Abstract
Following cervical spinal cord injury at C2 (SH hemisection model) there is progressive recovery of phrenic activity. Neuroplasticity in the postsynaptic expression of neurotransmitter receptors may contribute to functional recovery. Phrenic motoneurons express multiple serotonergic (5-HTR) and glutamatergic (GluR) receptors, but the timing and possible role of these different neurotransmitter receptor subtypes in the neuroplasticity following SH are not clear. The current study was designed to test the hypothesis that there is an increased expression of serotonergic and glutamatergic neurotransmitter receptors within phrenic motoneurons after SH. In adult male rats, phrenic motoneurons were labeled retrogradely by intrapleural injection of Alexa 488-conjugated cholera toxin B. In thin (10 μm) frozen sections of the spinal cord, fluorescently-labeled phrenic motoneurons were visualized for laser capture microdissection (LCM). Using quantitative real-time RT-PCR in LCM samples, the time course of changes in 5-HTR and GluR mRNA expression was determined in phrenic motoneurons up to 21 days post-SH. Expression of 5-HTR subtypes 1b, 2a and 2c and GluR subtypes AMPA, NMDA, mGluR1 and mGluR5 was evident in phrenic motoneurons from control and SH rats. Phrenic motoneuron expression of 5-HTR2a increased ~8-fold (relative to control) at 14 days post-SH, whereas NMDA expression increased ~16-fold by 21-days post-SH. There were no other significant changes in receptor expression at any time post-SH. This is the first study to systematically document changes in motoneuron expression of multiple neurotransmitter receptors involved in regulation of motoneuron excitability. By providing information on the neuroplasticity of receptors expressed in a motoneuron pool that is inactivated by a higher-level spinal cord injury, appropriate pharmacological targets can be identified to alter motoneuron excitability.
Keywords: Neuroplasticity, Neurotransmitter, Laser capture microdissection, Quantitative real-time RT-PCR, AMPA, NMDA, 5-HTR2a, Spinal hemisection, Motor neuron
Introduction
Upper cervical spinal cord injury (SCI) disrupts descending premotor excitatory drive to phrenic motoneurons, leading to ipsilateral diaphragm muscle paralysis. Over time, there is gradual recovery of rhythmic diaphragm activity following a spinal cord hemisection at C2 (SH) (Fuller et al., 2006; Golder and Mitchell, 2005; Nantwi et al., 1999; Porter, 1895). This example of neuroplasticity results in recovery of motor function, albeit partial, after SCI. Functional recovery after SH may reflect restoration of descending excitatory inputs from contralateral pathways, the “crossed-phrenic phenomenon” (Goshgarian, 1981; Rosenblueth et al., 1938). In addition, functional recovery may reflect changes in phrenic motoneuron expression of excitatory neurotransmitter and neuromodulatory receptors (Alilain and Goshgarian, 2008; Fuller et al., 2005; Murray et al., 2010). In the present study, we show that quantitation of motoneuron expression of specific receptor subtypes is now possible with the use of quantitative real-time RT-PCR measurements in retrogradely-labeled phrenic motoneurons sampled using laser capture microdissection (LCM).
The primary excitatory synaptic input to phrenic motoneurons is glutamatergic, likely acting via α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) subtype receptors (Greer et al., 1991; Liu et al., 1990; Murphy et al., 1996). Phrenic motoneurons also express the N-methyl-d-aspartate (NMDA) subtype of glutamate receptors and the metabotropic (G-protein coupled) receptor subtypes mGluR1 and mGluR5 (Dong and Feldman, 1999; McCrimmon et al., 1989). Serotonin receptors (5-HTR) also play an important role in several forms of phrenic motor plasticity, including recovery from SCI (Fuller et al., 2001; Mitchell et al., 1992; Zhou et al., 2001). Based primarily on pharmacological experiments and mRNA measurements in spinal cord homogenates, 5-HTR expression in phrenic motoneurons is thought to include 5-HTR1b, 5-HTR2a and 5-HTR2c receptor subtypes (Basura et al., 2001; Fuller et al., 2005). The current study was designed to test the hypothesis that increased expression of serotonergic and glutamatergic neurotransmitter receptors within phrenic motoneurons occurs after SH. This study was conducted to validate quantitative measurements of receptor mRNA levels in phrenic motoneurons sampled by LCM and determine the time course of multiple serotonergic and glutamatergic receptor subtype expression in a well-established model of SCI.
Materials and methods
Adult male Sprague–Dawley rats (wt. ~300 g) were assigned to two different experimental groups: control (CTL, n=7) and spinal cord hemisection at C2 (SH, n =23). All animal protocols were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the Mayo Clinic.
Spinal cord hemisection (SH)
Details of the surgical procedure and validation of the technique were reported previously (Mantilla et al., 2007; Miyata et al., 1995; Prakash et al., 1999). Briefly, rats were anesthetized using intramuscular ketamine (90 mg/kg) and xylazine (10 mg/kg). Under sterile conditions, a dorsal C2 laminectomy was performed using a dissecting microscope (Carl Zeiss Inc., Thornwood NY) and the right anterolateral cord was cut at C2 with a surgical microknife. The hemisection was anterior to the dorsal root entry zone fissure (i.e., involving only the lateral and ventral funiculi, while preserving the dorsal funiculus). Animals were allowed to recover with a heating pad. Acetaminophen (100–300 mg/kg) was administered orally for the first 3 days and buprenorphine (0.1 mg/kg) was injected i.m. as needed.
Diaphragm EMG recordings
Three days prior to the SH surgery, a pair of electrode wires were implanted into the midcostal regions of both the right and left sides of the diaphragm muscle for chronic EMG recordings, as previously reported (Dow et al., 2006, 2009; Mantilla et al., 2011; Trelease et al., 1982). The electrode wires (insulated multi-stranded stainless steel, 0.28 mm diameter—model AS631, Cooner Wire Inc.) were stripped of insulation for 2 mm and secured such that the uninsulated portion remained within the muscle. The electrode wires were tunneled subcutaneously to the back of the animal where they were externalized.
Diaphragm EMG activity was assessed at the time of surgery (SH) to document absence of ipsilateral activity. In addition, diaphragm EMG activity was recorded in passively-restrained, awake animals at 3 days post-SH (SH3D), at which time absence of eupneic rhythmic diaphragm EMG activity was used to verify the completeness of the surgical hemisection. At regular intervals (7, 14 or 21 days post-SH), diaphragm EMG recordings were used to determine recovery of ipsilateral rhythmic eupneic diaphragm EMG activity. In all cases, EMG signals were differentially amplified (2000×) and band-pass filtered between 20 Hz and 1 kHz (Model 2124, DATA Inc.), analog to digital converted at 2 kHz and recorded using LabView-based software with data acquisition hardware (National Instruments, Austin, TX). The root-mean-squared (RMS) was calculated for 50-ms windows to determine the amplitude of any diaphragm EMG activity (Mantilla et al., 2011).
Retrograde labeling of phrenic motoneurons
Phrenic motoneurons were labeled by intrapleural injection of Alexa Fluor 488-conjugated cholera toxin subunit B (CTB; Invitrogen), as previously described (Mantilla et al., 2009). Briefly, 15 μl of a 0.2% solution of Alexa Fluor 488-CTB were injected bilaterally using a Hamilton syringe. The pleural space was injected between the 7th and 8th ribs three days prior to the terminal experiment. We previously confirmed that this intrapleural technique only labels phrenic motoneurons in the cervical spinal cord (Mantilla et al., 2009).
Laser capture microdissection (LCM) of phrenic motoneurons
At the terminal experiment, animals were anesthetized as above, euthanized by exsanguination and the cervical spinal cord (C2–C7) was quickly excised and frozen in liquid N2 under RNase-free conditions. Spinal cords were cut in 10 μm thick rostrocaudal sections in such manner that the left and right sides could be easily identified. Sections were placed on pre-chilled slides (SuperFrost, Fisher Scientific, Pittsburgh, PA) and stored at −80 °C until immediately before microdissection. Slides were placed in serial alcohol dehydration steps followed by xylene treatment according to the manufacturer’s protocol (Arcturus LCM, Applied Biosystems, Life Technologies Corp., Carlsbad, CA). Individual, retrogradely-labeled phrenic motoneurons were visualized under direct epifluorescence illumination and microdissected onto Capsure HS LCM caps (Arcturus) using an Arcturus XT LCM microdissection system (Fig. 1). In most cases, it was possible to use a single cap for 3–4 spinal cord sections. On average, four caps were obtained from the right side of the spinal cord (ipsilateral to SH) for each animal. Caps were stored at −80 °C until RNA extraction.
Fig. 1.
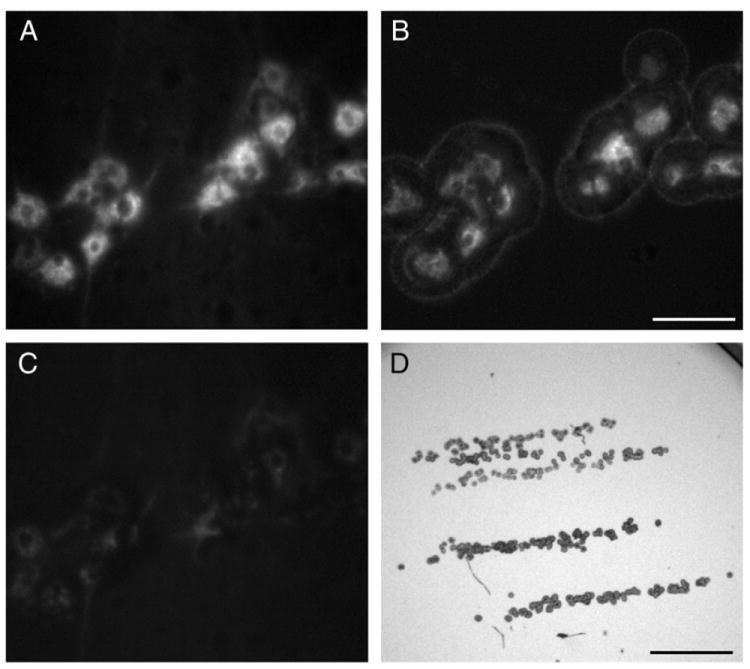
Representative photomicrographs showing labeled phrenic motoneurons that were targeted for laser capture microdissection (LCM). A. Phrenic motoneurons were labeled by intrapleural injection of Alexa 488-conjugated cholera toxin subunit B and visualized using epifluorescence. B. Selective capture of phrenic motoneurons was confirmed visually in the LCM cap. C. Complete capture of phrenic motoneurons is evident by lack of Alexa 488 fluorescence in the section of the spinal cord tissue. D. LCM cap at low magnification shows column of phrenic motoneurons captured from multiple (four) sections from the same animal. Bar, 100 μm in A–C; 1 mm in D.
Phrenic motoneuron RNA extraction
Total RNA was extracted from LCM samples using the RNeasy Micro kit (Qiagen Inc., Valencia, CA) following the manufacturer’s protocol with the addition of on-column DNase digestion. The RNA from all caps from each animal was pooled for subsequent analyses.
Reverse transcription
Total RNA was reverse transcribed using the Transcriptor First Strand cDNA Synthesis kit (Roche Applied Science, Indianapolis, IN) following the manufacturer recommended protocol. Briefly, 1.0 μl of sample RNA, 50 pmol anchored-oligo(dT)18 primer, 1.2 nmol random hexamer primer, and RNase free sterile water added to 13 μl were incubated at 65 °C for 10 min and chilled on ice for 1 min. A mix with 1 × Transcriptor reverse transcriptase reaction buffer, 20 units Protector RNase inhibitor, 1 mM each dNTP and 10 U Transcriptor reverse transcriptase was then added to each sample and incubated at room temperature for 10 min followed by 20 min at 55 °C. Samples were then heated to 85 °C for 5 min and placed on ice. All reverse transcription reactions were done in duplicate for each sample.
Real-time PCR
For gene expression analysis, 1 μl of the reverse transcription reaction was added to a reaction mix containing LightCycler 480 1 × SYBR Green I Master (Roche), and 0.5 μM of the respective primer pair (5′ and 3′ primers are listed in Table 1). The following receptor subtypes were studied: 5-HTR1b, 5-HTR2a, 5-HTR2c, AMPA, NMDA, mGluR1 and mGluR5. The ribosomal protein S16 (RPS16) was used as reference gene. Amplification and quantitation of mRNA was performed on a LightCycler 480 (all gene transcripts but NMDA) or LightCycler 2.0 (NMDA and RPS16). Parameters for amplification of all products were identical: an initial, 5-min pre-amplification incubation at 95 °C was followed by 60 cycles of primer annealing at 60 °C for 12 s, PCR product extension at 72 °C for 18 s and denaturing at 95 °C for 15 s. Amplification fluorescence was measured immediately following the PCR product extension step and plotted for quantitative analyses (see below).
Table 1.
Primers used for amplification of serotonergic and glutamatergic receptor mRNA in laser capture microdissected phrenic motoneurons.
| Gene | RGD ID | GenBank accession # | Primer location
|
|
|---|---|---|---|---|
| 5′ primer | 3′ primer | |||
| RPS16 | Rps16 | X17665 | 170–190 | 380–399 |
| 5-HTR1b | Htr1b | X62944 | 559–574 | 898–913 |
| 5-HTR2a | Htr2a | M30705 | 1009–1024 | 1214–1230 |
| 5-HTR2c | Htr2c | U35315 | 732–751 | 958–974 |
| AMPA (GluR2)a | Gria2 | AF164344 | 786–803 | 959–974 |
| NMDA-R1a | Grin1 | U08261 | 2358–2375 | 2603–2618 |
| mGluR1 | Grm1 | X57569 | 3705–3720 | 3896–3912 |
| mGluR5 | Grm5 | D10891 | 2251–2267 | 2523–2538 |
RGD: Rat Genome Database (Twigger et al., 2007); RPS16: ribosomal protein S16; 5-HTR: serotonergic receptor subtypes 1b, 2a and 2c (5-HTR1b, 5-HTR2a and 5-HTR2c, respectively); AMPA: 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid; NMDA: N-methyl-d-aspartate; mGluR; metabotropic glutamate receptor subtypes 1 and 5 (mGluR1 and mGluR5, respectively).
Note that for AMPA and NMDA receptors, expression of the GluR2 and NR1 subunits, respectively, was actually determined.
Following amplification, a melting curve analysis was performed to verify amplification product specificity. Briefly, amplification products were denatured at 95 °C, and then quickly cooled to 65 °C for 15 s. Products were then heated at a rate of 0.1 °C s−1 to a final temperature of 95 °C while continuously measuring fluorescence. The derivative of the melting curve fluorescence (−dF/dT) was plotted versus temperature thus yielding melting peaks indicative of products generated during amplification (Figs. 2A and 4A). The lack of primer–dimer complexes and the presence of an appropriate single peak in the resultant melting curve were indicators of correct amplification products. The specificity of the PCR product was also verified by DNA electrophoresis (correct product size; Figs. 2B and 4B) and by sequencing. Lack of genomic DNA amplification was confirmed by including no reverse transcriptase samples (all samples underwent DNase digestion). All PCR reactions were performed in duplicate for each reverse transcription product.
Fig. 2.
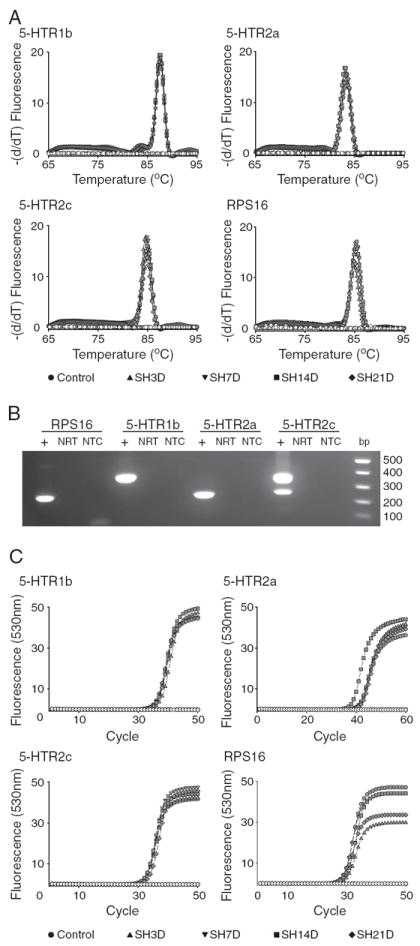
Real-time RT-PCR was used to quantify the expression of ribosomal protein S16 (RPS16) and serotonergic (5-HTR subtypes 1b, 2a and 2c) receptors in microdissected phrenic motoneurons. Experimental groups include control and spinal hemisection (SH) groups at 3, 7, 14 and 21 days post-injury. A. Melting curve analysis for each transcript showing specificity of amplification of the corresponding primer pairs (Table 1) across experimental groups (see label). B. Agarose gel electrophoresis of PCR products was used to verify transcript size. C. Representative amplification curves for serotonergic receptor transcripts and RPS16 across all experimental groups. Specificity of the PCR product was verified by including control reactions that did not include the template (NTC in B) or the RT enzyme (NRT in B; open symbols in A and C). Quantification of receptor expression was performed using the second derivative method for crossing-point (CP) analyses (see Materials and methods for details). The difference between the crossing points for each receptor transcript and RPS16 was used to determine expression levels (ΔCP) for each sample and transcript.
Fig. 4.
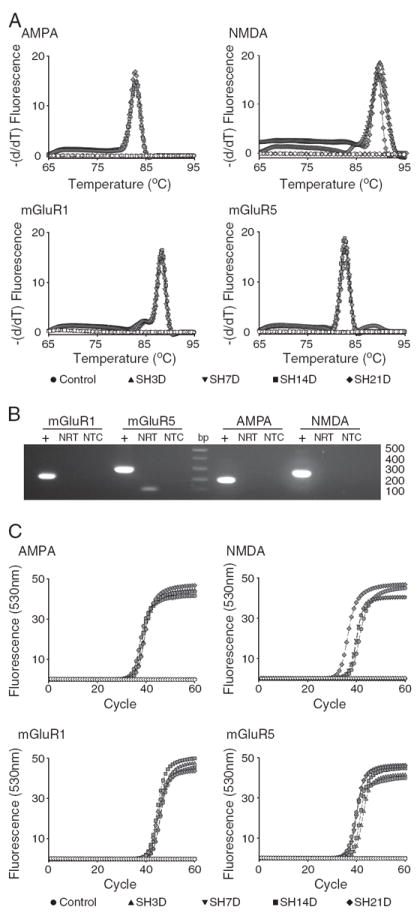
Real-time RT-PCR was used to quantify the expression of glutamatergic receptors (AMPA, NMDA, mGluR1 and mGluR5) in microdissected phrenic motoneurons. A. Melting curve analysis for each transcript showing specificity of amplification of the corresponding primer pairs (Table 1). B. Agarose gel electrophoresis of PCR products was used to verify transcript size. C. Representative amplification curves for glutamatergic receptor transcripts across all experimental groups. Quantification of receptor expression and verification of PCR product specificity was performed as in Fig. 2.
Quantitative analyses
An amplification fluorescence plot was generated for each sample with LightCycler 480 software (version 1.5, Roche) (Figs. 2C and 4C). The second derivative method of the amplification curve was used to determine the threshold cycle (i.e., crossing point—CP) at which amplification of each gene product transitions from the linear phase to the logarithmic phase. Comparison of the CP of the gene product to that of the RPS16 standard run concurrently permitted determination of a ΔCP for each sample and transcript. The ΔCP reflects the relative mRNA concentration for each serotonergic and glutamatergic receptor subtype transcript in each sample. An average ΔCP was then calculated for each transcript and animal. Data were thus clustered per animal and represent 3.9±1.0 (mean±SD) LCM caps and 4.4±2.2 PCR reactions for each transcript.
In order to ensure quantitative measurements of receptor subtype mRNA expression, a real-time RT-PCR technique was used without a pre-amplification step. Although there was limited mRNA available for repeated measurements of different transcripts, all measurements were conducted in duplicate for each RT and PCR reaction (i.e., at least 4 measurements per transcript for each animal). In addition, an extensive validation was performed of the quantitative real-time RT-PCR technique using melting curve analyses, transcript sequencing, gel electrophoresis of the PCR product, and reaction controls lacking template or the RT enzyme. The technique provides quantitative measurements for multiple mRNA transcripts in the same pool of phrenic motoneurons, thus permitting measurements that could not be obtained with previous techniques such as in situ hybridization or analyses of receptor subtype expression using whole tissue homogenates of the spinal cord.
Serotonergic and glutamatergic receptor subtype expression was compared over time following SH by one-way analysis of variance (ANOVA) using JMP Statistical software (JMP 8.0, SAS Institute Inc., Cary, NC). When appropriate, post hoc analyses were conducted using Tukey–Kramer’s honestly significant difference (HSD) test. The correlation between transcripts was calculated by linear regression after calculating a Z-score for each transcript and sample in the entire population. The degree of correlation is expressed as the r2 value, with the effectiveness of the model (difference from the mean response) determined by ANOVA. Statistical significance was considered at p<0.05. Data are presented as mean (±SE), unless otherwise indicated.
Results
Diaphragm EMG activity following SH
A total of 23 SH animals were implanted with bilateral diaphragm EMG electrodes for chronic monitoring of rhythmic respiratory-related activity. Diaphragm EMG recordings were used to verify the completeness of the SH surgery at the time of surgery and 3 days post-SH. No animals displayed ipsilateral rhythmic EMG activity during eupnea at SH3D. Animals were randomly allocated to each experimental group, and repeated diaphragm EMG recordings were performed at 3, 7, 14 and 21 days post-SH as applicable. Over time post-SH, a subset of animals displayed recovery of ipsilateral rhythmic diaphragm EMG activity, such that by SH14D three (out of six) animals displayed functional recovery. By SH21D three (out of six) animals displayed functional recovery, one of which displayed ipsilateral diaphragm EMG activity by SH14D. There was no attempt at quantifying the extent of recovery of rhythmic activity, although in all cases it was greatly reduced in amplitude compared to baseline.
Labeling of phrenic motoneurons and microdissection
Representative photomicrographs showing labeled phrenic motoneurons that were targeted for LCM are shown in Fig. 1. Phrenic motoneurons were labeled by intrapleural injection of Alexa 488-conjugated cholera toxin subunit B and were clearly visualized by Alexa 488 fluorescence. As expected, phrenic motoneurons were located in a rostrocaudal column within the gray matter of cervical spinal cord segments C3–C5 (Kinkead et al., 1998; Mantilla et al., 2009; Prakash et al., 1993, 2000; Zhan et al., 1989). Complete capture of phrenic motoneurons was confirmed by visual inspection of the LCM cap as well as the remaining spinal cord tissue as seen in Fig. 1. On average, 16.08±1.31 mm3 were captured per animal, corresponding to an estimated 205±17 phrenic motoneurons. The number of animals in each experimental group is shown in Table 2. Note that some transcripts were not detectable in all animals.
Table 2.
Distribution of animals used for analyses of serotonergic and glutamatergic receptor expression in control and over time following spinal cord hemisection at C2 (SH).
| Group | 5HTR-1b | 5HTR-2a | 5HTR-2c | AMPA | NMDA | mGlurR1 | mGlurR5 |
|---|---|---|---|---|---|---|---|
| Control | 7 (4) | 7 | 7 | 7 | 6 | 7 | 7 (6) |
| SH3D | 5 (3) | 5 | 5 | 5 | 5 | 5 (3) | 5 |
| SH7D | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| SH14D | 6 (4) | 6 | 6 | 6 | * | 6 (5) | 5 (4) |
| SH21D | 6 (4) | 6 | 6 | 6 | 6 | 6 | 6 |
The number of animals examined for receptor mRNA expression in phrenic motoneurons microdissected by LCM for each transcript and experimental group are shown in each cell. The number in parenthesis indicates the number of animals in which transcript was detected by RT-PCR if not detectable in all animals in that group. In particular, mRNA expression was not consistently detectable for the 5- HTR1b, mGluR1 and mGluR5 receptors in all animals. Note that mRNA was not available from all animals for some transcripts:
No mRNA remained for analysis of NMDA receptor expression in these animals.
Serotonergic receptor expression in phrenic motoneurons
Real-time RT-PCR was used to quantify the expression of serotonergic receptors (5-HTR subtypes 1b, 2a and 2c) and RPS16 in microdissected phrenic motoneurons. The specificity of amplification of the corresponding primer pairs (Table 1) was verified using 3 different methods. A melting curve analysis for each transcript was performed for each PCR reaction, where a single peak at an appropriate temperature was identified (Fig. 2A). Agarose gel electrophoresis of the PCR products was used to verify transcript size (Fig. 2B). A single band of an appropriate size (base pairs) was obtained for the 5-HTR1b, 5-HTR2a and RPS16 transcripts. Two bands were obtained for 5-HTR2c consistent with reported splice variants for the sequence amplified (Canton et al., 1996). Finally, the correct DNA sequence for each transcript was also verified.
Representative amplification curves for serotonergic receptor transcripts and RPS16 are shown in Fig. 2C for all experimental groups. RPS16 expression was similar across animals (overall mean CP: 28.69±0.19). No differences in RPS16 transcript expression were evident across groups (p>0.05).
Relative expression for serotonergic receptors following SH is shown in Fig. 3. There were no significant differences in serotonergic receptor expression in microdissected phrenic motoneurons over time following SH, except for the 5-HTR2a receptor which was significantly increased at SH14D compared to all other experimental groups (p<0.0001; one-way ANOVA, post hoc Tukey–Kramer HSD). The change in ΔCP for phrenic motoneurons at SH14D represents a ~8-fold (95% CI: 4–12) increase in 5-HTR2a expression relative to control (Fig. 3B).
Fig. 3.
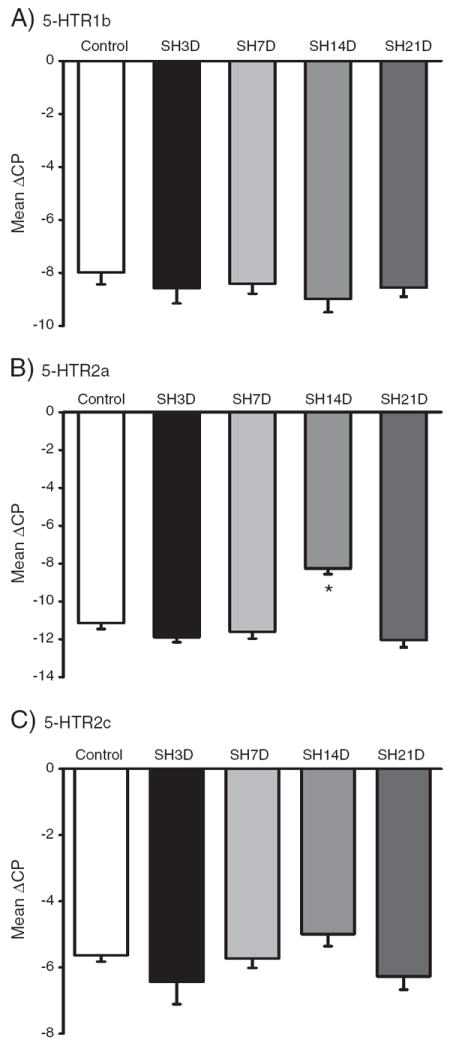
Serotonergic receptor expression in microdissected phrenic motoneurons following spinal hemisection at C2 (SH). A–C. Expression of the 5-HTR1b, 5-HTR2a and 5-HTR2c receptor subtypes in control and following SH (3D-21D post-SH). For each transcript, data are mean±SE of ΔCP across animals. *, p<0.05 vs. other experimental groups (one-way ANOVA, post hoc Tukey–Kramer’s HSD test).
Glutamatergic receptor expression in phrenic motoneurons
Real-time RT-PCR was used to quantify the expression of glutamatergic receptors (AMPA, NMDA, mGluR1 and mGluR5) in microdissected phrenic motoneurons. As described for serotonergic receptors, the specificity of amplification of the corresponding primer pairs (Table 1) was verified using 3 different methods. A single peak at an appropriate temperature was identified using melting curve analysis (Fig. 4A), correct transcript size was evident using agarose gel electrophoresis of the PCR products (Fig. 4B) and correct sequence was verified for each transcript. Representative amplification curves for glutamatergic receptor transcripts are shown in Fig. 4C for all experimental groups.
The relative expression for glutamatergic receptors following SH is shown in Fig. 5. There were no significant differences in glutamatergic receptor expression in microdissected phrenic motoneurons over time following SH, except for the NMDA receptor which was significantly increased at SH21D compared to all other experimental groups (p<0.007; one-way ANOVA, post hoc Tukey–Kramer HSD). The change in ΔCP would correspond to a ~16-fold (95% CI: 12–20) increase in NMDA expression in phrenic motoneurons by 21-days after SH (Fig. 5B).
Fig. 5.
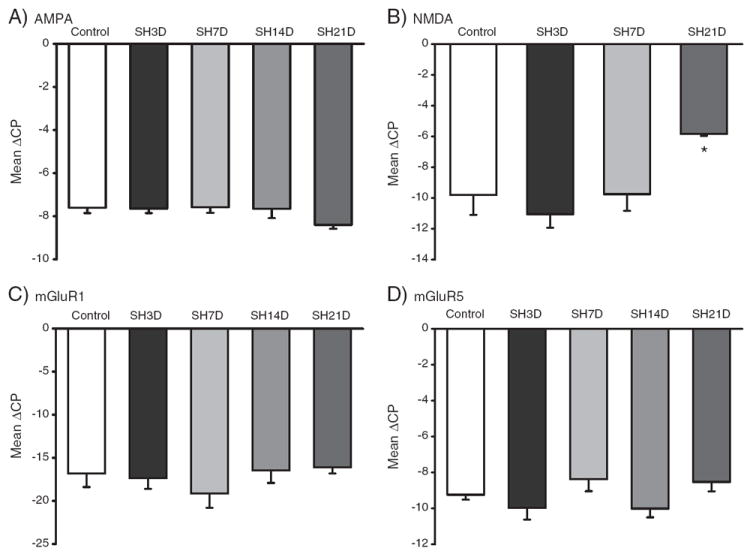
Glutamatergic receptor expression in microdissected phrenic motoneurons following SH. A–D. Expression of the AMPA, NMDA, mGluR1 and mGluR5 receptor subtypes in control and following SH (3D-21D post-SH). For each transcript, data are mean±SE of ΔCP across animals. *, p<0.05 vs. other experimental groups (one-way ANOVA, post hoc Tukey–Kramer’s HSD test).
Neurotransmitter receptor expression following SH
Possible correlations between transcript expression for either serotonergic or glutamatergic receptors were determined using pairwise comparisons of Z-scores for each transcript and sample in the entire population (Figs. 6 and 7).
Fig. 6.
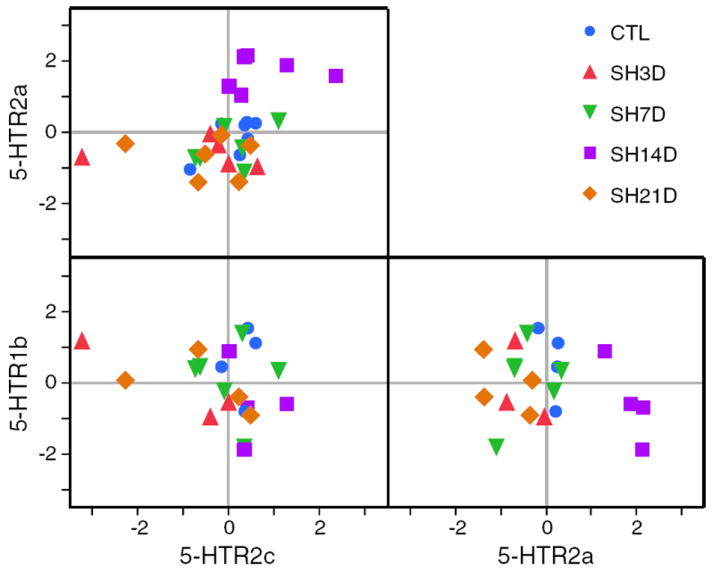
Correlation between phrenic motoneuron expression of serotonergic receptors (5-HTR1b, 5-HTR2a and 5-HTR-2c) for all animals in each group. Each point represents the Z-score for transcript expression for an individual animal. For each animal transcript, Z-scores were calculated from the mean ΔCP across PCR reactions for that animal and overall mean and SD for all animals. Changes in mRNA transcript expression for two transcripts are shown in each box. Overall correlations in receptor expression were determined using r2. In addition, correlated changes in expression (increase or decrease) across experimental groups are evident by clustering within a quadrant (right upper quadrant represents increased expression for both transcripts; left lower quadrant, decreased expression).
Fig. 7.
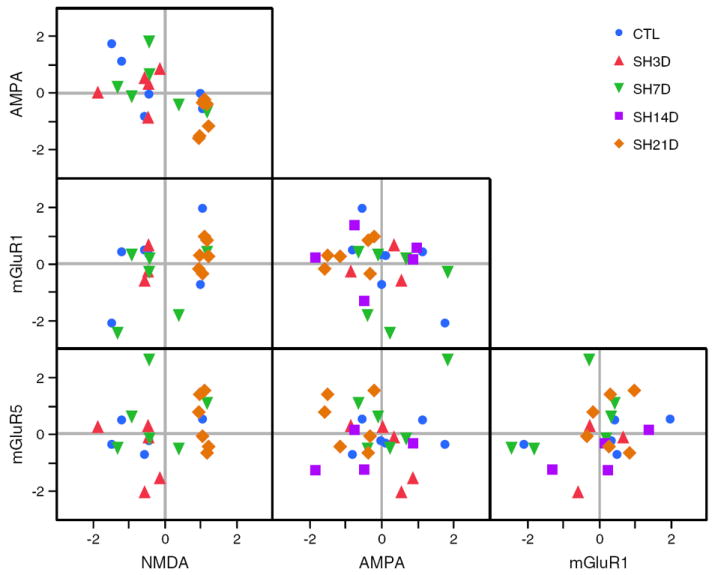
Correlation between phrenic motoneuron expression of glutamatergic receptors (AMPA, NMDA, mGluR1 and mGluR5) for all animals in each group. Each point represents the Z-score for transcript expression for an individual animal. For each animal transcript, Z-scores were calculated from the mean ΔCP across PCR reactions for that animal and overall mean and SD for all animals. Changes in mRNA transcript expression for two transcripts are shown in each box. Correlated expression (increase or decrease) results in grouping within a quadrant (right upper quadrant represents increased expression for both transcripts; left lower quadrant, decreased expression).
Across all experimental groups, there was a significant, positive, albeit weak, correlation between 5-HTR2a and 5-HTR2c mRNA expression (slope=0.45; r2=0.20; p=0.013; Fig. 6). No significant correlations were evident for other serotonergic receptor pairings. Clustering of SH14D animals is evident in the right upper quadrant of the 5-HTR2a vs. 5-HTR2c graph, reflecting an increased relative expression of both of these receptors in all animals in this experimental group.
For glutamatergic receptors (Fig. 7), there was a significant, negative correlation between AMPA and NMDA receptors (slope=−0.66; r2=0.36; p=0.003). In addition, there was a weak, positive correlation between NMDA and mGluR1 receptors (slope=0.40; r2=0.19; p=0.048). No significant correlations were evident for other glutamatergic receptor pairings. Clustering of SH21D animals is evident in the right lower quadrant of the AMPA vs. NMDA graph, reflecting increased NMDA expression with concomitant decreased AMPA receptor expression in all animals in this experimental group. There was no evidence of clustering in the NMDA vs. mGluR1 graph across experimental groups.
Discussion
Phrenic motoneuron expression of specific subtypes of serotonergic and glutamatergic receptors transiently increases following cervical spinal cord hemisection at C2. By using laser capture microdissection of retrogradely-labeled phrenic motoneurons and quantitative real-time RT-PCR, changes in the expression of multiple neurotransmitter receptors were studied over time following SH. This is the first study, to our knowledge, which systematically documented changes in motoneuron expression of multiple neurotransmitter receptors involved in regulation of motoneuron excitability and plasticity. By providing information on the neuroplasticity of receptors expressed in a well-defined motoneuron pool that is inactivated by a higher-level SCI, appropriate pharmacological targets can be identified to alter motoneuron excitability.
The present study directly examined the expression of multiple serotonergic and glutamatergic receptor subtypes and the time course of the changes in receptor mRNA levels in phrenic motoneurons following SH. Serotonergic pathways play an important role in several forms of phrenic motor plasticity (Fuller et al., 2000, 2001; Mitchell et al., 1992), including recovery from SCI (Basura et al., 2001; Fuller et al., 2005; Hadley et al., 1999; Zhou et al., 2001). Glutamatergic pathways are also important in the neuroplasticity of phrenic motor output (Alilain and Goshgarian, 2008; McGuire et al., 2008). However, the cellular substrate for serotonergic or glutamatergic activity following SCI has not been systematically studied. In particular, the results of studies using pharmacological inhibitors have very little cellular specificity, and it may not be possible to determine whether receptor activation occurs at phrenic motoneurons. By examining multiple receptor subtypes in a well-established model of SCI, the time course of changes in phrenic motoneuron receptor expression can be determined.
Receptor mRNA subtype expression in phrenic motoneurons
In the present study, phrenic motoneurons were labeled by intrapleural injection (Mantilla et al., 2009) and captured by LCM. The intrapleural injection technique was validated previously, with CTB labeling only phrenic motoneurons in the cervical spinal cord ventral horn. Indeed, near complete labeling of the phrenic motor pool was possible. Using a fluorescently-conjugated CTB, LCM was possible without immunohistochemical processing of the tissue samples. As expected, extensive and widespread labeling of the phrenic motor pool was evident (Fig. 1). Based on the volume of tissue captured, ~200 motoneurons were sampled ipsilateral to SH in all animals. These results are comparable to the total number of phrenic motoneurons on each side of the rat spinal cord, as labeled by dipping the phrenic nerve in retrograde tracer (Mantilla et al., 2009). Thus, mRNA measurements in the LCM sample of phrenic motoneurons are representative of the entire phrenic motor pool.
Serotonergic receptor expression in phrenic motoneurons
Several previous studies reported serotonergic receptor expression in cervical spinal cord homogenates containing the phrenic motor pool (Basura et al., 2001; Fuller et al., 2005). Although 5-HT2a and 5-HT2c receptor expression was documented in cervical spinal motoneurons, co-localization to retrogradely-labeled phrenic motoneurons has not been consistently documented (Basura et al., 2001). Other studies used functional measures of phrenic motor output or intracellular phrenic motoneuron recordings to determine the effects of serotonergic agonist and antagonist treatment in the region of the phrenic motor pool (Di Pasquale et al., 1997; Fuller et al., 2000; Kinkead et al., 1998). Based on these observations, phrenic motoneurons are generally thought to express 5-HTR1b, 5-HTR2a and 5-HTR2c receptor subtypes. In the present study, expression of these serotonergic receptor subtypes was verified in phrenic motoneurons, although levels of 5-HTR1b were below detectable limits in 3 (out of 7) control rats. Serotonergic receptors (in particular, 5-HTR2a and 5-HT2c) are important regulators of motoneuron excitability (Rekling et al., 2000) by facilitating persistent Ca2+ inward currents (Murray et al., 2010). Such effects are also likely present in phrenic motoneurons of uninjured animals, and may be facilitated post-SCI.
Previous studies examined the role of serotonergic receptors in the neuroplasticity of phrenic motor output following SCI (Basura et al., 2001; Fuller et al., 2005; Zhou and Goshgarian, 1999; Zhou et al., 2001). Although serotonin-containing boutons increase gradually after SH (Golder and Mitchell, 2005), phrenic motoneuron expression of 5-HTR has not been consistently studied after SH. Following 24 h of SH, pharmacological inhibition of 5-HTR2a, not 5-HTR2c, blunted the recovery of phrenic nerve activity ipsilateral to SH (Basura et al., 2001). In the same study, mRNA levels for either 5-HTR subtype were not altered in labeled phrenic motoneurons as measured by in situ hybridization. Two-weeks post-SH, the density of 5-HTR2a immunoreactivity increased in the cervical spinal cord region containing phrenic motoneurons (Fuller et al., 2005). In general agreement, 5-HTR2a expression increased in phrenic motoneurons at SH14D and was no longer significantly elevated by SH21D. Expression of 5-HTR2a and 5-HTR2c was positively correlated, and indeed both of these receptor subtypes were increased in all SH14D animals compared to the other experimental groups. This pattern of coupled regulation of 5-HTR expression may contribute to the functional recovery of rhythmic phrenic nerve activity after SH. For instance, expression of constitutively-active 5-HTR2c isoforms accounted for sustained muscle contractions and spasms of the rat tail 6–12 weeks after an S2 transection (Murray et al., 2010). Whether phrenic motoneurons express such constitutively-active isoforms after SH remains to be determined.
Glutamatergic receptor expression in phrenic motoneurons
Several previous studies reported glutamatergic receptor expression in the cervical spinal cord region containing the phrenic motor pool. Both pharmacological and anatomical studies indicate that the primary excitatory synaptic input to phrenic motoneurons is glutamatergic (Chitravanshi and Sapru, 1996; Greer et al., 1991; Liu et al., 1990; Murphy et al., 1996; Robinson and Ellenberger, 1997). Immunoreactivity to both AMPA and NMDA glutamate receptor subtypes was shown in phrenic motoneurons (Alilain and Goshgarian, 2008). The metabotropic (G-protein coupled) receptor subtypes mGluR1 and mGluR5 are also thought to be primarily postsynaptic on phrenic motoneurons (Dong and Feldman, 1999; McCrimmon et al., 1989). In the present study, expression of these glutamatergic receptor subtypes was verified in phrenic motoneurons.
Previous studies examined glutamatergic receptor expression in the phrenic motor pool following SH. Using quantitative electron microscopic analyses, reduced numbers of glutamatergic boutons were reported 30 days post-SH (Tai and Goshgarian, 1996). Cervical spinal cord expression of NMDA receptors increased 12–16 weeks after SH, whereas AMPA receptor expression decreased (Alilain and Goshgarian, 2008). Although immunoreactivity was localized to labeled phrenic motoneurons, quantitative measurements were not possible at the motoneuron level. In agreement, NMDA receptor expression increased in phrenic motoneurons by SH21D in the present study. In addition, there was a significant, negative correlation between NMDA and AMPA receptors for all animals. In SH21D animals, there was a coupled increase in NMDA receptor and decrease in AMPA receptor expression compared to animals in other experimental groups. A switch in ionotropic glutamatergic transmission from AMPA to NMDA receptors can increase motoneuron excitability via prolongation of excitatory postsynaptic currents and slower desensitization of the receptor (Rekling et al., 2000).
Neuroplasticity following SH
The SH model of SCI is a well-established model of incomplete injury where the ipsilateral phrenic motoneuron pool is inactivated by disruption of its descending excitatory drive. In the present study, animals were chronically instrumented with bilateral diaphragm EMG electrodes in order to verify the absence of ipsilateral diaphragm activity 3 days post-SH (Mantilla et al., 2011; Miyata et al., 1995) and thus the completeness of the SH injury. All animals in the present study displayed absence of ipsilateral EMG activity at SH3D. Over time post-SH, half of the SH14D and half of the SH21D animals displayed ipsilateral diaphragm EMG activity. The timing and conditions necessary for recovery of rhythmic diaphragm activity after SH vary across previous studies, ranging from a few hours to weeks, and differences may relate to the extent of the injury caused by transecting and/or suctioning the ipsilateral cord (Fuller et al., 2006; Golder et al., 2003; Goshgarian, 1981; Nantwi et al., 1999; O’Hara and Goshgarian, 1991; Vinit et al., 2006; Zhou et al., 2001). Regardless, there is a gradual increase in the proportion of animals displaying recovery of ipsilateral diaphragm activity, which most likely reflects the process of neuroplasticity and strengthening of contralateral premotor drive to phrenic motoneurons. This strengthening of synaptic inputs may involve pre- and postsynaptic mechanisms. The current study systematically examined the postsynaptic (phrenic motoneuron) expression of multiple serotonergic and glutamatergic receptor subtypes and establishes the complex interplay between these two different neurotransmitter pathways. Neuroplasticity in the expression of serotonergic receptors reveals the importance of 5-HTR2a (and possibly 5-HTR2c) by 14 days post-SH, whereas NMDA upregulation (and concomitant AMPA downregulation) by 21 days post-SH may signal a transition in the underlying basis for functional recovery following SCI. Future studies should directly examine the complement of neurotransmitter expression in animals displaying functional recovery vs. those that do not as well as the pharmacological effect of the different receptors in functional recovery. The present study provides fundamental information regarding possible neurotransmitter receptor targets to study.
Conclusions and clinical relevance
The current study was designed to determine the expression of serotonergic and glutamatergic receptors within phrenic motoneurons ipsilateral to SH. Importantly, the progressive recovery of rhythmic diaphragm activity in the SH model generally reflects clinical conditions. According to the most recent update of the National Spinal Cord Injury Statistical Center report (2011), most cases of SCI involve the cervical spinal cord and are incomplete, resulting in varying levels of tetraplegia. Following cervical SCI, inability to sustain ventilation commonly results in the need for mechanical ventilation, which can be associated with inability to clear airway secretions and an increased risk for pneumonia. Indeed, despite improvements in the care of SCI patients, pneumonia continues to be the leading cause of death (DeVivo and Ivie, 1995; DeVivo et al., 1993). Functional recovery of ventilatory and expulsive non-ventilatory activity of respiratory muscles such as the diaphragm can significantly improve the life-expectancy and quality of life of patients with complete and even incomplete tetraplegia (Mantilla and Sieck, 2009; Sieck and Mantilla, 2009). Knowledge of the neuroplasticity of specific neurotransmitter pathways following SCI could be used to design novel and specific interventions to promote functional recovery.
Acknowledgments
The authors wish to thank Ms. Yun-Hua Fang for technical support. Supported by NIH grant HL096750, the Paralyzed Veterans of America Research Foundation and the Mayo Clinic Foundation.
References
- Alilain WJ, Goshgarian HG. Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp Neurol. 2008;212:348–357. doi: 10.1016/j.expneurol.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basura GJ, Zhou SY, Walker PD, Goshgarian HG. Distribution of serotonin 2A and 2C receptor mRNA expression in the cervical ventral horn and phrenic motoneurons following spinal cord hemisection. Exp Neurol. 2001;169:255–263. doi: 10.1006/exnr.2001.7682. [DOI] [PubMed] [Google Scholar]
- Canton H, Emeson RB, Barker EL, Backstrom JR, Lu JT, Chang MS, Sanders-Bush E. Identification, molecular cloning, and distribution of a short variant of the 5-hydroxytryptamine2C receptor produced by alternative splicing. Mol Pharmacol. 1996;50:799–807. [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. NMDA as well as non-NMDA receptors mediate the neurotransmission of inspiratory drive to phrenic motoneurons in the adult rat. Brain Res. 1996;715:104–112. doi: 10.1016/0006-8993(95)01565-5. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Ivie CS., III Life expectancy of ventilator-dependent persons with spinal cord injuries. Chest. 1995;108:226–232. doi: 10.1378/chest.108.1.226. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Black KJ, Stover SL. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil. 1993;74:248–254. [PubMed] [Google Scholar]
- Di Pasquale E, Lindsay A, Feldman J, Monteau R, Hilaire G. Serotonergic inhibition of phrenic motoneuron activity: an in vitro study in neonatal rat. Neurosci Lett. 1997;230:29–32. doi: 10.1016/s0304-3940(97)00469-2. [DOI] [PubMed] [Google Scholar]
- Dong XW, Feldman JL. Distinct subtypes of metabotropic glutamate receptors mediate differential actions on excitability of spinal respiratory motoneurons. J Neurosci. 1999;19:5173–5184. doi: 10.1523/JNEUROSCI.19-13-05173.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow DE, Mantilla CB, Zhan WZ, Sieck GC. EMG-based detection of inspiration in the rat diaphragm muscle. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1204–1207. doi: 10.1109/IEMBS.2006.260688. [DOI] [PubMed] [Google Scholar]
- Dow DE, Zhan WZ, Sieck GC, Mantilla CB. Correlation of respiratory activity of contralateral diaphragm muscles for evaluation of recovery following hemiparesis. Conf Proc IEEE Eng Med Biol Soc. 2009;1:404–407. doi: 10.1109/IEMBS.2009.5334892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci. 2003;23:2494–2501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol. 1981;72:211–225. doi: 10.1016/0014-4886(81)90139-4. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol. 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley SD, Walker PD, Goshgarian HG. Effects of serotonin inhibition on neuronal and astrocyte plasticity in the phrenic nucleus 4 h following C2 spinal cord hemisection. Exp Neurol. 1999;160:433–445. doi: 10.1006/exnr.1999.7238. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci. 1998;18:8436–8443. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Feldman JL, Smith JC. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J Neurophysiol. 1990;64:423–436. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Neuromuscular adaptations to respiratory muscle inactivity. Respir Physiol Neurobiol. 2009;169:133–140. doi: 10.1016/j.resp.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Rowley KL, Zhan WZ, Fahim MA, Sieck GC. Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity. Neuroscience. 2007;146:178–189. doi: 10.1016/j.neuroscience.2007.01.048. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods. 2009;182:244–249. doi: 10.1016/j.jneumeth.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Hurtado-Palomino JN, Zhan WZ, Sieck GC. Chronic assessment of diaphragm muscle EMG activity across motor behaviors. Respir Physiol Neurobiol. 2011;177:176–182. doi: 10.1016/j.resp.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Smith JC, Feldman JL. Involvement of excitatory amino acids in neurotransmission of inspiratory drive to spinal respiratory motoneurons. J Neurosci. 1989;9:1910–1921. doi: 10.1523/JNEUROSCI.09-06-01910.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M, Liu C, Cao Y, Ling L. Formation and maintenance of ventilatory long-term facilitation require NMDA but not non-NMDA receptors in awake rats. J Appl Physiol. 2008;105:942–950. doi: 10.1152/japplphysiol.01274.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Sloan HE, Jiang C, Miletic V, Hayashi F, Lipski J. 5-Hydroxytryptophan (5-HTP) augments spontaneous and evoked phrenic motoneuron discharge in spinalized rats. Neurosci Lett. 1992;141:75–78. doi: 10.1016/0304-3940(92)90338-8. [DOI] [PubMed] [Google Scholar]
- Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol. 1995;79:1640–1649. doi: 10.1152/jappl.1995.79.5.1640. [DOI] [PubMed] [Google Scholar]
- Murphy SM, Pilowsky PM, Llewellyn-Smith IJ. Vesicle shape and amino acids in synaptic inputs to phrenic motoneurons: do all inputs contain either glutamate or GABA? J Comp Neurol. 1996;373:200–219. doi: 10.1002/(SICI)1096-9861(19960916)373:2<200::AID-CNE4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D’Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med. 2010;16:694–700. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy A, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehabil Neural Repair. 1999;13:225–234. [Google Scholar]
- National Spinal Cord Injury Statistical Center. Spinal Cord Injury: Facts and Figures at a Glance. University of Alabama; Birmingham: 2011. [Google Scholar]
- O’Hara TEJ, Goshgarian HG. Quantitative assessment of phrenic nerve functional recovery mediated by the crossed phrenic reflex at various time intervals after spinal cord injury. Exp Neurol. 1991;111:244–250. doi: 10.1016/0014-4886(91)90012-2. [DOI] [PubMed] [Google Scholar]
- Porter J. The path of the respiratory impulse from the bulb to the phrenic nuclei. J Physiol (Lond) 1895;17:455–485. doi: 10.1113/jphysiol.1895.sp000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Smithson KG, Sieck GC. Measurements of motoneuron somal volumes using laser confocal microscopy: comparisons with shape-based stereological estimations. Neuroimage. 1993;1:95–107. doi: 10.1006/nimg.1993.1003. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Miyata H, Zhan WZ, Sieck GC. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve. 1999;22:307–319. doi: 10.1002/(sici)1097-4598(199903)22:3<307::aid-mus3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol. 2000;89:563–572. doi: 10.1152/jappl.2000.89.2.563. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D, Ellenberger H. Distribution of N-methyl-d-aspartate and non-Nmethyl-d-aspartate glutamate receptor subunits on respiratory motor and premotor neurons in the rat. J Comp Neurol. 1997;389:94–116. doi: 10.1002/(sici)1096-9861(19971208)389:1<94::aid-cne7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Rosenblueth A, Klopps CT, Simeone FA. A further study of the crossed phrenic phenomenon. J Neurophysiol. 1938;1:508–520. [Google Scholar]
- Sieck GC, Mantilla CB. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir Physiol Neurobiol. 2009;169:218–225. doi: 10.1016/j.resp.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai Q, Goshgarian HG. Ultrastructural quantitative analysis of glutamatergic and GABAergic synaptic terminals in the phrenic nucleus after spinal cord injury. J Comp Neurol. 1996;372:343–355. doi: 10.1002/(SICI)1096-9861(19960826)372:3<343::AID-CNE2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Trelease RB, Sieck GC, Harper RM. A new technique for acute and chronic recording of crural diaphragm EMG in cats. Electroencephalogr Clin Neurophysiol. 1982;53:459–462. doi: 10.1016/0013-4694(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Twigger SN, Shimoyama M, Bromberg S, Kwitek AE, Jacob HJ. The Rat Genome Database, update 2007 - easing the path from disease to data and back again. Nucleic Acids Res. 2007;35:D658–D662. doi: 10.1093/nar/gkl988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinit S, Gauthier P, Stamegna JC, Kastner A. High cervical lateral spinal cord injury results in long-termipsilateral hemidiaphragm paralysis. J Neurotrauma. 2006;23:1137–1146. doi: 10.1089/neu.2006.23.1137. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Ellenberger HH, Feldman JL. Monoaminergic and GABAergic terminations in phrenic nucleus of rat identified by immunohistochemical labeling. Neuroscience. 1989;31:105–113. doi: 10.1016/0306-4522(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG. Effects of serotonin on crossed phrenic nerve activity in cervical spinal cord hemisected rats. Exp Neurol. 1999;160:446–453. doi: 10.1006/exnr.1999.7213. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Basura GJ, Goshgarian HG. Serotonin(2) receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol. 2001;91:2665–2673. doi: 10.1152/jappl.2001.91.6.2665. [DOI] [PubMed] [Google Scholar]


