Abstract
We report on the incidental observation of ectopic pancreas in a donor for islet cell transplantation. The donor’s clinical and imaging presentation was definitive for holoprosencephaly. This case report discusses a possible link between ectopic pancreas and holoprosencephaly.
Keywords: dorsal pancreas, hydrocephalus, islet isolation, islet transplantation, pancreas anomaly, ventral pancreas
Case Presentation
A 22-y-old male became a multiple-organ donor after diagnosis of brain death and obtaining a legal consent. His past medical history included hydrocephalus necessitating ventriculo-peritoneal shunt placement at birth and seizure disorder since 2 y of age, with a clinical diagnosis of holoprosencephaly (HPE), but was unremarkable for pancreatic disease. Laboratory tests revealed serum lipase level of 115 U/L and hemoglobin A1c of 4.6%. His body weight was 35 kg and height 160 cm. His pancreas was retrieved en bloc with the spleen and duodenum at a local hospital and transported to the Clinical Islet Laboratory at the University of Alberta for the purpose of islet isolation and transplantation. When the spleen and duodenum were dissected from the pancreas, it was noted that a small yellowish nodule presented at the posterior aspect of proximal duodenum, just below the pyloric ring (Fig. 1). This solid nodule, apparently not connected with the pancreas, could be easily dissected from the duodenal wall. The nodule was not subjected to islet isolation process, but was preserved for histological examination. During intraductal perfusion of the pancreas as a first step of islet isolation procedure, it was further noted that there was no communication between the ventral and dorsal ducts, leading to a diagnosis of pancreas divisum. The isolation procedure from this small pancreas (55 g) failed to yield enough islet mass for clinical transplantation. Histopathologic examination of the nodule revealed ectopic pancreas consisting of pancreatic acini, ducts, and islets (Fig. 2). Immunohistologically islets stained positive for both insulin and glucagon, but we could not detect islets containing abundant pancreatic polypeptide (PP) positive cells.
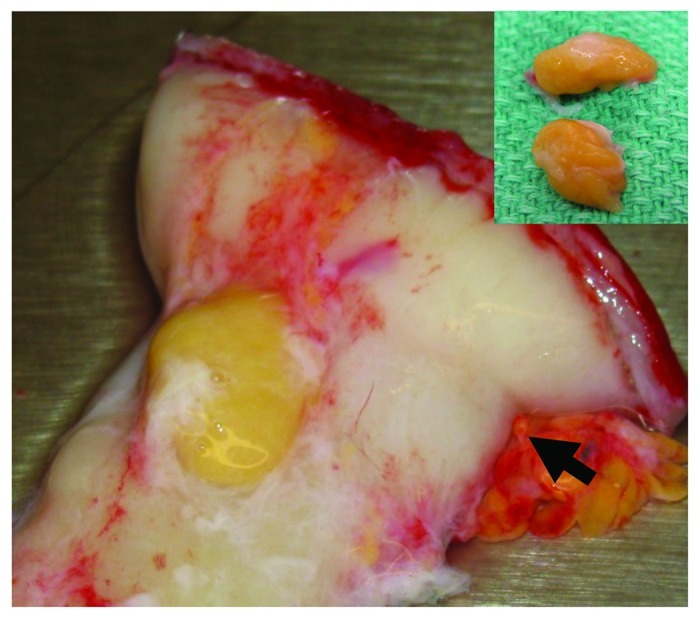
Figure 1. Ectopic pancreas is located on the posterior duodenum, just near the pyloric ring (arrow). Inset: A cut surface of resected ectopic pancreas shows no cystic lesions.
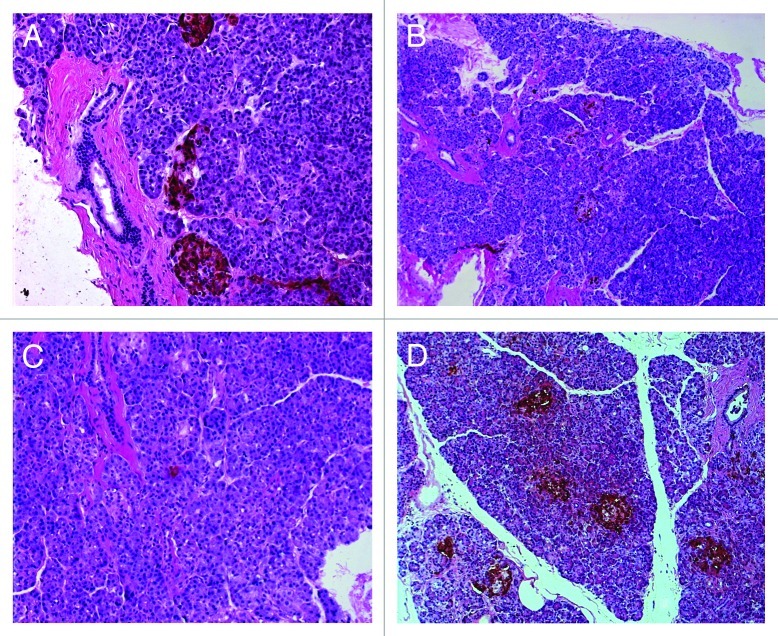
Figure 2. Ectopic pancreas stained for insulin (A), glucagon (B) and pancreatic polypeptide (C). Normal ventral pancreas from a different donor stained for pancreatic polypeptide shows pancreatic polypeptide enriched islets (D).
Discussion
HPE is a genetically and phenotypically heterogenous disorder involving the development of brain and face. Sonic hedgehog (Shh) is one of genes for responsible for HPE. Dominant and recessive mutations of Shh in humans lead to HPE.1 Shh is broadly expressed throughout embryonic development but is excluded from the pancreas during development. Thus increased Shh signaling can suppress the development of the pancreas2 while inhibition of Shh leads to ectopic development of the pancreatic tissue.3 Although we did not investigate the mutation of Shh and chromosomal abnormality, the present case suggests a link between HPE and development of ectopic pancreas.
Tsugu et al. have reported a young Japanese female with congenital brain malformation associated with intracranial ectopic pancreas.4 The patient underwent ventriculo-peritoneal shunt placement for hydrocephalus at birth, suggesting HPE. At age of 11, her brain tumor was resected and pathological examination revealed a non-functioning pancreatic tumor arising from intracranial ectopic pancreas. Interestingly a further detailed analysis showed pancreatic duodenal homebox 1was not expressed in the ectopic pancreatic tissue.5
A review described by Lemire indicates that there is one case of HPE associated with ectopic pancreas,6 but we note that the term “ectopic pancreas” was used to describe pancreas located caudally7 in the Lemire’s review.
The histogenetic mechanism of ectopic pancreas development remains unknown. One hypothesis postulates trans-commitment of non-pancreatic tissue progenitors to pancreatic lineage. Another theory proposes that pancreas fragments are separated from the original pancreas into the different sites during development of the pancreas. If the latter is the case, a question arises as to which anlage is the origin of the ectopic pancreas. To answer this question we examined the presence of PP-rich islets in the ectopic pancreas and found that very few PP-expressing cells were detected. Because it is well known that islets in the ventral pancreas contain abundant PP-cells in contrast to PP-poor islets in the dorsal panceras,8 the present case suggests that the origin is not likely to be the ventral pancreas.
There are several reported cases, including the present case, with ectopic pancreas accompanied with pancreas divisum.9 Considering an incidence of 1.3–22%,10 pancreas divisum may be now regarded as a normal variant rather than a true malformation. We believe that co-existence of the two entities in our case is just incidental and insignificant.
In summary, we report a case of ectopic pancreas in association with HPE. This case report is a valuable starting point for further investigation regarding a genetic link between the two conditions.
Acknowledgments
The Clinical Islet Transplant Program receives funding from the Juvenile Diabetes Research Foundation, from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, and from the Immune Tolerance Network. The Program is further supported by Province Wide Services through Alberta Health and Wellness. Generous philanthropic support is provided by the Roberts Family, the North American Foundation for the Cure of Diabetes, the Alberta Building Trades Council and from the Diabetes Research Institute Foundation Canada.
Glossary
Abbreviations:
- HPE
holoprosencephaly
- PP
pancreatic polypeptide
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/20478
References
- 1.Belloni E, Muenke M, Roessler E, Traverso G, Siegel-Bartelt J, Frumkin A, et al. Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet. 1996;14:353–6. doi: 10.1038/ng1196-353. [DOI] [PubMed] [Google Scholar]
- 2.Kawahira H, Scheel DW, Smith SB, German MS, Hebrok M. Hedgehog signaling regulates expansion of pancreatic epithelial cells. Dev Biol. 2005;280:111–21. doi: 10.1016/j.ydbio.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Kim SK, Melton DA. Pancreas development is promoted by cyclopamine, a hedgehog signaling inhibitor. Proc Natl Acad Sci U S A. 1998;95:13036–41. doi: 10.1073/pnas.95.22.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsugu H, Oshiro S, Kawaguchi H, Fukushima T, Nabeshima K, Matsumoto S, et al. Nonfunctioning endocrine tumor arising from intracranial ectopic pancreas associated with congenital brain malformation. Childs Nerv Syst. 2007;23:1337–40. doi: 10.1007/s00381-007-0391-9. [DOI] [PubMed] [Google Scholar]
- 5.Heller RS, Tsugu H, Nabeshima K, Madsen OD. Intracranial ectopic pancreatic tissue. Islets. 2010;2:65–71. doi: 10.4161/isl.2.2.10580. [DOI] [PubMed] [Google Scholar]
- 6.Lemire RJ, Cohen MM, Jr., Beckwith JB, Kokich VG, Siebert JR. The facial features of holoprosencephaly in anencephalic human specimens. I. Historical review and associated malformations. Teratology. 1981;23:297–303. doi: 10.1002/tera.1420230304. [DOI] [PubMed] [Google Scholar]
- 7.Hardey R. A case of acephalo-cyclopean monstrosity. Trans Obstet Soc London. 1863;4:213–21. [Google Scholar]
- 8.Slack JMW. Developmental biology of the pancreas. Development. 1995;121:1569–80. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- 9.Mizuta Y, Takeshima F, Yamao T, Isomoto H, Ohba K, Omagari K, et al. Cyst formation of duodenal heterotopic pancreas accompanied by pancreas divisum. Dig Dis Sci. 2004;49:1412–7. doi: 10.1023/B:DDAS.0000042239.28186.75. [DOI] [PubMed] [Google Scholar]
- 10.Kin T, Shapiro AM. Surgical aspects of human islet isolation. Islets. 2010;2:265–73. doi: 10.4161/isl.2.5.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]