak-to-trough, plasma-concentration ratios of oral antipsychotics varied from 1.47 (paliperidone extended-release, once daily) to 3.30 (active-moiety risperidone, once daily). Among long-acting injectable antipsychotics, the ratios varied from 1.56 (paliperidone palmitate, once monthly) to approximately 4 (olanzapine pamoate, once every four weeks). Among drugs with similar dosing intervals, longer Tmax and/or t1/2 generally correlated with less peak-to-trough fluctuation.
Conclusion: Peak-to-trough fluctuations in plasma concentrations vary widely and may be affected by differences in dosing, pharmacokinetic sampling, subjects’ phenotypes, concomitant medications, comorbid diseases, and formulation. These fluctuations may affect clinical response and tolerability. Along with other patient-specific and drug-specific factors, these fluctuations warrant consideration when selecting an antipsychotic and antipsychotic formulation. Further study is needed with more robust and generalizable peak-to-trough fluctuation data.
Keywords: Peak-to-trough fluctuation, antipsychotic, long-acting injectable, oral
Introduction
Dopamine type 2 (D2)–receptor occupancy has been shown to correlate with antipsychotic therapeutic response.1 A linear relationship is usually observed between antipsychotic dose and plasma concentration, and an asymptotic nonlinear (i.e., plateau-shaped) relationship exists between plasma concentration and D2-receptor occupancy.1–3 Most studies that explore this issue suggest that D2-receptor occupancy or plasma concentration correlates with tolerability, and some studies suggest a possible correlation with efficacy.1,2,4–8 For example, the antipsychotic effect of risperidone and haloperidol is reported to be evident in a range of 60 to 65 percent D2-receptor occupancy, with occupancy above 80 percent reported to increase the risk for developing extrapyramidal symptoms (EPSs)2 and other adverse events (AEs). More specifically, recent brain imaging studies suggest that the clinical efficacy of antipsychotic drugs was correlated to dopamine-receptor occupancy in the temporal cortex and striatum, whereas extrapyramidal symptoms were primarily related to striatal dopamine-receptor occupancy.9 An increase in plasma concentration peak-to-trough ratio may result in increased fluctuations in D2-receptor occupancy, although D2-receptor occupancy changes may lag behind plasma concentration changes due to delays in drug distribution between plasma and the central nervous system.4
If plasma concentration correlates with downstream pharmacologic effect on D2-receptor occupancy, antipsychotic formulations with smaller plasma-concentration fluctuations should cause smaller fluctuations in D2-receptor occupancy. This smaller fluctuation in occupancy may, in turn, lead to an improved pharmacodynamic profile. Formulations with a shorter half-life (t1/2) will have greater peak-to-trough fluctuations,5 given an equivalent dosing interval. When dose selection is optimized, the advantages of small plasma-concentration fluctuations often seen in controlled-release formulations include reduced peak concentrations (potentially decreasing the incidence of AEs), increased trough concentrations (potentially decreasing the chance of subtherapeutic drug concentrations), and the potential to improve adherence because of a possibly improved risk-benefit profile.10,11 As early as 1973, Johnson12 reported that reducing the dosing interval of fluphenazine decanoate (thereby reducing peak-to-trough fluctuation) could reduce AEs without diminishing efficacy. We hypothesized that this finding related to serum drug concentrations remaining below a theoretic threshold concentration may be associated with poor tolerability.12 Therapeutic drug monitoring groups have recommended a dosing interval that results in a peak-to-trough fluctuation of 2 or less for antipsychotics, although the proposed therapeutic ranges for specific medications vary in width.13,14
Long-acting injectable (LAI) antipsychotics have been developed with a wide variety of formulation technologies (e.g., covalent linkage of the active molecule to a fatty acid by esterification for paliperidone palmitate, haloperidol decanoate, and zuclopenthixol decanoate; ionic crystalline salt for olanzapine pamoate; microsphere technology for risperidone long-acting injection), suggesting the possibility of widely varied pharmacokinetic profiles.14–18
The aim of this analysis is to review the steady-state, peak-to-trough, plasma-concentration fluctuation and the pharmacokinetic stability of LAI antipsychotics and equivalent oral formulations and to review the clinical effects of peak-to-trough fluctuation. We present the steady-state, peak-to-trough fluctuation of antipsychotics over the dosing interval, including two pharmacokinetic parameters: time to maximum plasma concentration (Tmax) and t1/2. We present the peak-to-trough fluctuation over the dosing interval because this value likely affects the efficacy, safety, and tolerability of a drug. We present Tmax and t1/2 because these two parameters, along with dosing interval, substantially underlie peak-to-trough fluctuation.
Methods
Study selection. In an effort to identify and use all relevant published sources that could be used to validly determine steady-state, peak-to-trough, plasma-concentration fluctuations, the following literature search strategy was used: first, the references had to explicitly state the peak-to-trough fluctuation in the text or a figure or provide a figure depicting the plasma concentration over time that could be used to derive the mean steady-state, peak-to-trough fluctuation. Second, oral antipsychotics were only included if a suitable reference was identified for the corresponding LAI formulation. In addition, articles were not included if they only showed animal data or were case reports or only showed plasma concentrations before or after steady-state during titration or discontinuation. The first literature search of PubMed from 1960 through April 2011 consisted of the following terms: (clopenthixol or flupenthixol or fluphenazine or haloperidol or olanzapine or paliperidone or risperidone or zuclopenthixol) and (peak-to-trough or fluctuation index or [Cmax and Cmin] or steady state and pharmacokinetic). If suitable references for a given formulation were not identified in the first literature search, a second literature search of PubMed from 1960 through April 2011 was conducted for the remaining molecules and “pharmacokinetics.” One of the authors (J.J.S.) reviewed both literature searches. The reference lists from the identified articles were further examined to identify additional pertinent references that may not have been identified from the original search; unpublished data on Janssen Scientific Affairs, LLC products were also evaluated. In the event two or more appropriate references were identified, the peak-to-trough fluctuation was averaged across the studies.
Calculations. Whenever possible, peak-to-trough fluctuations were calculated as the ratio of the steady-state, mean maximum plasma concentration (Cmax) to the steady-state, mean minimum plasma concentration (Cmin) after administration according to the recommended dosing interval. Reported plasma concentrations represent the pharmacologically active moiety, when applicable. Peak-to-trough calculations for each medication are presented in Table 1.14,19–25 The key studies that yielded the Cmax and Cmin values for each antipsychotic are listed in Table 2.14,19–24
TABLE 1.
Peak-to-trough methods of calculation and data source
| DRUG | METHOD OF CALCULATION AND DATA SOURCE |
|---|---|
| LAI medications | |
| Haloperidol decanoate | Derived from the reported dose-normalized Cmax and Cmin19 |
| Olanzapine pamoate | Reported in the literature20 |
| Paliperidone palmitate | Predicted median Cmax and median Cmin in a PK model21 |
| Risperidone LAI | PK model22 |
| Zuclopenthixol decanoate | Reported in the literature14 |
| Oral medications | |
| Haloperidol | Calculated from Cmax and Cmin in the literature19 |
| Olanzapine | Derived from graph depicting steady-state PK23 |
| Paliperidone ER | Calculated from the steady-state mean Cmax and Cmin in a PK study24,25 |
| Risperidone | Calculated from the average peak-to-trough fluctuation of the active moiety derived from a PK study and predicted from a PK model22,24 |
LAI: long-acting injectable; Cmax: maximum plasma concentration; Cmin: minimum plasma concentration; ER: extended release; PK: pharmacokinetics.
TABLE 2.
Key clinical studies used to obtain pharmacokinetic data
| STUDY | DRUG(S) | NUMBER OF PATIENTS | DURATION OF STUDY |
|---|---|---|---|
| Nayak, 198719 | Haloperidol decanoate, haloperidol | 30 | ≥6 months |
| Taylor, 200920 | Olanzapine pamoate | NS | NS |
| Samtani, 200921 | Paliperidone palmitate | NA | NA |
| Mannaert, 200522 | Risperidone LAI, oral risperidone | 26 | 84 days |
| Poulsen, 199414 | Zuclopenthixol decanoate | 58 | Analysis was during long-term treatment |
| Callaghan, 199923 | Oral olanzapine | NS | NS |
| Berwaerts, 201024 | Paliperidone ER, oral risperidone | 38 for each drug | 6 days |
LAI: long-acting injection; ER: extended release; NA: not applicable (modeling study); NS: not stated (review article)
Results
An initial literature search resulted in identification of 147 publications for haloperidol (oral and LAI), paliperidone extended-release (ER), risperidone (oral and LAI), and zuclopenthixol (LAI). A second, broader literature search for the remaining agents and pharmacokinetics resulted in identification of 460 additional publications. From the second literature search, the authors identified suitable references for olanzapine and olanzapine pamoate. The authors combined these results with internal results for paliperidone palmitate.16–31 Most publications were not suitable for use in the calculation of peak-to-trough ratios because they did not meet the selection criteria outlined in the methods.
For most agents, only a single reference met the criteria. For zuclopenthixol decanoate, although a few different references reported the peak-to-trough fluctuation, the authors report the results from the most recent article because previous articles did not sample plasma concentrations at peak concentration. For oral risperidone, two articles met the search criteria and were appropriate for inclusion (one pharmacokinetic model and one pharmacokinetic study with human subject data);22 24 the average peak-to-trough fluctuation was derived from these two publications. No relevant values were identified for human, steady-state, peak-to-trough fluctuations in plasma concentrations for clopenthixol decanoate, flupenthixol decanoate, fluphenazine decanoate, fluphenazine enanthate, or oral zuclopenthixol.
The steady-state, peak-to-trough, plasma-concentration ratios of oral antipsychotics over the dosing interval varied from 1.47 (paliperidone extended release [ER], once daily) to 3.30 (active-moiety risperidone, once daily) (Figure 1).14,19–25 Among LAI antipsychotics, the steady-state ratios varied from 1.56 (paliperidone palmitate, once monthly) to approximately 4.00 (olanzapine pamoate, once every four weeks) (Figure 1).14,19–25
Figure 1.
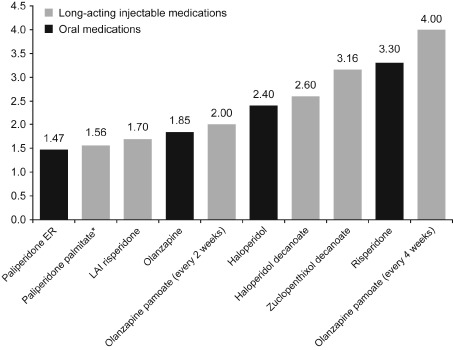
Steady-state, peak-to-trough fluctuation in plasma concentration over the recommended dosing interval.
Peak-to-trough fluctuations were calculated as the ratio of mean Cmax to mean Cmin at the steady-state following administration according to the recommended dosing interval. Source data.14,19–25
*Dose and injection site affect the pharmacokinetics (including peak-to-trough fluctuation) of paliperidone palmitate. Here the steady-state fluctuation of paliperidone palmitate 117mg was administered in the deltoid muscle. Administration of higher doses and/or gluteal administration may result in less steady-state, peak-to-trough fluctuation. Source data.27,40,41
Selected pharmacokinetic parameters are described in Table 3.14,16–19,26–31 Because t1/2 represents the time for plasma drug concentration to decrease by half, among drugs administered with identical dosing intervals, a formulation with a longer t1/2 will result in a smaller peak-to-trough fluctuation. Similarly, assuming an identical dosing interval, a longer time to maximum plasma concentration (Tmax) also may result in a smaller peak-to-trough fluctuation because the elimination phase would constitute a smaller proportion of the dosing interval (Figure 1 and Table 2).14,16–31
TABLE 3.
Selected pharmacokinetic parameters
| DRUG | Tmax | T1/2 |
|---|---|---|
| LAI medications, days | ||
| Haloperidol decanoate18 | 6 | 21 |
| Olanzapine pamoate16,26 | 4 | 30 |
| Paliperidone palmitate27 | 13 | 37* |
| Risperidone LAI†,17 | 35* | 4.5* |
| Zuclopenthixol decanoate14 | 3 | 7.4 |
| Oral medications, hours | ||
| Haloperidol19,28 | 4.9 | 25.6* |
| Olanzapine29 | 6 | 30 |
| Paliperidone ER31 | 24 | 23 |
| Risperidone†,30 | 1.3 | 19.5 |
LAI: long-acting injectable; ER: extended-release; T1/2: terminal half-life; Tmax: time to maximum plasma concentration
Where a range was reported, the midpoint of the range is presented here.
Risperidone: data reported for the active moiety (risperidone + 9-OH-risperidone).
Discussion
Our analyses show that the steady-state, plasma-concentration fluctuations of antipsychotics vary and are also related to how they have been formulated. Among the oral and long-acting antipsychotics evaluated here, steady-state, peak-to-trough plasma concentrations varied least with paliperidone ER and paliperidone palmitate. Among the antipsychotic formulations with a similar dosing interval that were evaluated, such as risperidone and paliperidone, a longer Tmax and/or t1/2 generally corresponded with less peak-to-trough fluctuation.
Over the entire dosing interval, some antipsychotics (e.g., haloperidol and paliperidone) had similar peak-to-trough fluctuations for both the LAI formulation and the oral formulation. For others (e.g., risperidone), the LAI form had less peak-to-trough fluctuation than the oral form (Figure 1).14,19–25
Bai et al32 hypothesized that improved adherence with risperidone LAI compared with oral antipsychotics may have been due to stable serum concentrations and a subsequent significant reduction in AEs.32 The majority of the literature supports a significant correlation between plasma concentration and/or large fluctuations in peak-to-trough plasma concentration with increased AEs,2,7,8,33,34 but some studies do not demonstrate this relationship.35,36
Long-acting or ER antipsychotics have a narrow peak-to-trough fluctuation and have been reported to be better tolerated than drugs with immediate release (i.e., with a larger peak-to-trough fluctuation). For example, comparisons of paliperidone ER and risperidone immediate-release (IR) may provide the best information on the impact of peak-to-trough fluctuation on tolerability because those two formulations use molecules with similar receptor binding profiles but widely different peak-to-trough fluctuations. In 2009, the National Institute for Health and Clinical Excellence compared the tolerability profile of oral atypical antipsychotics and reported a lower odds ratio of developing acute extrapyramidal symptoms with paliperidone ER (0.35) compared with risperidone (0.55) over one year.37 Moreover, a single-dose pharmacokinetic study of intravenous (IV), IR, and ER formulations of paliperidone reported a reduced rate of somnolence with the ER formulation compared with the IR and IV formulations (30% vs. 55–60%, respectively).38 Finally, a randomized, six-week, prospective, blinded-initiation study evaluated medication satisfaction as a primary outcome measure in a schizophrenia trial.39 Participants with suboptimal response to oral risperidone reported improved medication satisfaction after initiation of paliperidone ER at the two-week time point compared with participants who continued to receive oral risperidone.39 Improved patient satisfaction may be linked to the lower peak-to-trough fluctuations of paliperidone ER compared with oral risperidone.
This analysis has several limitations. Steady-state, peak-to-trough fluctuations reported in, or derived from, the literature can vary for many reasons, including differences in study design (e.g., duration, dosing interval evaluated, dose selected, dose stability, frequency of pharmacokinetic sampling). Subjects' phenotypes can be important, particularly for medications with complex metabolism via multiple enzymes. In addition, concomitant medications, substance abuse, and comorbid diseases (e.g., renal failure) may affect plasma concentrations and associated pharmacokinetic parameters. Because the rate of absorption varies depending on whether an antipsychotic is administered into the gluteal or deltoid muscle, or, more significantly, whether it is injected into fatty tissue rather than muscle, injection sites may influence peak-to-trough fluctuation. For example, dose and injection sites affect the pharmacokinetics (including peak-to-trough fluctuation) of paliperidone palmitate, and administration of higher doses and/or gluteal administration may result in less steady-state, peak-to-trough fluctuation.27,40,41
Not all available antipsychotic drugs were included in this study; for some drugs (clopenthixol decanoate, flupenthixol decanoate, fluphenazine decanoate, fluphenazine enanthate, or oral zuclopenthixol), no adequate studies were available or data were limited. Further, the total number of subjects in the key studies included here was less than 200; therefore, it may not be valid to extrapolate the conclusions of this study to all patients taking antipsychotic drugs.
The literature generally supports a correlation between plasma concentrations and tolerability; however, exact relationships for specific drugs have not been established. Furthermore, substantial interpatient variability in peak-to-trough fluctuation exists. This variability may also be dose related. Nevertheless, although the clinical significance of peak-to-trough profiles identified for antipsychotic treatments is not fully predictive of individual response, these fluctuations warrant consideration, along with other patient- and drug-specific factors, when selecting an antipsychotic and its formulation. As peak-to-trough fluctuations may be affected by differences in dosing, pharmacokinetic sampling, subjects' phenotypes, concomitant medications, comorbid diseases, and injection site, further study is needed to more clearly demonstrate relationships between changes in efficacy and safety parameters and peak-to-trough fluctuations in plasma concentrations of antipsychotic drugs.
Acknowledgments
The authors acknowledge Matthew Grzywacz, PhD, and Sheena Hunt, PhD, for providing writing and editorial assistance; and Mahesh Samtani, PhD, principal scientist, Johnson & Johnson Pharmaceutical Research and Development, for technical assistance.
Footnotes
FUNDING: Supported by Janssen Scientific Affairs, LLC.
FINANCIAL DISCLOSURES: Dr. Sheehan was an employee of Janssen Scientific Affairs, LLC, at the time of this analysis and is currently an employee of Bristol-Myers Squibb; Drs. Reilly, Fu, and Alphs are employees of Janssen Scientific Affairs, LLC, and are Johnson & Johnson stockholders.
References
- 1.Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 2.Medori R, Mannaert E, Grunder G. Plasma antipsychotic concentration and receptor occupancy, with special focus on risperidone long-acting injectable. Eur Neuropsychopharmacol. 2006;16(4):233–240. doi: 10.1016/j.euroneuro.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Mamo D, Kapur S, Keshavan M, et al. D2 receptor occupancy of olanzapine pamoate depot using positron emission tomography: an open-label study in patients with schizophrenia. Neuropsychopharmacology. 2008;33(2):298–304. doi: 10.1038/sj.npp.1301409. [DOI] [PubMed] [Google Scholar]
- 4.Olsen CK, Brennum LT, Kreilgaard M. Using pharmacokinetic-pharmacodynamic modelling as a tool for prediction of therapeutic effective plasma levels of antipsychotics. Eur J Pharmacol. 2008;584(2-3):318–327. doi: 10.1016/j.ejphar.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Catafau AM, Penengo MM, Nucci G, et al. Pharmacokinetics and time-course of D(2) receptor occupancy induced by atypical antipsychotics in stabilized schizophrenic patients. J Psychopharmacol (Oxf). 2008;22(8):882–894. doi: 10.1177/0269881107083810. [DOI] [PubMed] [Google Scholar]
- 6.Locatelli I, Kastelic M, Koprivsek J, et al. A population pharmacokinetic evaluation of the influence of CYP2D6 genotype on risperidone metabolism in patients with acute episode of schizophrenia. Eur J Pharm Sci. 2010;41(2):289–298. doi: 10.1016/j.ejps.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura R, Ueda N, Nakamura J. Possible relationship between combined plasma concentrations of risperidone plus 9-hydroxyrisperidone and extrapyramidal symptoms. Preliminary study. Neuropsychobiology. 2001;44(3):129–133. doi: 10.1159/000054932. [DOI] [PubMed] [Google Scholar]
- 8.Kakihara S, Yoshimura R, Shinkai K, et al. Prediction of response to risperidone treatment with respect to plasma concentrations of risperidone, catecholamine metabolites, and polymorphism of cytochrome P450 2D6. Int Clin Psychopharmacol. 2005;20(2):71–78. doi: 10.1097/00004850-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Stone JM, Davis JM, Leucht S, Pilowsky LS. Cortical dopamine D2/D3 receptors are a common site of action for antipsychotic drugs: an original patient data meta-analysis of the SPECT and PET in vivo receptor imaging literature. Schizophr Bull. 2009;35(4):789–797. doi: 10.1093/schbul/sbn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benet LZ, Kroeta DL, Sheiner LB. Pharmacokinetics: the dynamics of drug absorption, distribution, and elimination. In: Hardman JG, Limbirg LE, Molinoff PB, et al., editors. Goodman & Gillman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill; 1996. pp. 3–27. [Google Scholar]
- 11.Ereshefsky L, Mascarenas CA. Comparison of the effects of different routes of antipsychotic administration on pharmacokinetics and pharmacodynamics. J Clin Psychiatry. 2003;64:18–23. [PubMed] [Google Scholar]
- 12.Johnson DA. The side-effects of fluphenazine decanoate. Br J Psychiatry. 1973;123(576):519–522. doi: 10.1192/bjp.123.5.519. [DOI] [PubMed] [Google Scholar]
- 13.Baumann P, Hiemke C, Ulrich S, et al. The AGNP-TDM expert group consensus guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry. 2004;37(6):243–265. doi: 10.1055/s-2004-832687. [DOI] [PubMed] [Google Scholar]
- 14.Poulsen JH, Olesen OV, Larsen NE. Fluctuation of serum zuclopenthixol concentrations in patients treated with zuclopenthixol decanoate in viscoleo. Ther Drug Monit. 1994;16(2):155–159. doi: 10.1097/00007691-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Samtani MN, Vermeulen A, Stuyckens K. Population pharmacokinetics of intramuscular paliperidone palmitate in patients with schizophrenia: a novel once-monthly, long-acting formulation of an atypical antipsychotic. Clin Pharmacokinet. 2009;48(9):585–600. doi: 10.2165/11316870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Indianapolis, IN: Eli Lilly and Company; 2011. Zyprexa® Relprevv™ (olanzapine for extended release injectable suspension) [Google Scholar]
- 17.Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2010. Risperdal® Consta® (risperidone) long-acting injection. [Google Scholar]
- 18.Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2011. Haldol® Decanoate (haloperidol decanoate injection) [Google Scholar]
- 19.Nayak RK, Doose DR, Nair NP. The bioavailability and pharmacokinetics of oral and depot intramuscular haloperidol in schizophrenic patients. J Clin Pharmacol. 1987;27(2):144–150. doi: 10.1002/j.1552-4604.1987.tb02175.x. [DOI] [PubMed] [Google Scholar]
- 20.Taylor D. Psychopharmacology and adverse effects of antipsychotic long-acting injections: a review. Br J Psychiatry Suppl. 2009;52:S13–S19. doi: 10.1192/bjp.195.52.s13. [DOI] [PubMed] [Google Scholar]
- 21.Samtani MN, Haskins JT, Alphs L, et al. Maintenance dosing of once-monthly (4-weekly) paliperidone palmitate in schizophrenia: Pharmacokinetic rationale based on population simulations 2009. Poster presented: Annual Meeting of The College of Psychiatric and Neurologic Pharmacists; April 19-22, 2009; Jacksonville, FL. [Google Scholar]
- 22.Mannaert E, Vermeulen A, Remmerie B, et al. Pharmacokinetic profile of long-acting injectable risperidone at steady-state: comparison with oral administration. Encephale. 2005;31(5 Pt 1):609–615. doi: 10.1016/s0013-7006(05)82420-0. [DOI] [PubMed] [Google Scholar]
- 23.Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM. Olanzapine: pharmacokinetic and pharmacodynamic profile. Clin Pharmacokinet. 1999;37(3):177–193. doi: 10.2165/00003088-199937030-00001. [DOI] [PubMed] [Google Scholar]
- 24.Berwaerts J, Cleton A, Rossenu S, et al. A comparison of serum prolactin concentrations after administration of paliperidone extended-release and risperidone tablets in patients with schizophrenia. J Psychopharmacol. (Oxf) 2010;24(7):1011–1018. doi: 10.1177/0269881109106914. [DOI] [PubMed] [Google Scholar]
- 25. Johnson & Johnson Pharmaceutical Research and Development, LLC. Clinical study report SCH-101 EDMS-PSDB-2637921:5.0. Data on file.
- 26.Citrome L. Olanzapine pamoate: a stick in time? A review of the efficacy and safety profile of a new depot formulation of a second-generation antipsychotic. Int J Clin Pract. 2009;63(1):140–150. doi: 10.1111/j.1742-1241.2008.01900.x. [DOI] [PubMed] [Google Scholar]
- 27. Invega® Sustenna® (paliperidone palmitate) extended-release injectable suspension. 2011. Titusville, NJ: Janssen Pharmaceuticals, Inc.
- 28.Kudo S, Ishizaki T. Pharmacokinetics of haloperidol: an update. Clin Pharmacokinet. 1999;37(6):435–456. doi: 10.2165/00003088-199937060-00001. [DOI] [PubMed] [Google Scholar]
- 29. Zyprexa® (olanzapine) tablets and Zyprexa Zydis® (olanzapine) orally disintegrating tablets. 2011. Indianapolis, IN: Eli Lilly and Company.
- 30.Huang ML, Van Peer A, Woestenborghs R, et al. Pharmacokinetics of the novel antipsychotic agent risperidone and the prolactin response in healthy subjects. Clin Pharmacol Ther. 1993;54(3):257–268. doi: 10.1038/clpt.1993.146. [DOI] [PubMed] [Google Scholar]
- 31. Invega® (paliperidone) extended-release tablets. 2011. Titusville, NJ: Janssen Pharmaceuticals, Inc.
- 32.Bai YM, Ting CT, Chen JY, et al. Equivalent switching dose from oral risperidone to risperidone long-acting injection: a 48-week randomized, prospective, single-blind pharmacokinetic study. J Clin Psychiatry. 2007;68(8):1218–1225. doi: 10.4088/jcp.v68n0808. [DOI] [PubMed] [Google Scholar]
- 33.Yasui-Furukori N, Saito M, Nakagami T, et al. Clinical response to risperidone in relation to plasma drug concentrations in acutely exacerbated schizophrenic patients. J Psychopharmacol (Oxf) 2010;24(7):987–994. doi: 10.1177/0269881109104849. [DOI] [PubMed] [Google Scholar]
- 34.Spina E, Avenoso A, Facciola G, et al. Relationship between plasma risperidone and 9-hydroxyrisperidone concentrations and clinical response in patients with schizophrenia. Psychopharmacology (Berl) 2001;153(2):238–243. doi: 10.1007/s002130000576. [DOI] [PubMed] [Google Scholar]
- 35.Riedel M, Schwarz MJ, Strassnig M, et al. Risperidone plasma levels, clinical response and side-effects. Eur Arch Psychiatry Clin Neurosci. 2005;255(4):261–268. doi: 10.1007/s00406-004-0556-4. [DOI] [PubMed] [Google Scholar]
- 36.Klampfl K, Taurines R, Preuss A, et al. Serum concentrations, therapeutic response and side effects in children and adolescents with impulsive-aggressive symptoms during risperidone therapy. Pharmacopsychiatry. 2010;43(2):58–65. doi: 10.1055/s-0029-1239540. [DOI] [PubMed] [Google Scholar]
- 37. [October 12, 2010]. http://guidance.nice.org.uk/CG82 National Institute for Health and Clinical Excellence. Schizophrenia: The NICE guideline on core interventions in the treatment and management of schizophrenia in adults in primary and secondary care - updated edition. National Institute for Health and Clinical Excellence Web site 2010.
- 38. Rossenu S, Crauwels H, Cleton A, et al. Comparison of the pharmacokinetics of an oral immediate-release and extended-release formulation of paliperidone in healthy subjects 2010. Poster presented at: Annual Meeting and Exposition or the American Association of Pharmaceutical Sciences; October 29-November 02, 2006; San Antonio, TX. [Google Scholar]
- 39.Canuso CM, Grinspan A, Kalali A, et al. Medication satisfaction in schizophrenia: a blinded-initiation study of paliperidone extended release in patients suboptimally responsive to risperidone. Int Clin Psychopharmacol. 2010;25(3):155–164. doi: 10.1097/YIC.0b013e3283372977. [DOI] [PubMed] [Google Scholar]
- 40. Cleton A, Rossenu S, Crauwels H, et al. Assessment of the dose proportionality if paliperidone palmate 25,50,100 and 150 mg eq. a new long-acting injectable antipsychotic, following administration in the deltoid or gluteal muscles. Poster presented at: American Society of Clinical Pharmacology and Therapeutics: April 2-5, 2008; Orlando, Florida. [Google Scholar]
- 41. Johnson & Johnson Pharmaceutical Research and Development, LLC. Clinical study report PSY-1004 EDMS-PSDB-6213530:3.0. Data on file.


