Abstract
Background: As the debate over the effectiveness of prostate-specific antigen (PSA) screening for prostate cancer continues, it is increasingly important to understand how PSA screening occurs in general-practice settings.
Methods: We conducted a retrospective cohort study within Kaiser Permanente Southern California, a large integrated health care system. Men aged 35 years and older at baseline, in 1998, were eligible. The proportion of men who underwent PSA screening was estimated and compared across groups defined by patient and physician characteristics. We also evaluated trends in screening across time and serum PSA levels for all subgroups.
Results: Of 2,061,047 men, 572,306 (28%) underwent PSA screening from 1998 through 2007. Patterns of PSA screening varied modestly by age, race, and physician. The lowest frequencies of screening occurred among men younger than age 45 years (19%) and men ages 85 years and older (13%). PSA screening was most common among white men (33.5%) and in men seen by physicians of the same race/ethnicity (32%), compared with men with physicians of disparate race/ethnicity (26%, p < 0.001). PSA screening increased over time for all racial/ethnic groups and among men age 75 years and older but decreased over time for men younger than age 75 years old.
Conclusions: Nearly 1 in 4 eligible men underwent PSA screening from 1998 through 2007, and screening varied only modestly by patient and physician characteristics. Estimates of the frequency of PSA screening in general-practice settings can inform the debate and provide useful insight as to how changes in cancer screening guidelines would alter practice patterns in an increasingly integrated health care environment.
Introduction
Despite its importance as the most commonly diagnosed noncutaneous cancer and the second leading cause of cancer death among men in the US, no definitive screening tool for prostate cancer exists.1–3 Digital rectal examination (DRE) and measurement of serum prostate-specific antigen (PSA) levels are imperfect but widely used methods of early detection. Current patterns of use of these screening tools have not been well characterized, complicating our understanding of the effects of early detection. Given the questionable benefit of PSA screening regarding prostate cancer mortality4,5 and the discussion surrounding the guidelines that inform its use,6 understanding the utilization of this test is imperative.
Central to the discussion regarding early detection of prostate cancer is the inability to distinguish between indolent prostate cancer that does not require treatment and aggressive prostate cancer that does require definitive treatment. The issues of overdetection and overtreatment of early stage prostate cancer are further compounded by the questionable accuracy of serum PSA measurements. Current estimates of the sensitivity and specificity of serum PSA testing for prostate cancer screening, based on the Prostate Cancer Prevention Trial, are 21% and 88.6%, respectively.7–9 As a result, some men with false-positive results undergo invasive and unnecessary work-ups (eg, prostatic ultrasound-guided biopsy). Furthermore, many men with indolent prostate cancer receive invasive therapies that often result in treatment-related complications such as erectile dysfunction and incontinence.10–13
Despite the potential limitations of PSA testing, prostate cancer mortality has decreased by 4% annually since its introduction.2 Controversy persists nonetheless, because the influence of PSA testing on prostate cancer mortality is questionable.6,14–20 Two recently published randomized clinical trials, the Prostate, Lung, Colon, and Ovarian Cancer Screening Trial and the European Randomized Study for Screening Prostate Cancer suggest that PSA testing does not decrease prostate cancer mortality.4,5 In light of these findings, the American Urological Association and the American Cancer Society have updated their prostate cancer screening guidelines. The American Urological Association recommends PSA and DRE screening begin at age 40 years, given a life expectancy of at least another 10 years, and at a younger age for men with certain risk factors (eg, African-American men or men with a family history of prostate cancer).21 The American Cancer Society takes a more conservative stance, recommending that men with low risk begin discussing the pros and cons of screening with their physician at age 50 years.22 Taking this conservative stance further, the US Preventive Services Task Force recently concluded that there is insufficient evidence to recommend screening.23 Further complicating the issue, payers and governmental agencies have attempted to intervene either through reimbursement policy or by requiring insurers to provide coverage for PSA testing.24–26 This ambiguity has made it difficult for health care systems and physicians both in the US and in Europe to develop consistent and appropriate approaches to prostate cancer screening.
Despite the discussion surrounding the use of PSA to screen for prostate cancer, estimates of PSA screening rates in the US are generally limited to surveys or institutional studies.17,24,25 Furthermore, these estimates are based on samples that are small and that often lack diversity, limiting their generalizability. National guidelines can have the greatest effect in large general practices, but the implementation of PSA testing in this setting remains poorly characterized.26–29 Therefore, the goal of this study was to characterize prostate cancer screening practices in a large managed health care system that promulgates and enforces practice guidelines across an integrated care network. This study was approved by the Kaiser Permanente Southern California (KPSC) institutional review board.
Methods
Setting and Study Population
KPSC is a large managed care organization that spans from Bakersfield, in the southern San Joaquin Valley, to San Diego, at the Mexican border. KPSC currently serves more than 3.4 million members with a racial and ethnic composition similar to that of Southern California. Health care is mostly delivered in 1 of 14 Medical Centers or affiliated outpatient facilities. A small fraction of emergent and specialty care is received from contracted physicians or through reimbursement claims. Regardless of the setting, detailed information on all diagnoses, procedures, test and biopsy results, pathology reports, treatments, and outcomes is tracked in electronic data systems.
Men who were 1) active KPSC members for at least 1 day during the period 1998 to 2007; 2) at least age 35 years on January 1, 1998; 3) at least age 45 years upon termination of membership or at the conclusion of the study period; and 4) without a prostate cancer diagnosis before baseline (ICD-9 code 185) were eligible for inclusion (N = 2,061,047). PSA data were captured from electronic medical records, including tests performed from the date of first eligibility (based on age and membership) until termination of membership or prostate cancer diagnosis (censoring).
Measurements
Demographic information was obtained from electronic medical records. Physician race/ethnicity (white, black, Asian, Hispanic, and other) and medical specialty, categorized as family medicine, internal medicine, or other, were ascertained from electronic provider files. During the study period, serum PSA levels were measured in ng/mL, using three immunoassays: AxSYM (Abbott Laboratories; Abbott Park, IL; 1998–2003), Immulite (Siemens Medical Solutions; Malveryn, PA; 2003–5), and Elecsys (Roche Diagnostics; Indianapolis, IN; 2005–7). All serum PSA measurements from tests that were performed from the beginning of study eligibility through the end of follow-up (or censoring) were extracted from electronic health plan files. To confirm the consistency of the test results, we randomly selected a 100-patient sample from tested men for chart abstraction. In addition, we abstracted DRE results, physician interpretations, and indications for testing.
… men age 85 years and older, had the lowest proportion of PSA testing: 13%.
Statistical Analysis
Demographic characteristics of men who had PSA tests during the study period were compared with those of men who did not, using the χ2 test and two-sided t test where appropriate. The proportion of men who had a PSA test was then calculated as the number of men with at least one PSA test divided by the total number of men eligible for PSA screening (as defined by the age and membership inclusion criteria) during the study period. The proportion of men screened was then calculated by demographic and physician characteristics. The proportions were similarly estimated over specific time periods (1998–2000, 2001–2003, and 2004–2007), with each proportion based on the first serum PSA measurement within the time period. The distributions of men with serum PSA values above the corresponding age-specific reference ranges (ASRRs) or greater than or equal to 4 ng/mL were also determined. All analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC), with an α-level of 0.05.
Results
In this cohort of men eligible for prostate cancer screening, the duration of enrollment in the Health Plan from 1998 through 2007 was 6.46 years. Approximately 27% of men had at least one PSA test during the study period. Patterns of PSA testing differed significantly by age (Table 1), with lower proportions observed in the oldest and youngest groups (p < 0.001). Men aged 45–74 years, constituting the majority of men screened, had similar testing proportions, approximately 36%, when age groups were divided by deciles. Only 19% of men younger than age 45 years underwent PSA tests, while 28% of men aged 75–84 years were tested. The oldest subgroup, men age 85 years and older, had the lowest proportion of PSA testing: 13%. In addition, the overall proportion of PSA testing varied slightly across racial groups, with white men (33.5%) having the highest proportion of PSA screening, followed by black men (30.4%), Asian men (30.0%), and Hispanic men (28.5%, p < 0.001, Table 1).
Table 1.
Characteristics of 2,061,047 men enrolled in KPSC 1998–2007, by PSA screening status
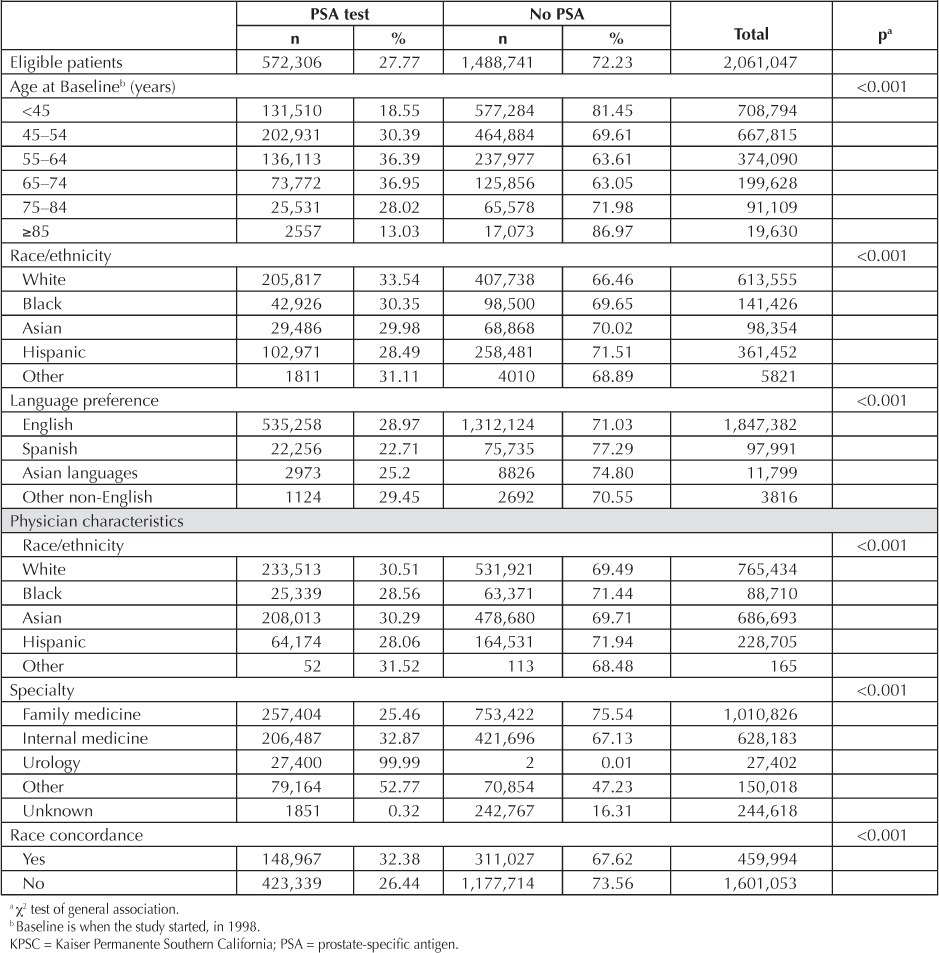
The percentage of men who had a PSA test differed minimally across physician specialty and race/ethnicity. PSA testing was more common in men receiving care from family physicians than in patients cared for by internal medicine physicians (45.0% vs 36.1%, p < 0.001). Although patients seen by black and Hispanic physicians had a screening rate of 28% (28.56% and 28.06%), those treated by white and Asian physicians were screened at a slightly higher rate (30.5% and 30.3%, respectively). Patients with physicians who shared the same race/ethnicity were more likely to be screened (32.4%) than patients with physicians of a different race (26.4%, p < 0.001, Table 1).
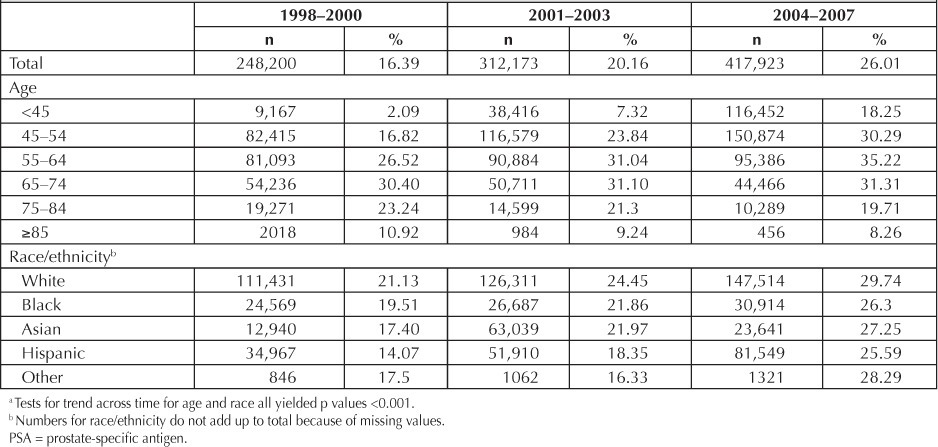
Table 2 presents rates of PSA screening during 3 progressive time periods beginning in 1998 and ending in 2007. During the study period, PSA testing rose from 16.4% to 20.2%, to 26.0%. Screening rates increased over time for black men (19.5%, 21.9%, and 26.3%), white men (21.1%, 24.5%, 29.7%), Hispanic men (14.1%, 18.4%, and 25.6%), and Asian men (17.4%, 22.0%, and 27.3%). PSA testing among the youngest men (<45 years) rose from 2.1% to 18.3% during the study period. Concurrently, screening for men older than age 55 years consistently decreased. Most Medical Centers had initial testing rates in the range of 13.7% through 20.8% and rates ranging from 22.7% through 30.2% in the most recent period (data not shown).
Table 2.
Proportions of men who participated in PSA testing, among those who were eligible, over time by age and race a
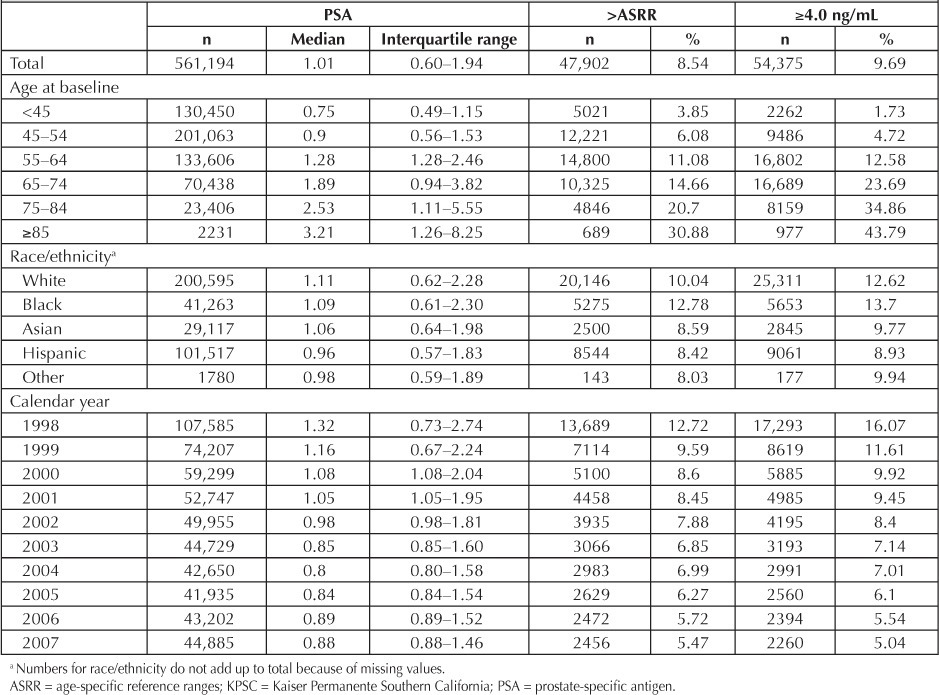
The distribution of PSA levels over the entire study period is presented in Table 3. The median overall serum PSA level was 1.01 ng/mL during the study period. The proportions of initial serum PSA levels greater than 4.0 ng/mL or exceeding the ASRR were 9.7% and 8.5%, respectively. Elevated serum PSA levels (>4 ng/mL or >ASRR) were more frequent among black men (13.7% and 12.8%) than white men (12.6% and 10.0%), Asians (9.8% and 8.6%), and Hispanics (8.9% and 8.4%). Older men had substantially higher proportions of elevated PSA levels than younger men (p < 0.001). We compared men younger than age 45 years to older subgroups defined by 10-year intervals extending to age 85 years. The proportion of PSA results above the ASRR increased considerably with age (range, 3.9%–30.9%). The proportion of men with results exceeding 4 ng/mL diminished from 16.1% in 1998 to 5.0% in 2007. Comparably, 12.7% and 5.5% of screened men had results exceeding the ASRR in 1998 and 2007, respectively (Table 3).
Table 3.
Distribution of initial PSA levels and PSA levels exceeding the ASRR or greater than 4.0 ng/mL, among men with a history of PSA testing in KPSC, 1998–2007
We performed validation studies of the electronic medical records via chart abstracting for a sample of 100 patients who had a PSA test ordered and completed. Of the 46 patients who underwent a DRE, 3 (6.5%) had abnormal findings.
Discussion
In this descriptive analysis of prostate cancer screening practices in a large managed care organization with a predilection for protocol and guideline-driven clinical practice, we found that nearly one in four eligible KPSC members underwent PSA testing from 1998 through 2007. PSA screening increased considerably over time and varied modestly across certain populations. This analysis of real-world practice could prove particularly useful in assessing the cost-effectiveness of prostate cancer screening as it is currently applied and the impact of emerging advances in cancer detection, and in anticipating how changes in screening guidelines will alter practice patterns in an increasingly coordinated health care environment.
Few population-based studies have assessed how screening is implemented in general-practice settings.15,30,31 Without direct observational data on screening patterns, researchers typically rely on billing and survey data or focus on physician or patient attitudes toward screening.24,26,28,32–34 Estimates using Medicare data put rates of PSA testing at 34% and 25% for white and black men over age 65 years, respectively.35 The Behavioral Risk Factor Surveillance Survey, a comprehensive national assessment of cancer screening, found that 49.3% of men underwent PSA testing within the previous 2 years of being questioned, in 2004.25 Despite its methodologic rigor, the Behavioral Risk Factor Surveillance Survey was limited by its self-report design and was subject to participation bias.
Interestingly, differences in rates of PSA testing between blacks and whites, which have been inconsistently reported in other studies, were not apparent in our study.35,36 Race was a very minor factor: the proportion of whites who were screened was 10% greater relative to blacks, Hispanics, and Asians, all of whom had similar rates of testing. This study does, however, highlight the need to better understand patterns of testing among minority racial/ethnic groups. Furthermore, some may argue that the greater risk of prostate cancer among black men should lead to higher rather than similar rates of testing relative to other racial groups. However, this variability could reflect appropriate differences in screening practices that are based on our understanding of prostate cancer risk factors and competing recommendations.
Age was a significant factor in this analysis, with the youngest and oldest men less likely to undergo PSA testing. In a study of self-reported data from the National Health Interview Survey, Ross et al showed that the rate of PSA testing for men aged 40 to 49 years was 16%, whereas men aged 50 to 69 years had a rate of 49%.37 Variability in rates of PSA testing by age may have the most potential for interventions aimed at standardizing prostate cancer screening practices. Surprisingly, the rate of PSA testing for older men (≥85 years) was 13% and increased over the most recent study period, representing an opportunity for patient and physician education based on the multiple guidelines that argue against screening in this age group.
Reasons for the variability in PSA testing rates among the various subpopulations in the present study are not immediately evident. However, earlier literature suggests that educational attainment, marital status, poverty, usual source of medical care, family history of prostate cancer, and comorbidities may all play a role.38–41 Clinical uncertainty, conflicting guidelines, physician beliefs, and patient preferences are also proffered.24,28,42–44 Insurance status and having a personal physician have been found to be associated with the likelihood of PSA testing.45 Patient-physician concordance has been suggested to increase PSA testing rates.46 These factors taken together suggest a disadvantage of certain populations (eg, those of low socioeconomic status and racial and ethnic minorities) in accessing or negotiating available services for prostate cancer detection.
Looking to modifiable factors, patient and physician perceptions of the efficacy of PSA testing may affect physician screening practices and adherence to guidelines.32 Certainly, differences in screening practices can result from variability in patient demographics and risk factors, however individual and organizational knowledge and preferences must also be considered. In fact, in our small validation sample of men who had undergone PSA testing, only 46% also had a concomitant DRE, raising the question of patient preferences and physician perceptions regarding the relative utility of symptomatic evaluations of prostate cancer.
Although this study characterizes the use of PSA testing in a large, general-practice setting, there are potential limitations that should be considered. It was not possible to differentiate between screening and diagnostic PSA testing or to identify the underlying rationale for performing a physical exam. Nonetheless, the chart review–based validation sample demonstrated that less than half of those who underwent PSA testing also had a DRE, and few of them had abnormal findings. Thus, the continued role of physical examination in prostate cancer screening may be questionable. Although the managed care organization setting was an advantage of this study because it provided access to data necessary to characterize the evolution of screening practices, the generalizability of our study is limited. KPSC members are a fully insured population, albeit a diverse one with coordinated care services. Additionally, we were not able to capture data for PSA testing performed outside of KPSC. However, managed care organizations, which provide similar care as universal health care systems, encourage patients to obtain services through general practitioners and within the system. For KPSC, this means members seek fewer tests and services outside the network. Finally, because the inclusion criteria specified that men only had to be members for one day during the study period and reach age 45 before membership termination, the denominator of men eligible for PSA screening in this study may be inflated. As a result, estimates of PSA screening rates in this study may be conservative.
Conclusions
Among this large, managed care sample, approximately one quarter of eligible men underwent PSA testing from 1998 through 2007. Lower rates of screening among racial minorities and younger men and persistent testing among men age 75 years and older may be opportunities for practice-based interventions aimed at optimizing PSA screening practices.
Disclosure Statement
This research was supported by research grants from Beckman Coulter, American College of Surgeons, and the Robert Wood Johnson Foundation Clinical Scholars Program.
Acknowledgments
The authors would like to thank May Lui, PhD; Julie Stern, MPH for their contribution to this manuscript.
Leslie E Parker, ELS, provided editorial assistance.
References
- 1.Grönberg H. Prostate cancer epidemiology. Lancet. 2003 Mar;361(9360):859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 2.Ries L, Melbert D, Krapch M, et al., editors. Bethesda MD: National Cancer Institute; SEER Cancer Statistics Review [monograph on the Internet] (eds) updated 2007 [cited 2012 Jun 11]. Available from: http://seer.cancer.gov/csr/1975_2004/ [Google Scholar]
- 3.Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol. 1993 Aug;150(2 Pt 1):379–85. doi: 10.1016/s0022-5347(17)35487-3. [DOI] [PubMed] [Google Scholar]
- 4.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009 Mar 26;360(13):1310–9. doi: 10.1056/NEJMoa0810696. PLCO Project Team. Erratum in: N Engl J Med 2009 Apr 23;360(17):1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009 Mar 26;360(13):1320–8. doi: 10.1056/NEJMoa0810084. ERSPC Investigators. [DOI] [PubMed] [Google Scholar]
- 6.US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008 Aug 5;149(3):185–91. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 7.Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006 Apr 19;98(8):529–34. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 8.Thompson IM, Ankerst DP, Etzioni R, Wang T. It's time to abandon an upper limit of normal for prostate specific antigen: assessing the risk of prostate cancer. J Urol. 2008 Oct;180(4):1219–22. doi: 10.1016/j.juro.2008.07.089. [DOI] [PubMed] [Google Scholar]
- 9.Thompson IM, Tangen CM, Ankerst DP, et al. The performance of prostate specific antigen for predicting prostate cancer is maintained after a prior negative prostate biopsy. J Urol. 2008 Aug;180(2):544–7. doi: 10.1016/j.juro.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Bacon CG, Giovannucci E, Testa M, Kawachi I. The impact of cancer treatment on quality of life outcomes for patients with localized prostate cancer. J Urol. 2001 Nov;166(5):1804–10. [PubMed] [Google Scholar]
- 11.Barry MJ, Albertsen PC, Bagshaw MA, et al. Outcomes for men with clinically nonmetastatic prostate carcinoma managed with radical prostactectomy, external beam radiotherapy, or expectant management: a retrospective analysis. Cancer. 2001 Jun 14;91(12):2302–14. doi: 10.1002/1097-0142(20010615)91:12<2302::aid-cncr1262>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 12.Davison BJ, So AI, Goldenberg SL. Quality of life, sexual function and decisional regret at 1 year after surgical treatment for localized prostate cancer. BJU Int. 2007 Oct;100(4):780–5. doi: 10.1111/j.1464-410X.2007.07043.x. [DOI] [PubMed] [Google Scholar]
- 13.Penson DF, Litwin MS, Aaronson NK. Health related quality of life in men with prostate cancer. J Urol. 2003;169(5):1653–61. doi: 10.1097/01.ju.0000061964.49961.55. [DOI] [PubMed] [Google Scholar]
- 14.Concato J, Wells CK, Horwitz RI, et al. The effectiveness of screening for prostate cancer: a nested case-control study. Arch Intern Med. 2006 Jan 9;166(1):38–43. doi: 10.1001/archinte.166.1.38. [DOI] [PubMed] [Google Scholar]
- 15.Lin K, Lipsitz R, Miller T, Janakiraman S. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the US Preventive Services Task Force. Ann Intern Med. 2008 Aug 5;149(3):192–9. doi: 10.7326/0003-4819-149-3-200808050-00009. US Preventive Services Task Force. [DOI] [PubMed] [Google Scholar]
- 16.Merrill RM, Lyon JL. Explaining the difference in prostate cancer mortality rates between white and black men in the United States. Urology. 2000 May;55(5):730–5. doi: 10.1016/s0090-4295(99)00564-6. [DOI] [PubMed] [Google Scholar]
- 17.Sirovich BE, Schwartz LM, Woloshin S. Screening men for prostate and colorectal cancer in the United States: does practice reflect the evidence? JAMA. 2003 May 19;289(11):1414–20. doi: 10.1001/jama.289.11.1414. [DOI] [PubMed] [Google Scholar]
- 18.Bergstralh EJ, Roberts RO, Farmer SA, Slezak JM, Lieber MM, Jacobsen SJ. Population-based case-control study of PSA and DRE screening on prostate cancer mortality. Urology. 2007 Nov;70(5):936–41. doi: 10.1016/j.urology.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agalliu I, Weiss NS, Lin DW, Stanford JL. Prostate cancer mortality in relation to screening by prostate-specific antigen testing and digital rectal examination: a population-based study in middle-aged men. Cancer Causes Control. 2007 Nov;18(9):931–7. doi: 10.1007/s10552-007-9031-7. [DOI] [PubMed] [Google Scholar]
- 20.Weinmann S, Richert-Boe KE, Van Den Eeden SK, et al. Screening by prostate-specific antigen and digital rectal examination in relation to prostate cancer mortality: a case-control study. Epidemiology. 2005 May;16(3):367–76. doi: 10.1097/01.ede.0000158395.05136.02. Erratum in: Epidemiology 2005 Jul;16(4):515. [DOI] [PubMed] [Google Scholar]
- 21.Greene KL, Albertsen PC, Babaian RJ, et al. Prostate specific antigen best practice statement: 2009 update. J Urol. 2009 Nov;182(5):2232–41. doi: 10.1016/j.juro.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 22.Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for early detection of cancer, 2006. CA Cancer J Clin. 2006 Jan–Feb;56(1):11–25. doi: 10.3322/canjclin.56.1.11. [DOI] [PubMed] [Google Scholar]
- 23.Chou R, Croswell JM, Dana T, et al. Screening for prostate cancer: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2011 Dec 6;155(11):762–71. doi: 10.7326/0003-4819-155-11-201112060-00375. [DOI] [PubMed] [Google Scholar]
- 24.Voss JD, Schectman JM. Prostate cancer screening practices and beliefs. J Gen Intern Med. 2001 Dec;16(12):831–7. doi: 10.1111/j.1525-1497.2001.10133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behavioral risk factor surveillance system ... survey data. Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion; 1984–1995. [Google Scholar]
- 26.Moran WP, Cohen SJ, Preisser JS, Wofford JL, Shelton BJ, McClatchey MW. Factors influencing use of the prostate-specific antigen screening test in primary care. Am J Manag Care. 2000 Mar;6(3):315–24. [PubMed] [Google Scholar]
- 27.McKnight JT, Tietze PH, Adcock BB, Maxwell AJ, Smith WO, Nagy MC. Screening for prostate cancer: a comparison of urologists and primary care physicians. South Med J. 1996 Sep;89(9):885–8. [PubMed] [Google Scholar]
- 28.Fowler FJ, Jr, Bin L, Collins MM, et al. Prostate cancer screening and beliefs about treatment efficacy: a national survey of primary care physicians and urologists. Am J Med. 1998 Jun;104(6):526–32. doi: 10.1016/s0002-9343(98)00124-7. [DOI] [PubMed] [Google Scholar]
- 29.Ross LE, Coates RJ, Breen N, Uhler RJ, Potosky AL, Blackman D. Prostate-specific antigen test use reported in the 2000 National Health Interview Survey. Prev Med. 2004 Jun;38(6):732–44. doi: 10.1016/j.ypmed.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Ankerst DP, Miyamoto R, Nair PV, Pollock BH, Thompson IM, Parekh DJ. Yearly prostate specific antigen and digital rectal examination fluctuations in a screened population. J Urol. 2009 May;181(15):2071–5. doi: 10.1016/j.juro.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002 Dec 3;137(11):917–29. doi: 10.7326/0003-4819-137-11-200212030-00014. [DOI] [PubMed] [Google Scholar]
- 32.Chan EC, Barry MJ, Vernon SW, Ahn C. Brief report: physicians and their personal prostate cancer-screening practices with prostate-specific antigen. J Gen Intern Med. 2006 Mar;21(3):257–9. doi: 10.1111/j.1525-1497.2006.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purvis Cooper C, Merritt TL, Ross LE, John LV, Jorgensen CM. To screen or not to screen, when clinical guidelines disagree: primary care physicians' use of the PSA test. Prev Med. 2004 Feb;38(2):182–91. doi: 10.1016/j.ypmed.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman RM, Papenfuss MR, Buller DB, Moon TE. Attitudes and practices of primary care physicians for prostate cancer screening. Am J Prev Med. 1996 Jul–Aug;12(4):277–81. [PubMed] [Google Scholar]
- 35.Etzioni R, Berry KM, Legler JM, Shaw P. Prostate-specific antigen testing in black and white men: an analysis of Medicare claims from 1991–1998. Urology. 2002 Feb;59(2):251–5. doi: 10.1016/s0090-4295(01)01516-3. [DOI] [PubMed] [Google Scholar]
- 36.Pan CC, Lee JS, Chan JL, Sandler HM, Underwood W, McLaughlin PW. The association between presentation PSA and race in two sequential time periods in prostate cancer patients seen at a university hospital and its community affiliates. Int J Radiat Oncol Biol Phys. 2003 Dec 1;57(5):1292–6. doi: 10.1016/s0360-3016(03)00771-5. [DOI] [PubMed] [Google Scholar]
- 37.Ross LE, Berkowitz Z, Ekwueme DU. Use of the prostate-specific antigen test among US men: findings from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008 Mar;17(3):636–44. doi: 10.1158/1055-9965.EPI-07-2709. [DOI] [PubMed] [Google Scholar]
- 38.Steenland K, Rodriguez C, Mondul A, Calle EE, Thun M. Prostate cancer incidence and survival in relation to education (United States) Cancer Causes Control. 2004 Nov;15(9):939–45. doi: 10.1007/s10552-004-2231-5. [DOI] [PubMed] [Google Scholar]
- 39.Bennett CL, Ferreira MR, Davis TC, et al. Relation between literacy, race, and stage of presentation among low-income patients with prostate cancer. J Clin Oncol. 1998 Sep;16(9):3101–4. doi: 10.1200/JCO.1998.16.9.3101. [DOI] [PubMed] [Google Scholar]
- 40.Roetzheim RG, Pal N, Tennant C, et al. Effects of health insurance and race on early detection of cancer. J Natl Cancer Inst. 1999 Aug 18;91(16):1409–15. doi: 10.1093/jnci/91.16.1409. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control. 2003 Oct;14(8):761–6. doi: 10.1023/a:1026321923883. [DOI] [PubMed] [Google Scholar]
- 42.Drummond FJ, Carsin AE, Sharp L, Comber H. Factors prompting PSA-testing of asymptomatic men in a country with no guidelines: a national survey of general practitioners. BMC Family Practice. 2009 Jan 12;10:3. doi: 10.1186/1471-2296-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demark-Wahnefried W, Strigo T, Catoe K, et al. Knowledge, beliefs, and prior screening behavior among blacks and whites reporting for prostate cancer screening. Urology. 1995 Sep;46(3):346–51. doi: 10.1016/S0090-4295(99)80218-0. [DOI] [PubMed] [Google Scholar]
- 44.Farrell MH, Murphy MA, Schneider CE. How underlying patient beliefs can affect physician-patient communication about prostate-specific antigen testing. Eff Clin Pract. 2002 May–Jun;5(3):120–9. [PubMed] [Google Scholar]
- 45.Carlos RC, Underwood W, 3rd, Fendrick AM, Bernstein SJ. Behavioral associations between prostate and colon cancer screening. J Am Coll Surg. 2005 Feb;200(2):216–23. doi: 10.1016/j.jamcollsurg.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 46.LaVeist TA, Nuru-Jeter A, Jones KE. The association of doctor-patient race concordance with health services utilization. J Public Health Policy. 2003;24(3–4):312–23. [PubMed] [Google Scholar]


