Abstract
Equol is an isoflavone (IF) metabolite produced by intestinal microbiota in a subset of people consuming dietary soy. Equol producers may show different responses to soy foods and phenotypes related to cancer risk. Here, we assessed the effects of soy IF, endogenous microbial equol production, and dietary racemic equol in a 3 × 2 × 2 factorial experiment using gnotobiotic apoE-null mice (n = 9–11/group/sex). At age 3–6 wk, equol-producing microbiota were introduced to one-half of the colony (n = 122). At age 6 wk, mice were randomized to receive a diet that contained 1 of 3 protein sources: casein and lactalbumin, alcohol-washed soy protein (low IF), and intact soy protein (high IF), with total IF amounts of 0, 42, and 566 mg/kg diet, respectively. One-half of each diet group also received racemic equol (291 mg/kg diet). After 16 wk of dietary treatment, serum isoflavonoid profiles varied with sex, soy IF amount, and intestinal microbiota status. There were no treatment effects on tissues of male mice. In females, reproductive tissue phenotypes differed by equol-producing ability (i.e., microbiota status) but not dietary equol or IF content. Equol producers had lower uterine weight, vaginal epithelial thickness, total uterine area, endometrial area, and endometrial luminal epithelial height compared with nonproducers (P < 0.05 for all), with an association between microbiota status and estrous cycle (P > chi-square = 0.03). Exogenous equol reduced expression of progesterone receptor (PGR) and the proliferation marker Ki67 (P < 0.0001) in vaginal epithelium and endometrium; for endogenous equol, only PGR was reduced (P < 0.0005). Our findings indicate that equol diminishes estrogen-dependent tissue responses in apoE-null mice.
Introduction
Epidemiological evidence has shown that the incidence of chronic diseases such as cancer and cardiovascular disease is lower in Asia than in Western countries. Lifestyle factors, including diet, have been identified as potential determinants (1, 2). Soy-based foods rich in phytoestrogen isoflavones (IF)7 are a primary component of many Asian but not Western diets (3). The intake of soy and the primary soy IF, genistein and daidzein, has been widely studied in association with cancer and other chronic diseases, but the results have been mixed and inconclusive (4). The lack of consensus regarding the health effects of soy IF interventions is due to a variety of potential factors, including variation in IF formulations across studies and interindividual differences in metabolism and response to soy diet (5).
Equol is a metabolite of daidzein produced by intestinal bacteria in ∼30% of adult non-Asian and nonvegetarian populations consuming dietary soy protein (6, 7). Approximately 10–30% are intermittent equol producers (8, 9). Inter-individual variation in the ability to produce equol is a consequence of difference in gut microbial community. Several bacterial strains that can produce equol have been identified in vitro (10); however, the nature of the bacteria and how a person harbors them are incompletely understood. Factors such as dietary habit may modulate the composition and activity of gut microbes, hence affecting equol production (7). Recent evidence suggests that the ability to metabolize daidzein into equol may influence the health-related responses to soy exposure (11). However, it is unclear whether such effects are driven by equol directly or if equol production simply serves as a marker for other intestinal microbiota-mediated effects. Equol has distinct biological activity compared with daidzein and genistein. It binds to estrogen receptors (ER) α and β with greater affinity than daidzein (12) and has antiandrogenic properties by binding to dihydrotestosterone (13). Equol producers and nonproducers may exhibit different phenotypes related to cancer risk and respond differently to IF, potentially accounting for some of the individual variation in response to soy foods and their related health effects. Still, the direct effects of equol or equol production are poorly understood. The intestinal microbiota is capable of producing only the S-(-) enantiomer of equol (S-equol), whereas the commercially available synthetic form of equol is a racemic R/S-(±) mixture. In healthy humans, racemic equol differs from the individual enantiomers with respect to bioavailability and pharmacokinetics (14), suggesting that endogenously produced and exogenous equol may have distinct biological activities.
Equol-producer status has now become an important consideration in the interpretation of soy study results. Furthermore, interest in identifying factors contributing to the equol-producer phenotypes and their association with human health has increased alongside development of equol-based dietary supplements (15, 16). The purpose of this study was to evaluate the effects of endogenously produced equol and exogenous racemic equol on reproductive tissues in mice fed a soy IF diet. The work was carried out in apoE-null mice, which are susceptible to atherosclerosis; the atherosclerosis-related findings will be reported elsewhere.
Materials and Methods
Mice and diets.
ApoE-null mice were used in the study. All breeding and procedures involving mice were conducted at Taconic Biotechnology in their Association for the Assessment and Accreditation of Laboratory Animal Care-accredited facility in Germantown and Rensselaer, NY and were approved by the Taconic Institutional Animal Care and Use Committee. Tissue-related procedures were performed at Wake Forest School of Medicine. The gnotobiotic apoE-null mouse breeding colony was initially established by exposure of Altered Schaedler Flora (ASF) (17) to germfree apoE-null mice at 7 wk of age via aqueous fecal suspension from an ASF-maintained donor. Preliminary assessment of serum equol concentrations indicated that mice harboring ASF were not equol producers (equol nonproducers).
This study utilized the offspring (n = 243) of apoE-null breeders harboring ASF. At age 3–6 wk, one-half of the colony (n = 122) was exposed to intestinal microbiota from normal apoE-null mice via fecal material to generate an equol-producing phenotype (equol producer). Throughout the study, mice were maintained under gnotobiotic conditions in plastic film isolators and the microbial status was monitored monthly by fecal culture. The microbial status of the nonproducers was verified under Taconic’s Defined Flora microbiological health standard, whereas equol producers were maintained at the Murine Pathogen Free status (18).
At age 6 wk, both equol producers and nonproducers were randomly assigned to dietary treatment groups (Supplemental Fig. 1) with n = 9–11/group, per sex. The diets (Supplemental Table 1) used 1 of 3 protein sources: 1) casein and lactalbumin (CL); 2) alcohol-washed soy protein [low IF (LIF)]; or 3) intact soy protein [high IF (HIF)]. The total IF amounts in the diets were 0, 42, and 566 mg/kg diet, respectively. The ratio of daidzein : genistein : glycitein was 1 : 1.5 : 0.2. Additionally, the diets also contained 291 mg/kg racemic equol (i.e., CL+, LIF+, HIF+) or no equol (i.e., CL−, LIF−, HIF−). The exogenous equol was given as a synthetic product chemically synthesized from daidzein and comprised of a 50/50 racemic R/S (±) mixture. Diets were manufactured by Research Diets. Archer Daniels Midland Company provided the isolated soy proteins and analyzed the IF content in the isolates. Racemic R/S (±) equol was provided by Solae. We used a dosing strategy for IF in mg/kJ to adjust for the metabolic rate difference between humans and mice and to provide the IF dose relevant to human exposure. Human daily energy consumption was considered to be 7530 kJ/d and our diet compositions were designed to create a dosing strategy of ∼275 mg IF/7530 kJ for HIF, 20 mg IF/7530 kJ for LIF, and 140 mg equol/7530 kJ for exogenous equol. Additionally, we assessed the effect of soy IF on microbiome of the equol producers at 2 wk post-dietary treatment (Supplemental Methods).
Following 16 wk of dietary treatment, mice were killed by CO2 asphyxiation. Mice were weighed and blood collection was done by cardiac puncture. Tissues collected were ovaries, oviduct, uterus, vagina, testicles, epididymis, and accessory glands of male reproductive tract (prostate, seminal vesicles, and coagulating glands). Tissues were fixed in 10% neutral buffered formalin. After 24 h, tissues were removed from formalin and stored in 70% ethanol. Uteri and testes were weighed as markers of estrogenic action on the reproductive tract. All tissues were embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. Histopathologic examinations were done by board-certified veterinary pathologists (C.E.W. and J.M.C.).
We identified the estrous cycle stage (proestrus, estrus, metestrus, and diestrus) of each mouse based on histology of the vagina, uterus, and ovaries (19, 20). Mice that did not meet all the criteria of a cycle stage were noted as “undetermined stage” and excluded from cycle-related analyses.
Serum isoflavonoids.
Isoflavonoid concentrations were measured from serum samples using time-resolved fluorescence immunoassay at the Fred Hutchinson Cancer Research Center, as previously described (21). Serum equol, genistein, and daidzein concentrations were measured in subsets of mice. Equol was measured across all treatment groups (n = 5–10 per group, per sex); genistein was measured in the CL−, LIF−, and HIF− groups (n = 5–10 per group, per sex); and daidzein was measured only in mice fed a HIF− diet (n = 10 per group, per sex). The lowest levels of quantitation for equol, genistein, and daidzein were 0.66, 1.0, and, 0.5 nmol/L, respectively.
Tissue histomorphometry.
Hematoxylin and eosin-stained slides were used for histomorphometric evaluation of reproductive tissues with a computer-assisted technique as previously described (22) using a Infinity 3 digital camera (Lumenera) and Image Pro-Plus 5.1 software (Media Cybernetics). We measured cross-sectional uterine and endometrial areas, endometrial luminal epithelial height, and vaginal epithelial thickness as markers of estrogenic action on the reproductive tract. Cross-sectional areas were measured at 4× (total uterine area) and 10× (endometrial area) magnifications. Endometrial luminal epithelial height and vaginal epithelial thickness were measured at 20× magnification in 3 randomly chosen sites. All measurements were made unaware of the treatment groups.
Immunohistochemistry.
For immunohistochemistry, we used a biotin-streptavidin-alkaline phosphatase staining method modified for antigen retrieval from paraffin-embedded tissue, as previously described (22). Staining was done for Ki67 and progesterone receptor (PGR) as markers for cell proliferation and ER activity, respectively. Primary antibodies were rabbit monoclonal anti-Ki67SP6 (Thermo Scientific) and rabbit polyclonal anti-PR sc-538 (Santa Cruz Biotechnology). All measurements were made unaware of the treatment groups. Positively stained cells were given a score based on staining intensity to obtain a semiquantitative measurement of intensity and distribution by H-score calculation (22, 23).
Data analysis.
The experiment was a 3 × 2 × 2 factorial design to test the effects of soy IF, microbiota status, and exogenous equol. Measured outcomes were expressed as continuous variables; non-normally distributed data were log-transformed or square-rooted to improve distribution for analysis, and retransformed to original scale for presentation of results. We performed 1-way, 2-way, or 3-way ANOVA by Fit Model procedure in JMP (version 9.0.2, SAS Institute). Post hoc comparisons were made using least square means Student’s t test (between 2 treatment groups or 2 levels in a binary category) or Tukey’s honestly significant difference test (for pairwise comparisons involving >2 levels or 2 treatment groups). Estrous cycle stage was expressed as categorical variable. Contingency table and chi-square statistic tests (i.e., the Likelihood Ratio chi-square test and the Pearson chi-square test) were used to assess the association between estrous cycle and each fixed factor (i.e., soy IF, microbiota status, or exogenous equol). For female reproductive outcomes, we performed analyses both with and without estrous cycle as a covariate in the model. For serum isoflavonoid concentrations, we initially included sex as a fixed factor in the model and further analyzed the data separately by sex.
Two of 18 females fed a HIF+ diet showed histopathologic lesions considered to be unrelated to treatment (see results). These 2 mice were excluded from our dataset.
Results
Serum isoflavonoids.
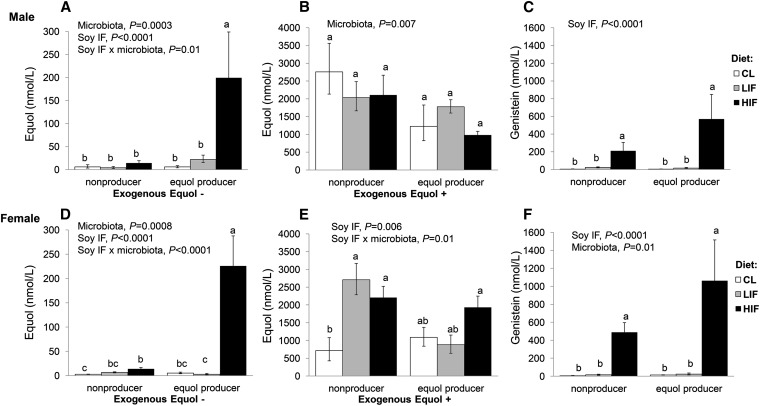
Importantly, we succeeded in developing equol-producing and nonequol-producing phenotypes in our mouse model (Fig. 1A,D). Without exogenous equol treatment, mice that were defined as equol producers had greater serum equol concentrations compared with nonproducers fed the same diet (P < 0.001 for both sexes). There was also a main effect of soy IF on serum equol concentrations (P < 0.0001 in both sexes) in which mice fed a HIF− diet had greater equol concentrations relative to mice fed LIF− and CL− diets. Among the equol producers, fecal microbial communities also differed between the mice fed HIF− and CL− diets (P < 0.001), but not by sex (Supplemental Fig. 2).
FIGURE 1.
Serum equol (A,B,D,E) and genistein (C,F) concentrations in male (A–C) and female (D–F) apoE-null mice after 16 wk of dietary soy treatment with (B,E) or without (A,D) equol by microbiota status. Values are means ± SEM, n = 5–10 or 5 (B,E)/group. Significant main effects and interactions are shown. Within each panel, labeled means without a common letter differ, P < 0.01 or P < 0.05 (E). CL, casein and lactalbumin; HIF, high isoflavone; IF, isoflavone; LIF, low isoflavone.
When exogenous racemic equol was given, the serum equol concentrations in both equol producers and nonproducers were higher than those without racemic equol (P < 0.0001 for main effect of exogenous equol) and notably higher (∼7- to 10-fold) than those produced endogenously by IF-fed mice with equol-producing intestinal bacteria (Fig. 1B,E). With exogenous equol, male equol producers had lower serum equol concentrations than nonproducers (P < 0.01 for the main effect of microbiota). A similar pattern was observed in the females, although the effect was not significant (P = 0.08).
Serum genistein and daidzein concentrations were higher in female mice than in males (P < 0.05 for both). We found a main effect of dietary soy IF on serum genistein concentration (P < 0.0001 in both sexes), whereby genistein was higher in mice fed a HIF− diet compared with LIF− and CL− diets (Fig. 1C,F). In the females, there was also a main effect of microbiota status (P < 0.05) in which equol producers had a higher serum genistein concentration than nonproducers. Although daidzein had a similar pattern, the difference by microbiota status was not significant in either males (P = 0.08) or females (P = 0.05). The males had mean serum daidzein concentrations of 158 nmol/L (nonproducers) and 459 nmol/L (equol producers). In female mice, the mean concentrations were 513 and 927 nmol/L in nonproducers and equol producers, respectively.
Histopathology.
Histopathologic lesions were present in few mice and did not differ across treatments. In females, lesions included vaginal mucification, vaginitis, uterine atrophy, endometritis, uterine and ovarian cysts, uterine gland dilatation, ovarian interstitial cell hyperplasia, persistent estrus, and mesonephric duct remnants. Two mice (one from each microbiota type) in the group that received a HIF+ diet had pyometra. Based on the fact that only a few mice had this lesion, we suspected that the finding was incidental. In males, lesions included prostatitis (in one mouse) and mild degenerative changes in the testes that were not related to treatment. No neoplasms were seen in this study.
Body weight.
In female mice, body weights did not differ by microbiota status or dietary treatments. In males, there was a main effect of soy IF diet (P < 0.05) in which mean body weights were 7% lower in the HIF group relative to CL (P < 0.05). There was also a main effect of microbiota (P < 0.05) limited to mice not receiving exogenous equol, whereby equol producers had 11% lower body weights compared with nonproducers (P < 0.005).
Tissue weight and histomorphometry.
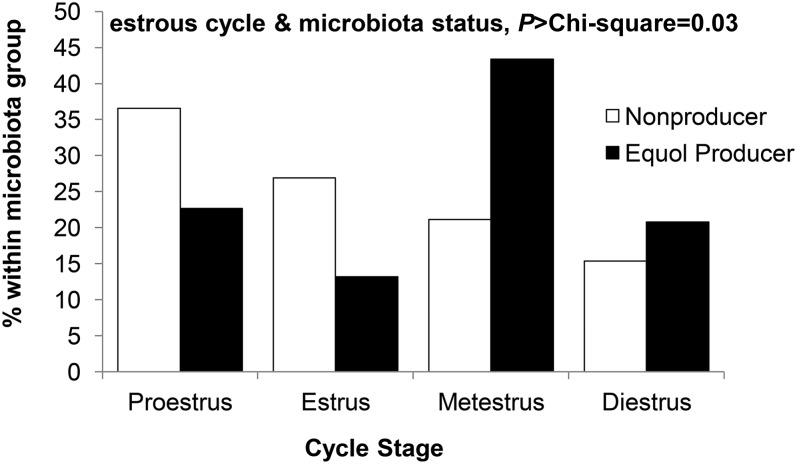
There was no significant effect of soy diet, microbiota status, or exogenous equol on testis weight. In the females, we found a main effect of microbiota status on reproductive tissues (Table 1). Uterine weight, total uterine area, endometrial area, endometrial luminal epithelial height, and vaginal epithelial thickness were lower in equol producers compared with nonproducers (P < 0.05 for all). Except for vaginal epithelial thickness, these measurements were not significantly affected by either soy IF or exogenous equol and there was no interaction between microbiota status and dietary treatments. There was a main effect of soy IF on vaginal epithelial thickness (P < 0.05), whereby HIF was greater than LIF (by 19%) and CL (by 15%) groups. Interestingly, the significant main effects of microbiota on histomorphometry measurements disappeared when estrous cycle stage was added as a covariate. Furthermore, we found that estrous cycle stage was significantly associated with microbiota status (P > chi-square = 0.03) but not with soy diet or exogenous equol. At the time of necropsy, equol producers were found predominantly in metestrus, whereas cycle stage across nonproducers was more evenly distributed, with the majority being in proestrus and estrus (Fig. 2).
TABLE 1.
Main effect of intestinal microbiota status on uterine weight and reproductive tissue histomorphometry in female apoE-null mice after 16 wk of treatment with dietary soy and equol1
| Microbiota status |
||||
| Nonproducer | Equol producer | P value2 | Adjusted P value3 | |
| Uterine weight, mg | 52.2 (49.6, 55.0)a | 45.8 (43.8, 47.9)b | 0.047 | 0.33 |
| Uterine area, mm2 | 1.58 (1.48, 1.68)a | 1.28 (1.21, 1.35)b | 0.015 | 0.30 |
| Endometrial area, mm2 | 0.87 (0.81, 0.94)a | 0.68 (0.64, 0.73)b | 0.014 | 0.30 |
| Endometrial luminal epithelial height, μm | 18.3 (17.9, 18.7)a | 17.0 (16.6, 17.4)b | 0.020 | 0.16 |
| Vaginal epithelial thickness, μm | 101.3 (97.3, 105.3)a | 88.3 (84.7, 91.9)b | 0.013 | 0.25 |
Values are mean (mean – SEM, mean + SEM), n = 54–59 (nonproducer) or 55–60 (equol producer). Labeled means in a row without a common letter differ, P < 0.05.
P value for main effect of intestinal microbiota status.
P value for main effect of intestinal microbiota status after adjusting for estrous cycle stage as a covariate.
FIGURE 2.
Distribution of estrous cycle stage by microbiota status in apoE-null mice after 16 wk of dietary soy and equol treatment. Values are percentage of mice within a microbiota group in a particular estrous cycle stage (proestrus, estrus, metestrus, diestrus) at the time of necropsy, n = 52 (nonproducer) or 53 (equol producer).
Immunohistochemistry.
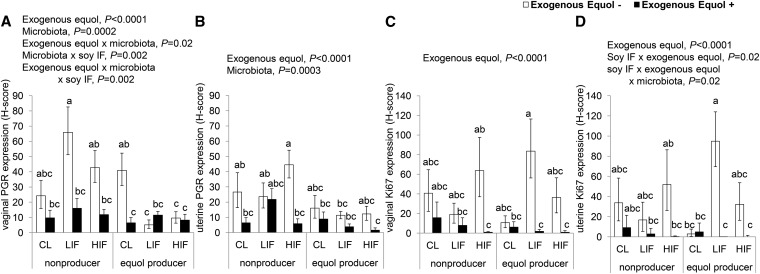
We next evaluated the expression of PGR as an estrogen-response marker in the vagina and uterus (Fig. 3A,B). We found main effects of exogenous equol and microbiota status but not soy diet. Exogenous equol lowered PGR expression in the vagina and uterus (P < 0.0001 in both tissues). A main effect of microbiota (P < 0.0005 in both tissues) was found, whereby equol producers had lower PGR expression than nonproducers. For vaginal epithelium, there was an interaction among soy diet, equol, and microbiota status (P-interaction < 0.005), equol and microbiota status (P-interaction < 0.05), and soy diet and microbiota (P-interaction < 0.005). In the absence of racemic equol, PGR expression was lower in the vagina of equol producers compared with nonproducers in both LIF− (P < 0.0001) and HIF− (P < 0.05). A similar pattern was observed in the uterus of the HIF− group, although not significant (P = 0.07). The main effects of exogenous equol and microbiota status remained significant after estrous cycle stage was added as a covariate in the model.
FIGURE 3.
Expression of PGR (A,B) and the proliferation marker Ki67 (C,D) by immunostaining in the vagina (A,C) and uterus (B,D) of apoE-null mice after 16 wk of dietary soy treatment with or without exogenous equol, by microbiota status. Values are means ± SEM, n = 8–11/group. Significant main effects and interactions are shown. Within each panel, labeled means without a common letter differ, P < 0.05. CL, casein and lactalbumin; HIF, high isoflavone; IF, isoflavone; LIF, low isoflavone; PGR, progesterone receptor.
Similar to PGR, we did not find a main effect of soy IF on the proliferation marker Ki67. There was a main effect of exogenous equol on Ki67 expression in the vagina and uterus (P < 0.0001 in both tissues) and it remained significant after estrous cycle stage was included as a covariate. Ki67 labeling was lower in mice that received exogenous equol compared with those without equol (Fig. 3C,D), regardless of soy IF dose. In uterine tissue, there were interactions among soy IF, microbiota status, and exogenous equol (P-interaction < 0.05) and between soy IF and microbiota status (P-interaction < 0.05).
Discussion
In this study, we evaluated the interactive effects of soy IF diet, endogenously produced equol, and exogenous racemic equol on reproductive tract measures in adult apoE-null mice. Treatment effects were seen only in female mice. Both endogenous and exogenous equol resulted in lower expression of markers of estrogenic action independent of soy IF dose, suggesting that equol may result in modest buffering effects on endogenous estrogen activity in the reproductive tract. A significant association was observed between microbiota status and estrous cycle stage, which may partially explain the observed effects.
This study utilized gnotobiotic apoE-null mice harboring ASF, a well-developed microbiota used for generating mice with standardized bacteria (17, 24). We showed that the equol-producing capacity in ASF-treated mice was minimal (serum equol concentration was <35 nmol/L following a HIF diet). Importantly, we managed to induce equol-producing capacity in these mice after the introduction of intestinal microbiota from normal apoE-null mice, thus providing genetically comparable equol producers. The serum equol concentration of these mice was low compared with normal apoE-null mice (25). However, this lower equol production was not considered to be a major limitation, because it is more comparable with that found in human equol producers. A person is categorized as “equol producer” if the serum equol concentration is >83 nmol/L following a soy-based meal, although these levels are typically <20% of total serum IF (11, 26).
Studies investigating the effects of soy IF and their metabolites on the reproductive system have reported equivocal or conflicting results. Most rodent studies have found that high doses of IF (>40 mg/kg body weight), genistein in particular, induced estrogen-like uterotropic effects (3). Similarly, genistein has been shown to increase vaginal epithelial height in rats (27). Studies of IF effects on the male rodent reproductive tract have been mixed, with some (25, 28, 29) but not others (30, 31) reporting estrogenic effects of IF. Studies of dietary soy IF or equol in male and female macaque monkeys have found no adverse effects on the reproductive tract, which is consistent with results from most human studies (32–37).
Our current results support the idea that soy IF do not have estrogen agonist effects in the reproductive tract of male or intact female mice when given in the diet, at doses relevant to human exposure, and within a soy protein matrix. This finding differed from our previous report that showed estrogenic effects of IF concentrates (100% aglycone) after 16 wk of treatment (25), highlighting the importance of IF form in the diet as a determinant of absorption, metabolism, and downstream tissue effects. Moreover, our results support the idea that equol, either racemic or naturally produced, does not elicit estrogen agonist effects in mice at physiologic concentrations (<3000 nmol/L).
An interesting new finding of this study was that the equol-producer phenotype, and not the soy diet itself, was associated with less estrogenic activity in the female reproductive system. We found lower uterine weight, uterine and endometrial size, endometrial luminal epithelial thickness, and vaginal epithelial thickness in mice with equol-producing intestinal microbiota. The relatively low serum equol concentrations (<300 nmol/L) associated with these effects suggest that equol-producing bacteria (rather than equol itself) altered endogenous estrogen exposure in some way. Gut bacteria have been shown to produce enzymes that metabolize estrogens during enterohepatic circulation (38–40). In a small human trial, equol producers had lower estrogen concentrations than nonproducers (41), which may be attributable to a higher fecal estrogen excretion (42). We did not measure circulating estrogen concentrations in our study and therefore cannot distinguish between such indirect intestinal microbiota effects and potential direct effects of S-equol.
Another potential contributing factor is alteration in estrous cycle. Whereas an equol-rich supplement (SE5-OH) did not alter the estrous cycle in mice (16), others have reported that soy consumption in premenopausal women resulted in longer menstrual cycle length and suppression of gonadotropins (43), which may indicate an effect of soy IF on the hypothalamo-pituitary gonadal axis. This effect, however, was small and could not be confirmed by subsequent studies. Our results show that reproductive cycles differ by equol-producing ability. This supports the hypothesis that the equol-producing phenotype may have important health-related effects, irrespective of the amount of soy consumed (12). Several studies in women have associated a soy-rich diet with reduced risk of endometrial cancer (44–46), whereas rodent studies have reported the opposite: increased risk with genistein (3, 47) and a mild uterotropic effect of dietary soy (3, 48) and racemic equol (49–51). Our findings support the idea that equol-producing ability may reduce the endometrial response to dietary soy IF.
Exogenous equol used in this study was a racemic mixture containing 50% S-equol and 50% R-(+)-equol (R-equol). S-equol binds more strongly to ERβ, whereas R-equol has more affinity to ERα (52, 53). A recent study showed that chronic feeding of S-equol had no effect on uterine weight, whereas R-equol did increase uterine weight in nonovariectomized rats (54). In contrast, we observed the opposite effect in which mice treated with racemic equol mixture had lower expression of PGR and proliferation marker Ki67 in the endometrium and vagina, suggesting less estrogenic exposure. Our finding may indicate that equol, like other isoflavonoids, can elicit both estrogenic and antiestrogenic responses depending on the concentration of circulating estrogens and other factors.
Our results showed equol concentrations were greater in equol producers (relative to nonproducers) and in mice that received diet with exogenous equol supplementation. These data confirm the success of the microbiota and dietary interventions and provide further support for the key role of gut microbiota in the equol-producing phenotype (55). We also found that soy IF had to be given in a high dose for our equol-producer model to produce endogenous equol. It should be noted that our LIF dose was equivalent to 20 mg IF/7530 kJ, which was only slightly lower than the typical daily IF consumption in an Asian population (25–50 mg/d) (56), but it did not result in significantly higher serum equol concentrations relative to the CL control group. This may need to be considered for future studies using this model.
Levels of circulating genistein and daidzein were marginally higher in the equol producers compared with nonproducers, which may indicate a higher efficiency of the equol-producing bacteria not only to convert daidzein to equol but also to hydrolyze the nonbioavailable daidzein glycosides in the soy diet to the bioavailable aglycones. Interestingly, serum equol concentrations in equol producers were lower than nonproducers in mice fed an exogenous equol. This may indicate that equol-producing bacteria continue to reduce equol as substrate to further breakdown products, reducing uptake into the circulation. Alternatively, a higher equol intake with the exogenous equol may possibly inhibit the enzymes involved in the production of equol from daidzein. Thus, daidzein may be shunted to O-desmethylangolensin and dihydrodaidzein, which we did not measure.
Although serum equol concentrations were the same across both sexes, we found a significant sex effect on genistein and daidzein metabolism. Females had higher genistein and daidzein concentrations than males. This is consistent with previous reports that showed women had longer excretion half-life values of daidzein and genistein (57) and a higher peak concentration with lower clearance rate of daidzein (6) compared with men. Our prior study using apoE-null mice showed a similar pattern for genistein, whereas equol tended to be higher in males (25). Overall, the results support the ideas that soy IF metabolism may be sex specific and that caution is warranted when generalizing results from dietary soy intervention studies to the opposite sex.
Reports on the potential action of equol as a natural selective estrogen modulator have contributed to the recent development of equol-rich supplements for postmenopausal symptoms (14, 16, 58, 59). It is not clearly understood, however, how equol affects the reproductive tract, particularly prior to menopause. Our results show that mice with equol-producing ability had less estrogenic reproductive tissue phenotypes relative to nonequol producers. We speculate that the findings may be associated with endogenous equol effects on estrous cycle and other factors related to endogenous estrogen exposure, which require further investigation.
Acknowledgments
The authors thank Hermina Borgerink, Jean Gardin, Lisa O’Donnell, Russell O’Donnell, and Joseph Finley for their technical assistance. M.R.A. and J.M.C. designed the study; F.N.D. and D.L.G. conducted the research; J.W.L. and M.A.J.H. generated serum isoflavonoid and fecal bacterial data; C.E.W. and J.M.C. performed histopathology evaluations; F.N.D. analyzed the data and wrote the manuscript with important contributions from C.E.W., A.A.F., and J.M.C.; and F.N.D. and J.M.C. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by the NIH R01 AT00639 (NCCAM) and R01 HL64746 (NHLBI). Soy protein isolates were donated by Archer Daniels Midland Company. Racemic equol mixture was donated by Solae, LLC.
Supplemental Methods, Supplemental Figures 1 and 2, and Supplemental Table 1 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: ASF, Altered Schaedler Flora; CL, casein and lactalbumin; ER, estrogen receptor; HIF, high isoflavone; IF, isoflavone; LIF, low isoflavone; PGR, progesterone receptor.
Literature Cited
- 1.Larkin T, Price WE, Astheimer L. The key importance of soy isoflavone bioavailability to understanding health benefits. Crit Rev Food Sci Nutr. 2008;48:538–52 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90 [DOI] [PubMed] [Google Scholar]
- 3.Wood CE, Barnes S, Cline JM. Phytoestrogen actions in the breast and uterus. In: Gilani GS, Anderson JJB, editors. Phytoestrogens and health. Champaign (IL): AOCS Press; 2002. p. 440–69 [Google Scholar]
- 4.Yuan JP, Wang JH, Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora: implications for health. Mol Nutr Food Res. 2007;51:765–81 [DOI] [PubMed] [Google Scholar]
- 5.Kurzer MS. Hormonal effects of soy in premenopausal women and men. J Nutr. 2002;132:S570–3 [DOI] [PubMed] [Google Scholar]
- 6.Cassidy A, Brown JE, Hawdon A, Faughnan MS, King LJ, Millward J, Zimmer-Nechemias L, Wolfe B, Setchell KD. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr. 2006;136:45–51 [DOI] [PubMed] [Google Scholar]
- 7.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36:27–32 [DOI] [PubMed] [Google Scholar]
- 8.Franke AA, Lai JF, Halm BM, Pagano I, Kono N, Mack WJ, Hodis HN. Equol production changes over time in postmenopausal women. J Nutr Biochem. 2012;23:573–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franke AA, Lai JF, Pagano I, Morimoto Y, Maskarinec G. Equol production changes over time in pre-menopausal women. Br J Nutr. 2012;107:1201–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Setchell KD, Clerici C. Equol: history, chemistry, and formation. J Nutr. 2010;140:S1355–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–84 [DOI] [PubMed] [Google Scholar]
- 12.Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood). 2005;230:155–70 [DOI] [PubMed] [Google Scholar]
- 13.Lund TD, Munson DJ, Haldy ME, Setchell KD, Lephart ED, Handa RJ. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol Reprod. 2004;70:1188–95 [DOI] [PubMed] [Google Scholar]
- 14.Setchell KD, Zhao X, Jha P, Heubi JE, Brown NM. The pharmacokinetic behavior of the soy isoflavone metabolite S-(-)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers. Am J Clin Nutr. 2009;90:1029–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lampe JW. Is equol the key to the efficacy of soy foods? Am J Clin Nutr. 2009;89:S1664–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matulka RA, Matsuura I, Uesugi T, Ueno T, Burdock G. Developmental and reproductive effects of SE5-OH: an equol-rich soy-based ingredient. J Toxicol. 2009;2009:307618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewhirst FE, Chien CC, Paster BJ, Ericson RL, Orcutt RP, Schauer DB, Fox JG. Phylogeny of the defined murine microbiota: altered Schaedler flora. Appl Environ Microbiol. 1999;65:3287–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taconic Farms Inc. Standards and designations: Taconic health standards; 2012 [cited 2012 June 21]. Available from: http://www.taconic.com/wmspage.cfm?parm1=265.
- 19.Li S, Davis B. Evaluating rodent vaginal and uterine histology in toxicity studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:246–52 [DOI] [PubMed] [Google Scholar]
- 20.Westwood FR. The female rat reproductive cycle: a practical histological guide to staging. Toxicol Pathol. 2008;36:375–84 [DOI] [PubMed] [Google Scholar]
- 21.Kreijkamp-Kaspers S, Kok L, Bots ML, Grobbee DE, Lampe JW, van der Schouw YT. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am J Clin Nutr. 2005;81:189–95 [DOI] [PubMed] [Google Scholar]
- 22.Wood CE, Lees CJ, Cline JM. Mammary gland and endometrial effects of testosterone in combination with oral estradiol and progesterone. Menopause. 2009;16:466–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budwit-Novotny DA, McCarty KS, Cox EB, Soper JT, Mutch DG, Creasman WT, Flowers JL, McCarty KS., Jr Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46:5419–25 [PubMed] [Google Scholar]
- 24.Orcutt RP, Gianni FJ, Judge RJ. Development of an “Altered Schaedler Flora” for NCI gnotobiotic rodents. Microecol Ther. 1987;17:59 [Google Scholar]
- 25.Cline JM, Franke AA, Register TC, Golden DL, Adams MR. Effects of dietary isoflavone aglycones on the reproductive tract of male and female mice. Toxicol Pathol. 2004;32:91–9 [DOI] [PubMed] [Google Scholar]
- 26.Niculescu MD, Pop EA, Fischer LM, Zeisel SH. Dietary isoflavones differentially induce gene expression changes in lymphocytes from postmenopausal women who form equol as compared with those who do not. J Nutr Biochem. 2007;18:380–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diel P, Smolnikar K, Schulz T, Laudenbach-Leschowski U, Michna H, Vollmer G. Phytoestrogens and carcinogenesis-differential effects of genistein in experimental models of normal and malignant rat endometrium. Hum Reprod. 2001;16:997–1006 [DOI] [PubMed] [Google Scholar]
- 28.Strauss L, Makela S, Joshi S, Huhtaniemi I, Santti R. Genistein exerts estrogen-like effects in male mouse reproductive tract. Mol Cell Endocrinol. 1998;144:83–93 [DOI] [PubMed] [Google Scholar]
- 29.Wisniewski AB, Cernetich A, Gearhart JP, Klein SL. Perinatal exposure to genistein alters reproductive development and aggressive behavior in male mice. Physiol Behav. 2005;84:327–34 [DOI] [PubMed] [Google Scholar]
- 30.Faqi AS, Johnson WD, Morrissey RL, McCormick DL. Reproductive toxicity assessment of chronic dietary exposure to soy isoflavones in male rats. Reprod Toxicol. 2004;18:605–11 [DOI] [PubMed] [Google Scholar]
- 31.Nagao T, Yoshimura S, Saito Y, Nakagomi M, Usumi K, Ono H. Reproductive effects in male and female rats of neonatal exposure to genistein. Reprod Toxicol. 2001;15:399–411 [DOI] [PubMed] [Google Scholar]
- 32.Balk JL, Whiteside DA, Naus G, DeFerrari E, Roberts JM. A pilot study of the effects of phytoestrogen supplementation on postmenopausal endometrium. J Soc Gynecol Investig. 2002;9:238–42 [PubMed] [Google Scholar]
- 33.Cline JM, Paschold JC, Anthony MS, Obasanjo IO, Adams MR. Effects of hormonal therapies and dietary soy phytoestrogens on vaginal cytology in surgically postmenopausal macaques. Fertil Steril. 1996;65:1031–5 [PubMed] [Google Scholar]
- 34.Nikander E, Rutanen EM, Nieminen P, Wahlstrom T, Ylikorkala O, Tiitinen A. Lack of effect of isoflavonoids on the vagina and endometrium in postmenopausal women. Fertil Steril. 2005;83:137–42 [DOI] [PubMed] [Google Scholar]
- 35.Perry DL, Spedick JM, McCoy TP, Adams MR, Franke AA, Cline JM. Dietary soy protein containing isoflavonoids does not adversely affect the reproductive tract of male cynomolgus macaques (Macaca fascicularis). J Nutr. 2007;137:1390–4 [DOI] [PubMed] [Google Scholar]
- 36.Wood CE, Kaplan JR, Stute P, Cline JM. Effects of soy on the mammary glands of premenopausal female monkeys. Fertil Steril. 2006;85 Suppl 1:1179–86 [DOI] [PubMed] [Google Scholar]
- 37.Mitchell JH, Cawood E, Kinniburgh D, Provan A, Collins AR, Irvine DS. Effect of a phytoestrogen food supplement on reproductive health in normal males. Clin Sci (Lond). 2001;100:613–8 [PubMed] [Google Scholar]
- 38.Lombardi P, Goldin B, Boutin E, Gorbach SL. Metabolism of androgens and estrogens by human fecal microorganisms. J Steroid Biochem. 1978;9:795–801 [DOI] [PubMed] [Google Scholar]
- 39.Gorbach SL. Estrogens, breast cancer, and intestinal flora. Rev Infect Dis. 1984;6 Suppl 1:S85–90 [DOI] [PubMed] [Google Scholar]
- 40.McBain AJ, Macfarlane GT. Ecological and physiological studies on large intestinal bacteria in relation to production of hydrolytic and reductive enzymes involved in formation of genotoxic metabolites. J Med Microbiol. 1998;47:407–16 [DOI] [PubMed] [Google Scholar]
- 41.Duncan AM, Merz-Demlow BE, Xu X, Phipps WR, Kurzer MS. Premenopausal equol excretors show plasma hormone profiles associated with lowered risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:581–6 [PubMed] [Google Scholar]
- 42.Adlercreutz H, Gorbach SL, Goldin BR, Woods MN, Dwyer JT, Hamalainen E. Estrogen metabolism and excretion in Oriental and Caucasian women. J Natl Cancer Inst. 1994;86:1076–82 [DOI] [PubMed] [Google Scholar]
- 43.Wu AH, Stanczyk FZ, Hendrich S, Murphy PA, Zhang C, Wan P, Pike MC. Effects of soy foods on ovarian function in premenopausal women. Br J Cancer. 2000;82:1879–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bandera EV, Williams MG, Sima C, Bayuga S, Pulick K, Wilcox H, Soslow R, Zauber AG, Olson SH. Phytoestrogen consumption and endometrial cancer risk: a population-based case-control study in New Jersey. Cancer Causes Control. 2009;20:1117–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horn-Ross PL, John EM, Canchola AJ, Stewart SL, Lee MM. Phytoestrogen intake and endometrial cancer risk. J Natl Cancer Inst. 2003;95:1158–64 [DOI] [PubMed] [Google Scholar]
- 46.Xu WH, Zheng W, Xiang YB, Ruan ZX, Cheng JR, Dai Q, Gao YT, Shu XO. Soya food intake and risk of endometrial cancer among Chinese women in Shanghai: population based case-control study. BMJ. 2004;328:1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newbold RR, Banks EP, Bullock B, Jefferson WN. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res. 2001;61:4325–8 [PubMed] [Google Scholar]
- 48.Gallo D, Cantelmo F, Distefano M, Ferlini C, Zannoni GF, Riva A, Morazzoni P, Bombardelli E, Mancuso S, Scambia G. Reproductive effects of dietary soy in female Wistar rats. Food Chem Toxicol. 1999;37:493–502 [DOI] [PubMed] [Google Scholar]
- 49.Legette LL, Martin BR, Shahnazari M, Lee WH, Helferich WG, Qian J, Waters DJ, Arabshahi A, Barnes S, Welch J, et al. Supplemental dietary racemic equol has modest benefits to bone but has mild uterotropic activity in ovariectomized rats. J Nutr. 2009;139:1908–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rachón D, Vortherms T, Seidlova-Wuttke D, Menche A, Wuttke W. Uterotropic effects of dietary equol administration in ovariectomized Sprague-Dawley rats. Climacteric. 2007;10:416–26 [DOI] [PubMed] [Google Scholar]
- 51.Selvaraj V, Zakroczymski MA, Naaz A, Mukai M, Ju YH, Doerge DR, Katzenellenbogen JA, Helferich WG, Cooke PS. Estrogenicity of the isoflavone metabolite equol on reproductive and non-reproductive organs in mice. Biol Reprod. 2004;71:966–72 [DOI] [PubMed] [Google Scholar]
- 52.Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–67 [DOI] [PubMed] [Google Scholar]
- 53.Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–9 [DOI] [PubMed] [Google Scholar]
- 54.Brown NM, Belles CA, Lindley SL, Zimmer-Nechemias LD, Zhao X, Witte DP, Kim MO, Setchell KD. The chemopreventive action of equol enantiomers in a chemically induced animal model of breast cancer. Carcinogenesis. 2010;31:886–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bowey E, Adlercreutz H, Rowland I. Metabolism of isoflavones and lignans by the gut microflora: a study in germ-free and human flora associated rats. Food Chem Toxicol. 2003;41:631–6 [DOI] [PubMed] [Google Scholar]
- 56.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1–12 [DOI] [PubMed] [Google Scholar]
- 57.Lu LJ, Anderson KE. Sex and long-term soy diets affect the metabolism and excretion of soy isoflavones in humans. Am J Clin Nutr. 1998;68:S1500–4 [DOI] [PubMed] [Google Scholar]
- 58.Ishiwata N, Melby MK, Mizuno S, Watanabe S. New equol supplement for relieving menopausal symptoms: randomized, placebo-controlled trial of Japanese women. Menopause. 2009;16:141–8 [DOI] [PubMed] [Google Scholar]
- 59.Onoda A, Ueno T, Uchiyama S, Hayashi SI, Kato K, Wake N. Effects of S-equol and natural S-equol supplement (SE5-OH) on the growth of MCF-7 in vitro and as tumors implanted into ovariectomized athymic mice. Food Chem Toxicol. 2011;49:2279–84 [DOI] [PubMed] [Google Scholar]