Abstract
Background: Ultrasound guidelines for hypertrophic pyloric stenosis (HPS) have fixed minimum measurements and do not account for variation in patient weight or age. We sought to determine if preoperative pyloric measurements correlated with weight and age in patients with surgically proven HPS.
Methods: A retrospective analysis was conducted of 189 patients with HPS treated at a single institution over a 5-year period (2005 to 2010). Pearson correlation and linear regression analyses were used to determine if there were statistically significant associations between these combinations of factors: age and pyloric muscle thickness, weight and pyloric muscle thickness, age and pyloric length, and weight and pyloric length.
Results: Patients' mean age was 4.6 weeks (range, 1 to 17 weeks). Their mean weight was 3.9 kg (range, 2.5 to 8.0 kg). Mean pyloric muscle thickness was 0.42 cm (range, 0.18 to 0.86 cm), and mean pyloric length was 1.89 cm (range, 0.8 to 2.8 cm). Pearson correlation coefficient analysis showed a significant relationship between age and muscle thickness (r = 0.35, p < 0.001) as well as weight and muscle thickness (r = 0.24, p = 0.001). No significant relationship existed between pyloric length and age or weight. Linear regression analysis demonstrated similar results.
Conclusion: In patients with HPS, pyloric muscle thickness was directly related to age and weight. Practitioners should be aware that smaller and younger infants with a clinical diagnosis of HPS may still truly have HPS even though the minimum diagnostic criterion for muscle thickness or length is not found on ultrasound.
Introduction
The current generally accepted ultrasound guidelines for hypertrophic pyloric stenosis (HPS) arise from work done by Rohrschneider et al.1 They found that pathologic limits were 3 mm for pyloric muscle thickness, 15 mm for pyloric length, 11 mm for pyloric diameter, and 12 mL for pyloric volume. Additionally, they concluded that muscle thickness was the most discriminating factor. However, clinical experience has led us to question the applicability of these findings to infants of varying ages and weights.
We postulated that infants with smaller weights and/or ages who had HPS may have had pyloric measurements that were within the normal accepted range (ie, measurements that by current standards are not diagnostic of HPS). This study was aimed at determining whether preoperative pyloric measurements correlated with weight and age in patients with surgically proven HPS.
Methods
The study design was a retrospective chart review. The institutional review board at our institution approved this study. Data from a single institution over a five-year period (2005 to 2020) were reviewed. Patients were identified by diagnosis codes indicating that they had HPS. Operative reports were reviewed to verify the diagnosis. Demographic data on admission for surgery were collected about individual patients. These included sex, age (postgestational age in weeks), weight (in kilograms), and duration of hospital admission. The muscle wall thickness and length of the pyloric channel (both in centimeters), as documented on the ultrasound report, were obtained for each patient. Patients who did not have pyloric ultrasound measurements recorded or who did not have pyloric stenosis at the time of operation were excluded.
Pearson correlation analysis was used to determine if there were statistically significant associations between the following combinations of factors: patient age and pyloric muscle thickness, patient weight and pyloric muscle thickness, age and pyloric length, and weight and pyloric length. A linear regression analysis also was performed to analyze these relationships. Pyloric length and muscle thickness were the dependent variables, and weight and age were the independent variables.
Results
A total of 189 patients were identified who met the study criteria and underwent either laparoscopic or open pyloromyotomy, during which the diagnosis of HPS was confirmed. Complete data were available for 165 patients, and thus this was the number of patients included in the statistical analysis. The patients' postgestational age ranged from 1 to 17 weeks, with a mean age of 4.6 weeks. Patients' weights ranged from 2.5 to 8.0 kg, whereas the mean weight was 3.9 kg. Pyloric muscle length ranged from 0.8 to 2.8 cm, and the mean pyloric length was 1.89 cm. The mean pyloric wall thickness was 0.42 cm, and the range was 0.18 to 0.86 cm.
In addition to the study patients, we encountered an additional 5 patients who underwent surgical exploration who were not found to have HPS at surgery. Their weights ranged from 3.2 to 4.6 kg, and their ages ranged from 2 to 7 weeks. Two of these had numerical criteria below the standard cutoff for the ultrasonic diagnosis of HPS (muscle length, 0.8 and 1.1 cm, respectively), and it is not clear why the decision was made to take them to surgery. Three patients had ultrasound measurements that were within the diagnostic range (muscle length, 1.2 to 1.7 cm, and thickness, 0.3 to 0.7 cm). Thus, there were 3 false-positive ultrasound studies, all of which occurred in infants who were heavier and older than the others.
Pearson correlation coefficient analysis showed that there was a statistically significant relationship between pyloric muscle wall thickness and patient age (r = 0.35, p < 0.001) as well as wall thickness and patient weight (r = 0.24, p = 0.001), as shown in Figure 1. The same analysis proved that there was no significant relationship between pyloric length and patient age (r = 0.07, p = 0.35) or weight (r = 0.09, p = 0.27), which is demonstrated in Figure 2.
Figure 1.
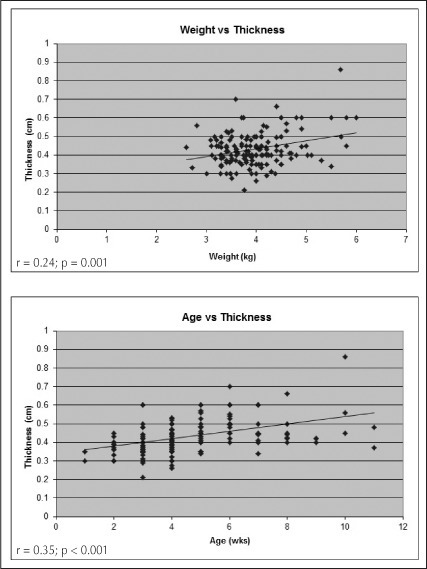
Pearson correlation graphs comparing patient weight (top) and age (bottom) vs pyloric muscle wall thickness.
Figure 2.
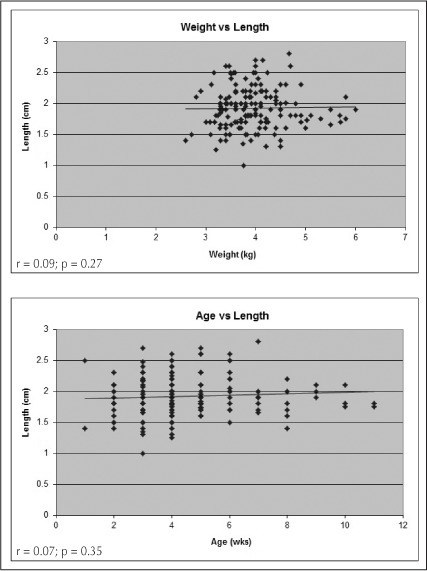
Pearson correlation graphs comparing patient weight (top) and age (bottom) vs pyloric length.
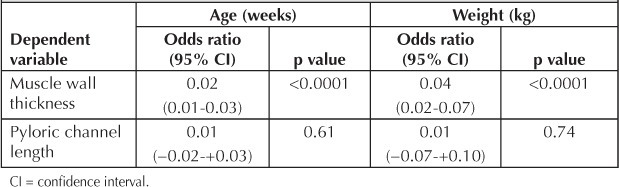
Linear regression analysis demonstrated that weight and age were predictors for increasing pyloric thickness (weight: p < 0.0001; 95% confidence interval [CI], 0.02 to 0.07; age: p < 0.0001; 95% CI, 0.01 to 0.03), as shown in Table 1. Linear regression confirmed that there was no relationship between pyloric length and age or weight (p = 0.61; 95% CI, −0.02 to +0.03; and p = 0.74; 95% CI, −0.07 to +0.10, respectively; Table 1).
Table 1.
Linear regression analysis of relationships between patient age and weight, and pyloric channel length and wall thickness
Discussion
When ultrasonographic images of the pylorus were initially being generated, it was clear that some standard criteria were required to allow the widespread adoption of this modality as a diagnostic tool. One of the original reports by Strauss et al2 reported 20 cases. Fifteen patients had a pyloric diameter greater than 1.5 cm and all of these had pyloric stenosis, whereas the 5 infants with a diameter of 1.5 cm or less did not have pyloric stenosis.2 It soon became clear that other measurements were possible and potentially useful, although muscle thickness was found to be the most discriminating and accurate criterion to make the diagnosis.1 Blumhagen et al3 noted significant overlap among the 319 infants they studied who did and did not have HPS.
There are many ultrasonographic findings that suggest HPS. These include failure of the stomach to empty and failure of the pylorus to open as well as an enlarged pyloric muscle. To the experienced radiologist and surgeon, the hypertrophied pylorus often looks obvious at first glance: “the hot dog in a bun” appearance. Unfortunately, these criteria are not objective, and therefore numerical criteria have become the standard for ultrasonographic diagnosis. We observed empirically that in smaller, younger infants, the muscle thickness and length criteria were occasionally not diagnostic even though the other criteria were strongly suggestive of HPS. Thus, we had the idea that sometimes the numbers can be misleading. In this series of more than 180 patients, there were 9 infants with surgically proven HPS with a muscle thickness at or below 3 mm and 15 infants with a pyloric channel length at or below 15 mm. Thus, 5% to 8% of patients with HPS had a numerically normal study. The question posed was whether a weight- or age-based criterion would be more accurate.
We attempted to develop a rigid criterion that would allow a diagnosis of HPS on the basis of age, weight, muscle thickness, and/or muscle length. The data in this large study clearly demonstrated that measurements of the pyloric length were quite varied and had no relationship to the age or weight of the infant. We believe that the primary reason for this is that when the antral muscle proximal to the pylorus is in spasm, it looks very similar to the pylorus, and therefore, measurements made by technologists may or may not include a portion of the antrum. This creates variability. We did find that muscle thickness showed a strong correlation to both the age and weight of the infant, but we could not find a foolproof method to avoid missing the diagnosis in smaller and younger infants. In these infants, other criteria must be considered.
Other authors have performed similar studies with varying results. In a study of 59 infants with pyloric stenosis, premature infants had a lower mean pyloric length, although it was not significantly lower, and the authors concluded that length did not correlate with prematurity.4 In a review of 91 infants, Haider et al5 found that the pyloric length was significantly greater in full-term infants than in preterm infants. They were unable to demonstrate a correlation between pyloric muscle thickness and prematurity.
The results from larger series mainly concur with the current study. Leaphart et al,6 in their analysis of ultrasound studies from 60 infants younger than age 21 days with proven pyloric stenosis, concluded that muscle thickness was significantly lower in younger vs older infants. However, their findings diverged from the current study in that they also found a relationship between muscle length and patient age. In concordance with our initial hypothesis, their results illustrated that the mean ultrasound measurements for younger newborns with pyloric stenosis fell within currently defined normal or borderline ranges. Another relatively large study that had results similar to the present study was that of Houben et al.7 In their evaluation of ultrasound findings of 100 pyloric stenosis infants, they stratified the size of the pylorus into 3 groups—short, moderate, and large—and compared these sizes with age. They proved that there was a statistically significant increase in the size of the pylorus with advancing age.
One of the weaknesses of this study is the failure to report on the pyloric measurements of several infants of varying ages and sizes who did not have proven HPS. This would have allowed for the development of a more accurate acceptable range that evaluates pyloric muscle thickness and quotes both normal and diagnostic values for infants on the basis of their weight. Future studies should be directed along these lines in order to develop diagnostic criteria that are accurate in various sizes and ages of infants. The real difficulty in developing standards for “normal” infants who are vomiting and may have HPS is in measuring the thickness and length of the normal pyloric muscle. The relaxed pylorus is difficult to distinguish from the adjacent antrum, and the ultrasonographer has difficulty taking the measurement accurately.
In conclusion, this study found that among HPS patients, pyloric muscle thickness was directly related to age and weight. There was no similar relationship for pyloric length. The collective experience of the authors of this study includes more than 1000 cases of pyloric stenosis. What we have seen is that HPS in smaller and younger infants can be a disease in evolution, and that repeating an ultrasound a few days later can be useful as the muscle thickens. In some infants, criteria other than muscle thickness can be used to make the diagnosis. For example, in a vomiting infant, electrolyte determinations, which reveal an alkalosis, are strongly suggestive of HPS. Unfortunately, concurrent dehydration with lactate production may neutralize the alkalosis, limiting its usefulness.
We support the currently accepted ultrasonographic diagnostic criterion of a pyloric muscle thickness of 3 mm or more. Reliance on a length of 12 mm or more can be misleading. When smaller neonates have a clinical picture consistent with HPS, a lower threshold for ultrasonographic diagnosis of HPS should be used. This will avoid delays in diagnosis and additional unnecessary studies. For primary care clinicians wondering whether a vomiting infant has HPS, consideration of numerous signs may be required to avoid missing the diagnosis.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Reference
- 1.Rohrschneider WK, Mittnacht H, Darge K, Tröger J. Pyloric muscle in asymptomatic infants: sonographic evaluation and discrimination from idiopathic hypertrophic pyloric stenosis. Pediatr Radiol. 1998 Jun;28(6):429–34. doi: 10.1007/s002470050377. [DOI] [PubMed] [Google Scholar]
- 2.Strauss S, Itzchak Y, Manor A, Heyman Z, Graif M. Sonography of hypertrophic pyloric stenosis. AJR Am J Roentgenol. 1981 Jun;136(6):1057–8. doi: 10.2214/ajr.136.6.1057. [DOI] [PubMed] [Google Scholar]
- 3.Blumhagen JD, Maclin L, Krauter D, Rosenbaum DM, Weinberger E. Sonographic diagnosis of hypertrophic pyloric stenosis. AJR Am J Roentgenol. 1988 Jun;150(6):1367–70. doi: 10.2214/ajr.150.6.1367. [DOI] [PubMed] [Google Scholar]
- 4.Forster N, Haddad RL, Choroomi S, Dilley AV, Pereira J. Use of ultrasound in 187 infants with suspected infantile hypertrophic pyloric stenosis. Australas Radiol. 2007 Dec;51(6):560–3. doi: 10.1111/j.1440-1673.2007.01872.x. [DOI] [PubMed] [Google Scholar]
- 5.Haider N, Spicer R, Grier R. Ultrasound diagnosis of infantile hypertrophic pyloric stenosis: determinants of pyloric length and effect of prematurity. Clin Radiol. 2002 Feb;57(2):136–9. doi: 10.1053/crad.2001.0853. [DOI] [PubMed] [Google Scholar]
- 6.Leaphart CL, Borland K, Kane TD, Hackam DJ. Hypertrophic pyloric stenosis in newborns younger than 21 days; remodeling the path of surgical intervention. J Pediatr Surg. 2008 Jun;43(6):998–1001. doi: 10.1016/j.jpedsurg.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Houben CH, Rudolf O, Misra D. Diagnosing hypertrophic pyloric stenosis: does size matter? Eur J Pediatr Surg. 1999 Dec;9(6):373–5. doi: 10.1055/s-2008-1072286. [DOI] [PubMed] [Google Scholar]


