Abstract
Objective
To determine whether hospital discharges for intussusception in children younger than 1 year have changed since the reintroduction of rotavirus vaccine in the United States.
Design
Serial cross-sectional analysis.
Setting
US hospitals.
Participants
Children younger than 1 year with a discharge diagnosis of intussusception identified in the Kids’ Inpatient Database, a series of nationally representative data sets of pediatric hospital discharges in the United States with 4 available years prior to vaccine reintroduction (1997, 2000, 2003, and 2006) and 1 year after (2009).
Main Exposures
Hospital discharge before vs after rotavirus vaccine reintroduction.
Outcome Measures
Total number and rate of hospital discharges for infants younger than 1 year with a diagnosis of intussusception (International Classification of Diseases, Ninth Revision, Clinical Modification code 560.0).
Results
From 1997 to 2006, there was no change in the total number of hospital discharges for intussusception, with a small decrease in the rate of intussusception discharges (41.6 [95% CI, 36.7–46.5] to 36.5 [95% CI, 31.7–41.2] per 100 000 infants). Based on the trend, the predicted rate of discharges for intussusception in 2009 was 36.0 (95% CI, 30.2–41.8) per 100 000 infants. The measured rate of hospital discharges for intussusception in 2009 was 33.3 (95% CI, 29.0–37.6) per 100 000 infants.
Conclusion
The reintroduction of rotavirus vaccine since 2006 has not resulted in a detectable increase in the number of hospital discharges for intussusception among US infants.
In 1998, a live, attenuated tetravalent rotavirus vaccine (RotaShield; Wyeth) was recommended for routine use in infants but was then withdrawn in 1999 after concerns arose suggesting an excess risk of intussusception among infants in the weeks following receipt of vaccine.1,2 As a result, larger prelicensure trials with more than 60 000 infants were performed for a pentavalent rotavirus vaccine (RotaTeq; Merck) and a monovalent rotavirus vaccine (Rotarix; GlaxoSmithKline), neither of which was associated with an increased risk of intussusception.3,4 Trial data led to licensure of both vaccines (RotaTeq, 2006; Rotarix, 2008) and the reintroduction of routine rotavirus vaccination for infants in the United States, with 2007 as the first full year of vaccination efforts.
To date, postlicensure surveillance studies in the United States have not detected an increased risk of intussusception among rotavirus vaccine recipients.5,6 In contrast, postlicensure active surveillance studies in 69 hospitals in Mexico and Brazil found a small increased risk of intussusception (1 in 51 000 to 1 in 68 000) for infants receiving the first or second dose of monovalent rotavirus vaccine.7 In addition, a recent study in Australia found an increased risk of intussusception in the weeks following the first dose of rotavirus vaccine but no overall increase in the risk of intussusception for vaccinated children aged 1 to 9 months.8
These concerns about intussusception re-emerge at the same time that rapid national adoption of rotavirus vaccination in the United States9 has been associated with significant reductions in diarrhea-associated outpatient and emergency department (ED) visits10–12 and hospitalizations11–15 during rotavirus seasons. The small but clinically significant increased risk of intussusception now reported from international studies, combined with the widespread adoption of rotavirus vaccine in the United States, raises concern for the possibility of increasing intussusception rates with continuing widespread use of rotavirus vaccine. Given the rare incidence of intussusception, it is plausible that surveillance networks may not have the reach or statistical power to identify significant changes in patterns of this condition.
Therefore, we sought to address the following question using national data: Has there been a change in hospitalizations for intussusception in infants after the reintroduction of rotavirus vaccine in the United States? To answer this question, we analyzed publicly available data from 3 different sources to examine intussusception rates over time and to estimate excess cases of intussusception that would be predicted based on available rotavirus vaccination coverage rates and the increased risk found in the recent international studies.
METHODS
STUDY DESIGN AND SETTING
We performed a serial cross-sectional analysis of pediatric discharges in 1997, 2000, 2003, 2006, and 2009 using the Health-care Cost and Utilization Project (HCUP) Kids’ Inpatient Database (KID), compiled by the Agency for Healthcare Research and Quality.16 The KID is a nationally representative database that samples 80% of pediatric discharges and 10% of uncomplicated births to increase the statistical power to detect and evaluate rare conditions among hospitalized children. Discharges are weighted based on the sampling scheme to permit inferences for a nationally representative population. In 2009, KID contained deidentified information for approximately 7.4 million weighted discharges from 4121 hospitals in 44 states. Readmissions are not identified in this data set. The HCUP provides additional sampling and weighting details.17
IDENTIFICATION OF SAMPLE
We included all hospitalizations for patients younger than 1 year with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code of 560.0 (intussusception) included as any of the discharge diagnoses. The analysis was restricted to infants for 3 reasons: (1) the rotavirus vaccine series is recommended to be administered at 2, 4, and 6 months of age for the pentavalent vaccine and 2 and 4 months for the monovalent vaccine; (2) previous epidemiological reports have suggested that most cases of intussusception related to the rotavirus vaccine occur within 1 to 2 weeks of administration2,7; and (3) 1 year was the finest gradation of age that is reliably reported in HCUP data.
As a proxy for the severity of cases, ICD-9-CM procedure codes were used to determine those hospitalizations for intussusception that required a surgical intervention. Surgical reduction was defined by procedure codes 46.80 to 46.82 (intraabdominal manipulation of the intestine, various levels). To better understand trends in surgical reduction, we also analyzed hospital discharges that had a code for radiologic reduction, which could potentially preempt the need for surgical intervention. Radiologic reduction was defined by procedure code 96.29 (reduction of intussusception of alimentary tract by fluoroscopy, enema, ultrasonography, water, or air) in 2000 to 2009. In 1997, the radiologic reduction procedure code was 93.39 (transanal enema, rectal irrigation). Inpatient mortality based on discharge disposition was also evaluated but could not be estimated because too few cases existed to provide reliable estimates.
To address potential case detection bias, a supplemental analysis was performed using data from the HCUP Nationwide Emergency Department Sample (NEDS) for all available years (2006–2008).18 A previous study found that more than 40% of intussusception cases were managed and discharged directly from ED or short-stay settings and could be missed in analyses using only inpatient discharge data.19 If the proportion of intussusception cases admitted from an ED setting is stable over time, inpatient data on intussusception should be a reasonable proxy for overall intussusception rates and should reflect trends over time. Intussusception cases were identified in NEDS using the same ICD-9-CM diagnosis codes described earlier for infants.
ANALYSIS OF TRENDS IN HOSPITALIZED CASE OF INTUSSUSCEPTION
Statistical analyses were performed using Stata 11.1 (Stata-Corp). All analyses were weighted by Agency for Healthcare Research and Quality–specified discharge values to obtain nationally representative estimates. The primary analysis was structured to compare the actual rate of population-based discharges for children younger than 1 year with a diagnosis of intussusception in 2009 with the predicted rate of discharges based on the trend in the 4 study years before the introduction of RotaTeq and Rotarix (1997, 2000, 2003, and 2006).20 We used methods recommended by HCUP to account for changes over the study years in the sampling frame of KID.20 Of note, RotaShield was introduced and withdrawn between the 2 initial years of KID and would be expected to have no influence on rates.1 The year 2006 was considered a prevaccine year because the Advisory Committee on Immunization Practices published recommendations for routine rotavirus immunization for infants in August 2006, with adoption by public and private payers in the following months. This makes it likely that very few infants received rotavirus vaccine during 2006; in fact, no national rotavirus vaccination data are available for the United States for 2006. We also performed a sensitivity analysis on the trend from 1997 to 2003, excluding the data from 2006 owing to some children receiving rotavirus vaccine during that year.
The number of weighted discharges for intussusception for children younger than 1 year was determined for 1997, 2000, 2003, 2006, and 2009, along with appropriate standard errors and confidence intervals. The trend in number of weighted discharges from 1997 to 2006 was assessed using variance-weighted regression.21,22 The predicted number of discharges for intussusception in 2009 was then determined from the regression model for the prior years. The number of weighted discharges was then adjusted by the population estimates from the US Census in each year23 (see eTable 1 for specific values; http://www.archpediatrics.com) to equal the rate of discharges for intussusception per 100 000 children in the population younger than 1 year. The predicted and actual rates of discharges were compared using the 95% confidence intervals. Similar analyses were performed for the proportion of hospital discharges for intussusception that included a procedure (surgical, radiologic, or any) for reduction.
To confirm the trend in overall discharges for intussusception in children younger than 1 year found in the KID, the HCUP National Inpatient Sample (NIS) was also evaluated using the total weighted discharges in all years between 1997 and 2006.24 The number of discharges and standard errors for the NIS are publicly available.19 The NIS has a different sampling strategy than the KID, with collection every year, and it is not focused on pediatric disease, which results in fewer observations to generate national estimates.25 As a result, in the NIS there is increased measurement error in the annual national estimates of pediatric discharges, but there are more years of data to include in the model. Variance-weighted regression was used in the same manner as described earlier. The results, presented in the eFigure, confirm findings with the KID data but with increased variation year to year reflecting the smaller sample size.
ANALYSES OF POTENTIAL INCREASE IN INTUSSUSCEPTION HOSPITALIZATIONS ASSOCIATED WITH ROTAVIRUS VACCINE
We performed an additional analysis to assess whether the risk of intussusception found in the large postlicensure studies in Mexico and Brazil would be detectable in data from the 2009 KID. For 2009, the potential increased rate of hospitalizations for intussusception after rotavirus vaccine reintroduction was estimated based on the population of infants younger than 1 year, the proportion of infants receiving at least 1 dose of rotavirus vaccine (72%),8 the excess risk of intussusception estimated in the most recent published studies (1 in 51 000–68 000),7 and the proportion of cases admitted to inpatient settings based on the 2008 NEDS data.
The potential increased rate of intussusception hospitalizations due to vaccine was summed, with the predicted rate of intussusception hospitalizations in 2009 based on the trend from 1997–2006 KID data. This was then compared with the measured rate of intussusception hospital discharges in the 2009 KID.
HUMAN SUBJECTS REVIEW
As an analysis of deidentified national data, this study was exempt from human subjects review.
RESULTS
HOSPITAL DISCHARGES FOR INTUSSUSCEPTION, 1997 TO 2009
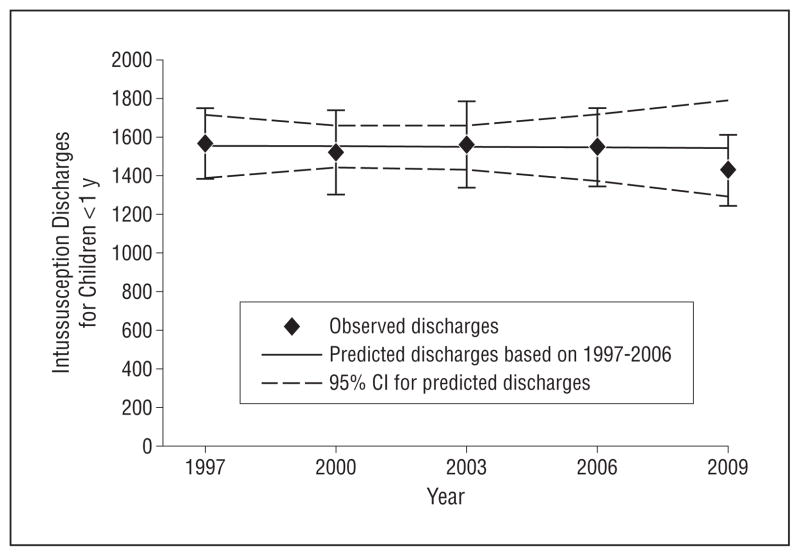
Based on data from KID, there was no change in the estimated total number of hospital discharges for intussusception among infants younger than 1 year prior to the reintroduction of rotavirus vaccine, from 1565 (95% CI, 1381–1750) in 1997 to 1548 (95% CI, 1345–1751) in 2006 (Figure). After population adjustment, there was a small decline in the rate of hospital discharges for intussusception from 1997 through 2006 (41.6 [95% CI, 36.7–46.5] to 36.5 [95% CI, 31.7–41.2] per 100 000 infants) (Table).
Figure.
Observed and predicted hospital discharges for intussusception for infants before (1997–2006) and after (2009) rotavirus vaccine reintroduction in the United States. CI indicates confidence interval.
Table.
Observed and Predicted Rates of Hospital Discharges for Intussusception for Infants Before and After Rotavirus Vaccine Reintroduction in the United States, 1997–2009
| Hospital Discharges for Intussusception, % (95% CI)
|
||||
|---|---|---|---|---|
| Rate of Discharges per 100 000 Children | Proportion of Discharges for Intussusception With Interventions
|
|||
| Any Procedure | Surgical Procedure | Radiologic Procedure | ||
| Before vaccine | ||||
| 1997 | 41.6 (36.7–46.5) | 43.2 (38.6–47.8)a | 40.4 (35.9–44.8) | 3.7 (1.6–5.7) |
| 2000 | 38.3 (32.8–43.7) | 52.1 (47.7–56.5) | 38.8 (34.5–43.1) | 14.8 (10.0–19.7) |
| 2003 | 38.3 (32.8–43.8) | 55.4 (50.1–60.8) | 41.4 (37.0–45.8) | 16.2 (11.1–21.3) |
| 2006 | 36.5 (31.7–41.2) | 58.5 (54.1–63.0) | 40.1 (36.4–43.8) | 22.0 (17.4–26.7) |
| After vaccine | ||||
| 2009 (Predicted)b | 36.0 (30.2–41.8) | 64.5 (58.8–70.1) | 40.4 (35.7–45.3) | 29.3 (24.0–43.6) |
| 2009 (Observed) | 33.3 (29.0–37.6) | 54.8 (50.7–58.9) | 36.3 (32.7–39.9) | 22.4 (18.6–26.3) |
Abbreviation: CI, confidence interval.
Infants may have received more than 1 procedure during a hospitalization; thus, the proportion with any procedure does not equal the sum of the proportions with surgical and radiologic procedures.
Prediction based on analysis of 1997–2006 data using variance-weighted regression.
Based on this trend, the predicted number of intussusception discharges in 2009 was 1543 (95% CI, 1295–1792), equivalent to a rate of 36.0 (95% CI, 30.2–41.8) per 100 000 infants. The measured number of discharges for intussusception in 2009 was 1428 (95% CI, 1244–1612), equal to a rate of 33.3 (95% CI, 29.0–37.6) per 100 000 infants, falling below the point estimate and within the confidence interval of the rate predicted by the trend prior to rotavirus vaccine reintroduction (Figure). Results from a sensitivity analysis excluding data from 2006 were consistent with these findings (eTable 1).
From 1997 to 2006, the proportion of discharges for intussusception with any radiologic or surgical intervention increased from 43.2% to 58.5% (Table). In 2009, the proportion of discharges for intussusception with any radiologic or surgical intervention was 54.8%, less than the proportion predicted by the trend from 1997 to 2006 (Table). The increase in the proportion of intussusception discharges with a procedure was driven by an increase in radiologic procedures from 3.7% of intussusception discharges in 1997 to 22.4% in 2009. The proportion of intussusception discharges with a surgical procedure was consistent from 1997 to 2006, at approximately 40%, with a slight decrease in 2009 to 36.3% (Table).
CASES OF INTUSSUSCEPTION IN THE ED SETTING
Based on the NEDS, the proportion of cases with intussusception discharged directly from the ED was 33% to 39% in 2006 to 2008. There was not a significant trend in the proportion of intussusception cases admitted vs discharged from EDs across this period. Full details are included in eTable 2. As a sensitivity analysis to assess the potential effects of varying ED discharge rates on the overall results for 2009, we estimated the number of intussusception cases that would have been absent from hospital discharge data if the proportion of intussusception cases discharged directly from the ED in 2009 was equivalent to the highest vs the lowest proportions in the 2006–2008 NEDS. We estimated that an additional 212 cases of intussusception would have been absent from the KID data in 2009 if ED discharge rates were at their highest rather than lowest levels. If this number of cases were added to the measured number of intussusception discharges from the 2009 KID (1428 + 212=1640), the total number of intussusception discharges is not significantly different from the number of discharges for 2009 predicted by the trend from 1997 to 2006 (1543 [95% CI, 1295–1792]).
POTENTIAL RISK OF INTUSSUSCEPTION HOSPITALIZATIONS WITH ROTAVIRUS VACCINATION
Assuming the rates of intussusception after rotavirus immunization reported in studies in Mexico and Brazil, an increase of 1.0 to 1.3 hospital discharges for intussusception per 100 000 infants would have been expected in 2009 in the United States compared with the baseline trend. When these estimates are added to the predicted rate of hospital discharges for intussusception in 2009, the result is an estimate of 37.4 to 37.7 hospital discharges for intussusception per 100 000 infants. These estimates are greater than the observed discharge rate for intussusception from the 2009 KID (33.3 [95% CI, 29.0–37.6] per 100 000 infants), again indicating no evidence for an increase in intussusception attributable to patterns of rotavirus vaccination.
COMMENT
In this study using the latest nationally representative data, there has been no detectable increase in the rate of hospital discharges for intussusception among infants younger than 1 year since the reintroduction of rotavirus vaccine in the United States. Our findings are consistent with prior studies showing intussusception rates in the range of 20 to 50 per 100 000 infants, depending on population and data source.26,27 Our results are also consistent with the gradual decrease in intussusception cases in the United States over time found in these studies,26,27 suggesting that this downward trend has continued after the widespread implementation of rotavirus vaccine. Additionally, our findings are consistent with a recent study in Australia that did not show an overall increased risk of intussusception among vaccinated children.8
It is intriguing that discharges for intussusception in 2009 fall below the rate predicted by the trend from 1997 to 2006 and well below the rate predicted by the trend from 1997 to 2006 augmented with the vaccine-related increased risk found in a prior study.7 It has been suggested that the effect of any increased intussusception risk found for rotavirus vaccine is difficult to predict at the population level2,28 because little is known about the risk of intussusception from wild-type rotavirus or other viruses as compared with rotavirus vaccine.29,30 Our analysis suggests that the reintroduction of rotavirus vaccine has not increased and may have contributed to a decrease in the total burden of intussusception-related hospitalizations in the United States.
Severe cases of intussusception require reduction by invasive techniques to prevent progression to ischemic bowel or even death. Fortunately, inpatient mortality from intussusception in this age group is so small that it could not be estimated in this population. Our analysis indicates that there was not an increase in the proportion of intussusception cases requiring a surgical procedure after reintroduction of rotavirus vaccine. While estimates of the proportion of cases that require intervention are not perfect surrogates for the severity of cases of intussusception because of variation in indications for procedures, they are indices that can be followed over time. Since 1997, there has been an increasing proportion of intussusception-related hospitalizations that have had radiologic reduction, while the proportion requiring surgical reduction has remained constant. The surgical rates observed herein are lower than previously described but follow a similar trend.27 At a minimum, these findings provide evidence that there has not been an increase in the severe forms of intussusception that require surgical intervention and may lead to additional morbidity and mortality.
The findings from this study are strengthened by the use of large, nationally representative data sets and results showing that the proportion of intussusception cases admitted vs discharged from EDs remained stable from 2006 to 2008. However, these analyses do have several limitations. There has only been 1 year of KID data (2009) after the reintroduction of rotavirus vaccine, limiting the strength of conclusions from the trend analysis. Retrospective identification of intussusception using administrative data is likely not as robust as prospective surveillance and case review.31,32 The use of administrative data makes it unclear, in particular, how much of the trends in surgical and radiologic interventions are attributable to changes in coding and billing practices vs changes in clinical practice. Our ability to assess the influence of outpatient management trends on intussusception hospitalization rates was limited by relatively few years of national ED data. Additionally, we cannot determine whether children in any of these data sets received rotavirus vaccine or the timing of vaccine receipt in relation to the hospitalization for intussusception.
Last, because of the width of the confidence intervals in the measured intussusception discharge rates, these results cannot unequivocally rule out a small increase in intussusception hospitalizations after the reintroduction of rotavirus vaccine consistent with the risk magnitude found in the studies in Mexico and Brazil. Nonetheless, this appears unlikely given that the measured rates of hospital discharges for intussusception in 2009 were well below the estimates that would be expected from the increased risk of intussusception after rotavirus vaccination found in those studies. Further work is warranted to clarify whether the risk of intussusception after rotavirus vaccine found in Mexico and Brazil also applies to the US population. If there is increased risk due to rotavirus vaccination in the United States, the risk is not large enough to result in a statistically detectable increase in hospitalizations for intussusception in children younger than 1 year.
To our knowledge, this is the first study to examine nationally representative hospitalization rates for intussusception among infants after the reintroduction of rotavirus vaccine in the United States. Although epidemiologic studies have indicated a small increased risk of intussusception immediately following the first and second doses of rotavirus vaccine, our findings suggest that this has not translated into an increase in the overall rate of hospitalizations for intussusception for infants in the first several years of widespread vaccination.
Supplementary Material
Acknowledgments
Funding/Support: Drs Zickafoose, Benneyworth, Riebschleger, and Espinosa were supported by training grant T32 HD07534 from the National Institute of Child Health and Human Development. Drs Riebschleger and Espinosa were also supported by grant NCRR UL1RR024986 from the Michigan Institute for Clinical and Health Research.
Footnotes
Author Contributions: Study concept and design: Zickafoose, Benneyworth, Riebschleger, and Davis. Acquisition of data: Benneyworth. Analysis and interpretation of data: Zickafoose, Benneyworth, Riebschleger, Espinosa, and Davis. Drafting of the manuscript: Zickafoose and Benneyworth. Critical revision of the manuscript for important intellectual content: Zickafoose, Benneyworth, Riebschleger, Espinosa, and Davis. Statistical analysis: Zickafoose, Benneyworth, and Espinosa. Administrative, technical, and material support: Davis.
Financial Disclosure: None reported.
Online-Only Material: The eTables and eFigure are available at http://www.archpediatrics.com.
Additional Contributions: We acknowledge the state organizations that contribute to HCUP (http://www.hcup-us.ahrq.gov/db/hcupdatapartners.jsp).
References
- 1.Centers for Disease Control and Prevention (CDC) Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999;48(43):1007. [PubMed] [Google Scholar]
- 2.Murphy BR, Morens DM, Simonsen L, Chanock RM, La Montagne JR, Kapikian AZ. Reappraisal of the association of intussusception with the licensed live rotavirus vaccine challenges initial conclusions. J Infect Dis. 2003;187(8):1301–1308. doi: 10.1086/367895. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 4.Vesikari T, Matson DO, Dennehy P, et al. Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 5.Belongia EA, Irving SA, Shui IM, et al. Vaccine Safety Datalink Investigation Group. Real-time surveillance to assess risk of intussusception and other adverse events after pentavalent, bovine-derived rotavirus vaccine. Pediatr Infect Dis J. 2010;29(1):1–5. doi: 10.1097/INF.0b013e3181af8605. [DOI] [PubMed] [Google Scholar]
- 6.Haber P, Patel M, Izurieta HS, et al. Postlicensure monitoring of intussusception after RotaTeq vaccination in the United States, February 1, 2006, to September 25, 2007. Pediatrics. 2008;121(6):1206–1212. doi: 10.1542/peds.2007-3793. [DOI] [PubMed] [Google Scholar]
- 7.Patel MM, López-Collada VR, Bulhões MM, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364(24):2283–2292. doi: 10.1056/NEJMoa1012952. [DOI] [PubMed] [Google Scholar]
- 8.Buttery JP, Danchin MH, Lee KJ, et al. PAEDS/APSU Study Group. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011;29(16):3061–3066. doi: 10.1016/j.vaccine.2011.01.088. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Rotavirus vaccination coverage among infants aged 5 months: immunization information system sentinel sites, United States, June 2006–June 2009. MMWR Morb Mortal Wkly Rep. 2010;59(17):521–524. [PubMed] [Google Scholar]
- 10.Bégué RE, Perrin K. Reduction in gastroenteritis with the use of pentavalent rotavirus vaccine in a primary practice. Pediatrics. 2010;126(1):e40–e45. doi: 10.1542/peds.2009-2069. [DOI] [PubMed] [Google Scholar]
- 11.Cortese MM, Tate JE, Simonsen L, Edelman L, Parashar UD. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatr Infect Dis J. 2010;29(6):489–494. doi: 10.1097/INF.0b013e3181d95b53. [DOI] [PubMed] [Google Scholar]
- 12.Staat MA, Payne DC, Donauer S, et al. New Vaccine Surveillance Network (NVSN) Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics. 2011;128(2):e267–e275. doi: 10.1542/peds.2010-3722. [DOI] [PubMed] [Google Scholar]
- 13.Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis. 2010;201(11):1617–1624. doi: 10.1086/652403. [DOI] [PubMed] [Google Scholar]
- 14.Tate JE, Cortese MM, Payne DC, et al. Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data. Pediatr Infect Dis J. 2011;30(1 suppl):S56–S60. doi: 10.1097/INF.0b013e3181fefdc0. [DOI] [PubMed] [Google Scholar]
- 15.Yen C, Tate JE, Wenk JD, Harris JM, II, Parashar UD. Diarrhea-associated hospitalizations among US children over 2 rotavirus seasons after vaccine introduction. Pediatrics. 2011;127(1):e9–e15. doi: 10.1542/peds.2010-1393. [DOI] [PubMed] [Google Scholar]
- 16.Introduction to the HCUP Kids’ Inpatient Database (KID) 2009. Rockville, MD: Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project (HCUP); 2011. [Google Scholar]
- 17.The Kids’ Inpatient Database. Healthcare Cost and Utilization Project (HCUP); [Accessed June 30, 2011]. Web site. www.hcup-us.ahrq.gov/kidoverview.jsp. [Google Scholar]
- 18.Welcome to HCUPnet. Healthcare Cost and Utilization Project (HCUP); [Accessed June 30, 2011]. Web site. http://hcupnet.ahrq.gov. [Google Scholar]
- 19.Cortese MM, Staat MA, Weinberg GA, et al. Underestimates of intussusception rates among US infants based on inpatient discharge data: implications for monitoring the safety of rotavirus vaccines. J Infect Dis. 2009;200(suppl 1):S264–S270. doi: 10.1086/605055. [DOI] [PubMed] [Google Scholar]
- 20.Using the KIDS’ Inpatient Database (KID) to Estimate Trends. Rockville, MD: Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project (HCUP); 2009. Report 2007–02. HCUP Methods Series. [Google Scholar]
- 21.Weisberg S. Applied Linear Regression. Hoboken, NJ: Wiley-Interscience; 2005. [Google Scholar]
- 22.Benneyworth BD, Gebremariam A, Clark SJ, Shanley TP, Davis MM. Inpatient health care utilization for children dependent on long-term mechanical ventilation. Pediatrics. 2011;127(6):e1533–e1541. doi: 10.1542/peds.2010-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Resident population estimates. US Census Bureau, Population Division; [Accessed July 28, 2011]. Web site. http://www.census.gov/popest/estimates.html. Published 2011. [Google Scholar]
- 24.Overview of the Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP); [Accessed July 1, 2011]. Web site. www.hcup-us.ahrq.gov/nisoverview.jsp. [Google Scholar]
- 25.Introduction to the HCUP Nationwide Inpatient Sample (NIS) 2009. Rockville, MD: Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project (HCUP); 2011. [Google Scholar]
- 26.Parashar UD, Holman RC, Cummings KC, et al. Trends in intussusception-associated hospitalizations and deaths among US infants. Pediatrics. 2000;106(6):1413–1421. doi: 10.1542/peds.106.6.1413. [DOI] [PubMed] [Google Scholar]
- 27.Tate JE, Simonsen L, Viboud C, et al. Trends in intussusception hospitalizations among US infants, 1993–2004: implications for monitoring the safety of the new rotavirus vaccination program. Pediatrics. 2008;121(5):e1125–e1132. doi: 10.1542/peds.2007-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenberg HB. Rotavirus vaccination and intussusception: act two. N Engl J Med. 2011;364(24):2354–2355. doi: 10.1056/NEJMe1105302. [DOI] [PubMed] [Google Scholar]
- 29.El-Hodhod MA, Nassar MF, Ezz El-Arab S, Ahmed EF. Rotavirus fecal antigen retrieval in infantile intussusception. Eur J Clin Microbiol Infect Dis. 2008;27 (9):879–881. doi: 10.1007/s10096-008-0506-6. [DOI] [PubMed] [Google Scholar]
- 30.Robinson CG, Hernanz-Schulman M, Zhu Y, Griffin MR, Gruber W, Edwards KM. Evaluation of anatomic changes in young children with natural rotavirus infection: is intussusception biologically plausible? J Infect Dis. 2004;189(8):1382–1387. doi: 10.1086/382655. [DOI] [PubMed] [Google Scholar]
- 31.Bines JE, Patel M, Parashar U. Assessment of postlicensure safety of rotavirus vaccines, with emphasis on intussusception. J Infect Dis. 2009;200(suppl 1):S282–S290. doi: 10.1086/605051. [DOI] [PubMed] [Google Scholar]
- 32.Bines JE, Kohl KS, Forster J, et al. Brighton Collaboration Intussusception Working Group. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004;22(5–6):569–574. doi: 10.1016/j.vaccine.2003.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.