Abstract
Several membrane trafficking proteins have been shown to participate in spindle assembly and stability during mitosis. Despite the fact that the role of some of them has been clarified, the requirement for these molecules in mitosis is still poorly understood.
Recently, we and others1,2 found that a key player in endocytosis, the small GTPase Rab5, controls the alignment of chromosomes on the metaphase plate both in mammalian cells and in the Drosophila model organism. Although the underlying mechanisms appear to be distinct, depletion of Rab5 affects progression through mitosis and the correct execution of chromosome segregation in the daughter cells in both systems, indicating that this function of Rab5 is conserved through evolution.
After outlining the common requirements for Rab5 in metazoans mitosis, I will comment on the involvement of Rab5 in spindle stability and in the localization of the centromere-associated protein CENP-F to kinetochores of mammalian cells.
Keywords: CENP-F, Kinetochore, Rab5, centrosome, chromosome, endosome, mitosis, spindle
A Common Requirement for Rab5 at the Onset of Mitosis
Rab5 is a member of the Rab family of small GTPases that controls the homeostasis of the endosomal compartment and the early steps of endocytosis of a large variety of receptors either cargoes or ligand-engaged. By recruiting downstream effectors, active-Rab5 regulates docking and fusion of early endosomes as well as their movement on microtubules.3-7 Work from Karen Oegema’s laboratory demonstrated that Rab5 is also required for structuring the endoplasmic reticulum (ER) in Caenorabditis elegans.8 The ER is a continuous network of tubules and sheets that includes the outer nuclear membrane. At mitosis, the ER clusters and during nuclear envelope breakdown some nuclear membrane proteins redistribute into the membrane system of the ER.9 In Caenorabditis elegans embryos, functional ablation of Rab5 alters mitotic ER clustering and inhibits nuclear envelope disassembly resulting in retention of B-type lamin at the nuclear membrane.8 During the first mitotic prophase of nematode embryos, the oocyte and sperm pronuclei migrate toward each other coincident with chromosome condensation. The pronuclear envelopes undergo a scission event in close proximity to the aligned chromosome and are cleared from the region between the chromosomes allowing the chromosomes to mix and form a single nucleus after segregation.10 In embryos depleted of Rab5 this scission event does not occur and oocyte- and sperm-derived chromosomes remain separate during their segregation on the spindle finally resulting in the formation of daughter cells with two nuclei each in which mixing of the genome failed.8 In the proposed model, Rab5 on endosomes might interact with effectors on the ER membrane “in trans” to promote their homotypic fusion. Rab5-depletion, by disrupting the morphology of the ER, might affect the diffusion of nuclear envelope components to the ER at the onset of mitosis thus inhibiting the nuclear envelope disassembly. Of note, the afore mentioned study shows that overexpression of a Rab5 constitutive-active mutant increases the number and size of mitotic ER clusters also in HeLa cells suggesting a role of Rab5 in nuclear envelope disassembly in mammals.8
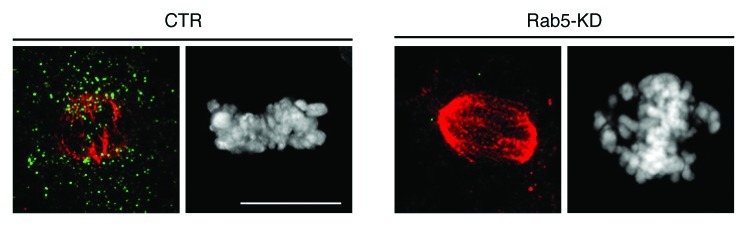
We confirmed that silencing of Rab5 retards the kinetics of nuclear envelope breakdown and lamin B disassembly also in mammalian cells. The delay in nuclear envelope disassembly results in temporary retention of mitotic proteins that localize at the nuclear membrane such as the centromere-associated protein CENP-F. This event is transient since the nuclear membrane appears to dissolve completely in the Rab5RNAi cells that enter prometaphase. In mitosis, Rab5-silencing impairs the alignment of chromosomes on the metaphase plate (an example is provided in Figure 1) delaying progression through mitosis and causing defective chromosome segregation in the daughter cells.
Figure 1. Functional ablation of Rab5 impairs chromosome alignment on the metaphase plate. U2OS cells were silenced with control oligos (CTR) or with Rab5 oligos (Rab5-KD) and treated with MG132 to block mitotic cells at metaphase. Pictures are Z-projections of confocal images of silenced cells stained with anti-α-tubulin (red) and anti-Rab5A (green) antibodies and DAPI (gray). In control cell all chromosomes are at the metaphase plate instead, in Rab5-KD cell chromosome congression is defective. Bar, in this and subsequent figures is 10 μm.
Rab5 participates to nuclear envelope disassembly also in Drosophila melanogaster. In this model organism, Rab5 associates with Lamin and Mud, the Drosophila counterpart of mammalian NuMA. Mud, a protein required for chromosome alignment, localizes to the nuclear envelope and translocates to the spindle poles in prophase and metaphase. In the Drosophila cells depleted of Rab5 the nuclear envelope does not disassemble properly at mitotic entry, the dispersal of Lamin is affected and Mud fails to accumulate at spindle poles. Furthermore, chromosome congression and mitotic progression are defective in these cells suggesting that Rab5, by contributing to disassembly of the nuclear lamina, allows the release and polar transport of Mud that, in turn, controls chromosome behavior during prometaphase.2
Notably, in the fruit fly, the nuclear membrane never disassembles completely but it fenestrates allowing spindle microtubules access to chromosomes in what is defined a “semi-open” mitosis.11 Similarly, also in Caenorabditis elegans nuclear envelope breakdown occurs very late, compared with vertebrates, fully disassembling only during mid-late anaphase.12 Despite these differences, the involvement of Rab5 in the regulation of nuclear envelope disassembly and in the release of nuclear envelope components seems to be a common requisite (Table 1). Importantly, these studies point at a novel function for Rab5 in alignment and proper segregation of chromosomes.
Table 1.
| Organism | Nuclear envelope disassembly and release of nuclear membrane components | Polar Transport | Kinetochore localization |
|---|---|---|---|
| C. elegans |
Yes |
- |
- |
| Drosophila |
Yes |
Yes (Mud) |
- |
| Homo sapiens | Yes | - | Yes (CENP-F) |
Rab5 is required for proper timing of nuclear envelope breakdown and lamina disassembly in metazoans. In Drosophila, it controls transport of Mud to spindle poles, in mammalian cells it localizes CENP-F to kinetochores.
Dynamic Localization of Rab5 during Mitosis
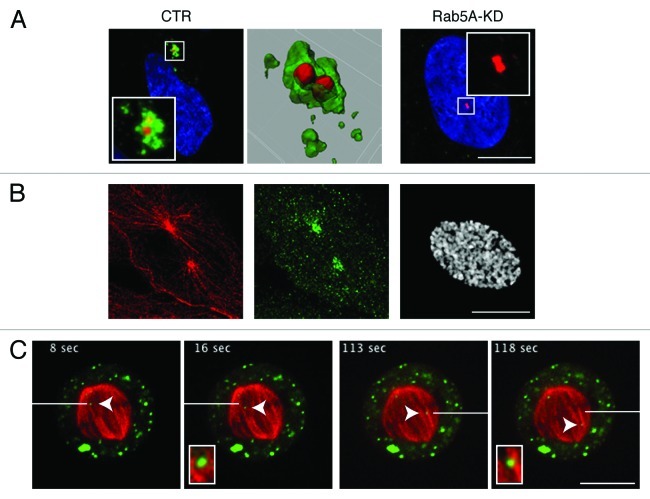
In mammalian cells, early endosomes are located beneath the plasma membrane. In addition to this location, Rab5-positive vesicles can also be detected around the centrosome (Fig. 2A). At late G2/prophase, they accumulate at duplicated centrosome and remain at spindle poles until nuclear envelope breakdown completes and the cell enters prometaphase (Fig. 2B). At this stage, while the bipolar spindle assembles, the number of Rab5-vesicles abruptly diminishes and, by metaphase, their clustering around poles is no longer visible. During metaphase a pool of Rab5-vesicles can be detected moving on spindle microtubules (Fig. 2C). Vesicles’ clustering around centrosome finally resumes at late telophase.
Figure 2. Rab5 localization around centrosome and in mitosis. (A) Confocal analysis of U2OS cells untreated (CTR) or silenced with Rab5A specific RNAi oligo (Rab5A-KD) stained with anti-Rab5A (green), anti-γ-tubulin (red) antibodies and DAPI (blue). The centrosomal region (boxed) is magnified in the insets. The magnification of the centrosomal region in control cell has been reconstructed with IMARIS 6.2 (Bitplane) and it is shown in the middle panel. (B) Confocal analysis of U2OS cell at prophase stained with anti-α-tubulin (red) and anti-Rab5A (green) antibodies and DAPI (gray) showing accumulation of Rab5 around spindle poles. (C) Selected frames from time-lapse movie of a mitotic U2OS cell at metaphase expressing YFP-Rab5A (green) and RFP-α-tubulin (red). A single z-section taken in the equatorial region of the cell is shown. Arrowheads point to moving vesicles (toward the metaphase plate, left panel, toward the pole, right panel). A reference white bar, positioned in each panel, indicates the point at which we started to track the vesicle. Time is in sec. Rab5-positive vesicles on spindle microtubules are boxed in and magnified in the insets.
Even if the function and cargoes composition of these vesicles still need to be addressed, an appealing hypothesis is that they accumulate at spindle poles at the onset of mitosis to subsequently re-distribute their cargo proteins along the spindle.
The behavior of Rab5-vesicles in Drosophila is slightly different from what we observed in mammalian cells because, in the fruit fly, Rab5-vesicles persist at spindle poles at metaphase and throughout the rest of mitosis.2
Rab5 Participates in Chromosome Congression and in Kinetochore Function
The initial observations that Rab5-positive vesicles move within the spindle in metaphase cells prompted us to investigate whether Rab5 might have a specific function in the early steps of cell division. We found that RNAi-mediated simultaneous depletion of the three human Rab5 isoforms impaired the alignment of chromosomes on the metaphase plate in U2OS cells (human osteosarcoma cell line). In agreement with findings showing that the function of the three Rab5 isoforms in endocytosis is redundant,13 silencing of a single isoform did not result in macroscopic mitotic defects.
Interestingly, the function of Rab5 in chromosome congression does not seem to be directly linked with endocytosis because, as shown for the effects of Rab5 depletion on ER morphology in Caenorabditis elegans,8 perturbation of endocytosis by other means does not affect chromosome alignment.
Chromosome alignment depends on the dynamic interaction of chromosomes and microtubules. In vertebrates, specialized chromosomal regions known as centromeres assemble the kinetochore, a large protein structure that mediates the attachment of chromosomes to a subset of spindle microtubules bundles called kinetochore-fibers. Kinetochore-fibers extend from the kinetochore to spindle poles where they are anchored.
To allow the precise and synchronous segregation of all chromosomes the cell needs to position them at the equator plane and wait, before driving the poleward movement of sister chromatids to the opposite poles, until every chromosome has been attached. This monitoring is achieved through a potent signaling system called the Spindle Assembly Checkpoint that delays mitosis in presence of unattached kinetochores. Kinetochores assemble during early prophase and disassemble by the end of mitosis with a timely ordered recruitment of various components. Among the protein complexes that constitute the kinetochore, HEC1 represents a main attachment site of kinetochore-fibers. Other molecules involved in stable microtubule capture at kinetochores are the centromere-associated proteins CENP-F and CENP-E (for reviews see refs.14,15).
We noticed that, in Rab5-silenced cells, the interkinetochore distance was reduced compared with control cells meaning that tension across kinetochores was defective. Kinetochore-fibers that are not stably attached to kinetochores depolymerize when cells are chilled at 4°C. Under these conditions, we observed that in Rab5-silenced cells a subset of unattached kinetochores or kinetochores showing aberrant attachment were present, similarly to what is reported for cells depleted of CENP-F.16 This result indicates that Rab5 participates in the stability of kinetochore-fibers.
Analysis of kinetochore localization of CENP-F and HEC1 revealed that the amount of CENP-F, but not of HEC1, at kinetochores of Rab5-silenced prometaphase cells was severely reduced.
CENP-F is a protein of the nuclear matrix that has no homologs in invertebrates. It is cell cycle-regulated, reaching maximum levels at G2/M.17 At the onset of mitosis, it is recruited to the nuclear membrane by a recently identified pathway that depends on the nuclear pore complex protein Nup133.18 In early prophase, CENP-F is also recruited to the maturing kinetochores. When the nuclear envelope disassembles, CENP-F is released into the mitotic cytoplasm and part of it accumulates at kinetochores during prometaphase. CENP-F remains at kinetochores until anaphase when it is degraded.17
The modalities of transient CENP-F recruitment to kinetochores are not completely understood. The kinase Bub1 is required for CENP-F localization to kinetochores.19,20 However, Bub1 itself is reduced at kinetochores depleted of CENP-F suggesting a complex regulation of these two molecules.16 Our findings show that Rab5 participates to CENP-F accumulation at kinetochores after nuclear envelope disassembly.
In mitosis, CENP-F contributes to kinetochore assembly and to stable microtubule capture at kinetochores. Silencing of CENP-F affects chromosome alignment, delays anaphase onset and results in chromosome missegregation.16,21-23 All these defects were recorded to comparable levels in the Rab5-silenced cells. In addition, kinetochore localization of the mitotic kinesin CENP-E, which is recruited downstream of CENP-F, was strongly reduced also in the Rab5-silenced cells.
Even if additional molecules might likely be involved in the Rab5-mediated events that control spindle stability, the results showing that Rab5 forms a transient complex with CENP-F at mitosis and the lack of additive effects in cells simultaneously depleted of Rab5 and CENP-F argue that CENP-F is a relevant biological target of Rab5 in chromosome congression.
How does Rab5 Participate in Kinetochore Function?
The “canonical” function of Rab5 is to regulate membrane dynamics, both at the endosome and coated pit level. The data showing that Rab5 is also necessary for chromosome alignment and kinetochore assembly pose the question of whether this “non-canonical” function of Rab5 is related to (or coordinated with) membrane dynamics or not. Active Rab5 is required for endosome transport along microtubules in interphase cells.7,24 We observed that some Rab5-positive vesicles also move on spindle microtubules during the early steps of mitosis. This might be linked to ordered endosomal inheritance in the forming daughter cells.25 However, another appealing hypothesis is that Rab5 might traffic a pool of CENP-F on spindle-associated vesicles releasing it in proximity of kinetochores.
In support of this hypothesis we observed that few native Rab5-CENP-F complexes could be detected adjacent to kinetochores. Furthermore, efficient targeting of CENP-F at kinetochores requires its farnesylation,26,27 a modification commonly related to protein recruitment to membranes. The limited number of Rab5/CENP-F complexes scored might be explained with their extremely dynamic spatial-temporal interaction. Of note, not all the kinetochores reach the same level of maturation simultaneously and this might also account for the observed limited number of Rab5 and CENP-F foci, compatible with a function of Rab5 in localizing a pool of CENP-F to kinetochores during kinetochore maturation. As reported in earlier studies on CENP-F localization,17,26 only a subset of CENP-F targets to kinetochores while the rest of the protein remains in the mitotic cytoplasm once the nuclear envelope disassembles. Since a fraction of CENP-F coimmunoprecipitates with Rab5 in mitosis this supports the possibility that Rab5, by entering in a complex with CENP-F, controls its transport to kinetochores after the completion of nuclear envelope disassembly.
While these hypotheses await experimental confirmation, they attract the attention toward a previously unrecognized profound level of interconnection among basic cellular functions.
Future Perspectives
Another Rab protein, Rab6A’, was previously found to participate in mitosis.28 Rab6A’ works in the retrograde Golgi transport route.29 At mitosis, depletion of Rab6A’ arrests cells at metaphase with aligned chromosomes. In these cells tension at kinetochores is unaffected suggesting no obvious alterations in spindle assembly. However, Rab6A’-silenced cells cannot progress through anaphase and display increased amount of Mad2 and p150Glued at kinetochores. Rab6A’ interacts with p150Glued, a subunit of the dynein-dynactin complex that is required for transport of checkpoint proteins, such as Mad2, off kinetochores at the metaphase/anaphase transition. Thus depletion of Rab6A’ might affect the removal of p150Glued from kinetochores thereby preventing inactivation of the spindle checkpoint and progression through mitosis.28
Despite the underlying molecular mechanisms still need to be elucidated, both Rab6A’ and Rab5 appear to participate, at different mitotic steps and with distinct modalities, to the localization of molecules that transiently associate with the outer kinetochore plate.
In a recent RNAi screening for novel mitotic hits in mammalian cells, other Rab family members have been identified.30 In this single-gene silencing approach, Rab5 is not listed among the potential mitotic hits, consistently with our results showing that simultaneous depletion of all the three Rab5 isoforms is needed to affect chromosome congression and to delay mitosis. While silencing of some Rabs (Rab37, Rab8a, Rab24) causes cell binucleation, likely reflecting defects in cytokinesis, a process known to rely on vesicular membrane transport,31 depletion of some others (Rab7, Rab22a and Rab25) causes mitotic arrest/delay.30 In this latter group is Rab22a, which belongs to an evolutionary conserved Rab-subfamily together with Rab5 and Rab2132 and shares common effectors with Rab5, leading to the question of whether it might also be involved in spindle stability.
In light of these findings, it would be interesting to investigate which is the role of these other Rabs in mitosis and if they might be required for localization of mitotic proteins.
Acknowledgments
This work and LL is supported by AIRC (Associazione Italiana per la Ricerca sul Cancro, START UP program), AICR (Association for International Cancer Research) and the Fondazione Piemontese per la Ricerca sul Cancro - ONLUS – Intramural Grant 5X1000 2008.
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/19987
References
- 1.Serio G, Margaria V, Jensen S, Oldani A, Bartek J, Bussolino F, et al. Small GTPase Rab5 participates in chromosome congression and regulates localization of the centromere-associated protein CENP-F to kinetochores. Proc Natl Acad Sci U S A. 2011;108:17337–42. doi: 10.1073/pnas.1103516108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capalbo L, D’Avino PP, Archambault V, Glover DM. Rab5 GTPase controls chromosome alignment through Lamin disassembly and relocation of the NuMA-like protein Mud to the poles during mitosis. Proc Natl Acad Sci U S A. 2011;108:17343–8. doi: 10.1073/pnas.1103720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, et al. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–28. doi: 10.1016/0092-8674(92)90306-W. [DOI] [PubMed] [Google Scholar]
- 4.Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–5. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- 5.Stenmark H, Parton RG, Steele-Mortimer O, Lütcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–96. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanzetti L, Palamidessi A, Areces L, Scita G, Di Fiore PP. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature. 2004;429:309–14. doi: 10.1038/nature02542. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen E, Severin F, Backer JM, Hyman AA, Zerial M. Rab5 regulates motility of early endosomes on microtubules. Nat Cell Biol. 1999;1:376–82. doi: 10.1038/14075. [DOI] [PubMed] [Google Scholar]
- 8.Audhya A, Desai A, Oegema K. A role for Rab5 in structuring the endoplasmic reticulum. J Cell Biol. 2007;178:43–56. doi: 10.1083/jcb.200701139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutay U, Hetzer MW. Reorganization of the nuclear envelope during open mitosis. Curr Opin Cell Biol. 2008;20:669–77. doi: 10.1016/j.ceb.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oegema K, Hyman AA. Cell division. WormBook. 2006:1–40. doi: 10.1895/wormbook.1.72.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsani KR, Karess RE, Dostatni N, Doye V. In vivo dynamics of Drosophila nuclear envelope components. Mol Biol Cell. 2008;19:3652–66. doi: 10.1091/mbc.E07-11-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KK, Gruenbaum Y, Spann P, Liu J, Wilson KL. C. elegans nuclear envelope proteins emerin, MAN1, lamin, and nucleoporins reveal unique timing of nuclear envelope breakdown during mitosis. Mol Biol Cell. 2000;11:3089–99. doi: 10.1091/mbc.11.9.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang F, Khvorova A, Marshall W, Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J Biol Chem. 2004;279:16657–61. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- 14.Musacchio A, Hardwick KG. The spindle checkpoint: structural insights into dynamic signalling. Nat Rev Mol Cell Biol. 2002;3:731–41. doi: 10.1038/nrm929. [DOI] [PubMed] [Google Scholar]
- 15.Walczak CE, Cai S, Khodjakov A. Mechanisms of chromosome behaviour during mitosis. Nat Rev Mol Cell Biol. 2010;11:91–102. doi: 10.1038/nrm2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bomont P, Maddox P, Shah JV, Desai AB, Cleveland DW. Unstable microtubule capture at kinetochores depleted of the centromere-associated protein CENP-F. EMBO J. 2005;24:3927–39. doi: 10.1038/sj.emboj.7600848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao H, Winkfein RJ, Mack G, Rattner JB, Yen TJ. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J Cell Biol. 1995;130:507–18. doi: 10.1083/jcb.130.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolhy S, Bouhlel I, Dultz E, Nayak T, Zuccolo M, Gatti X, et al. A Nup133-dependent NPC-anchored network tethers centrosomes to the nuclear envelope in prophase. J Cell Biol. 2011;192:855–71. doi: 10.1083/jcb.201007118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson VL, Scott MI, Holt SV, Hussein D, Taylor SS. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J Cell Sci. 2004;117:1577–89. doi: 10.1242/jcs.01006. [DOI] [PubMed] [Google Scholar]
- 20.Klebig C, Korinth D, Meraldi P. Bub1 regulates chromosome segregation in a kinetochore-independent manner. J Cell Biol. 2009;185:841–58. doi: 10.1083/jcb.200902128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Z, Guo J, Chen Q, Ding C, Du J, Zhu X. Silencing mitosin induces misaligned chromosomes, premature chromosome decondensation before anaphase onset, and mitotic cell death. Mol Cell Biol. 2005;25:4062–74. doi: 10.1128/MCB.25.10.4062-4074.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt SV, Vergnolle MA, Hussein D, Wozniak MJ, Allan VJ, Taylor SS. Silencing Cenp-F weakens centromeric cohesion, prevents chromosome alignment and activates the spindle checkpoint. J Cell Sci. 2005;118:4889–900. doi: 10.1242/jcs.02614. [DOI] [PubMed] [Google Scholar]
- 23.Laoukili J, Kooistra MR, Brás A, Kauw J, Kerkhoven RM, Morrison A, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–36. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 24.Hoepfner S, Severin F, Cabezas A, Habermann B, Runge A, Gillooly D, et al. Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell. 2005;121:437–50. doi: 10.1016/j.cell.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Dunster K, Toh BH, Sentry JW. Early endosomes, late endosomes, and lysosomes display distinct partitioning strategies of inheritance with similarities to Golgi-derived membranes. Eur J Cell Biol. 2002;81:117–24. doi: 10.1078/0171-9335-00232. [DOI] [PubMed] [Google Scholar]
- 26.Hussein D, Taylor SS. Farnesylation of Cenp-F is required for G2/M progression and degradation after mitosis. J Cell Sci. 2002;115:3403–14. doi: 10.1242/jcs.115.17.3403. [DOI] [PubMed] [Google Scholar]
- 27.Ashar HR, James L, Gray K, Carr D, Black S, Armstrong L, et al. Farnesyl transferase inhibitors block the farnesylation of CENP-E and CENP-F and alter the association of CENP-E with the microtubules. J Biol Chem. 2000;275:30451–7. doi: 10.1074/jbc.M003469200. [DOI] [PubMed] [Google Scholar]
- 28.Miserey-Lenkei S, Couëdel-Courteille A, Del Nery E, Bardin S, Piel M, Racine V, et al. A role for the Rab6A’ GTPase in the inactivation of the Mad2-spindle checkpoint. EMBO J. 2006;25:278–89. doi: 10.1038/sj.emboj.7600929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallard F, Tang BL, Galli T, Tenza D, Saint-Pol A, Yue X, et al. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Biol. 2002;156:653–64. doi: 10.1083/jcb.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neumann B, Walter T, Hériché JK, Bulkescher J, Erfle H, Conrad C, et al. Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes. Nature. 2010;464:721–7. doi: 10.1038/nature08869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albertson R, Riggs B, Sullivan W. Membrane traffic: a driving force in cytokinesis. Trends Cell Biol. 2005;15:92–101. doi: 10.1016/j.tcb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Pereira-Leal JB, Seabra MC. The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J Mol Biol. 2000;301:1077–87. doi: 10.1006/jmbi.2000.4010. [DOI] [PubMed] [Google Scholar]