Abstract
Aims/hypothesis: Islet amyloid polypeptide (IAPP) is a chief constituent of amyloid deposits in pancreatic islets, characteristic histopathology for type 2 diabetes. The goal of this study was to analyze islet cell composition in diabetic islets for the process of transforming water-soluble IAPP in β-cells to water-insoluble amyloid deposits by Immunocytochemical staining using different dilutions of anti-IAPP antibody. IAPP in β-cell granules may initiate β-cell necrosis through apoptosis to form interstitial amyloid deposits in type 2 diabetic islets.
Results: Control islets revealed twice as much β-cells as α-cells whereas 15 of 18 type 2 diabetic cases (83%) revealed α- cells as major cells in larger islets. Diabetic islets consisted of more larger islets with more σ-cells than β-cells, which contribute to hyperglucagonemia. In control islets, percentage of IAPP-positive cells against β-cells was 40–50% whereas percentage for type 2 diabetic islets was about 25%. Amyloid deposits in diabetic islets were not readily immunostained for IAPP using 1: 800 diluted antibody, however, 1: 400 and 1: 200 diluted solutions provided stronger immunostaining in early stages of islet amyloidogenesis after treating the deparaffinized sections with formic acid.
Methods: Using commercially available rabbit antihuman IAPP antibody, immunocytochemical staining was performed on 18 cases of pancreatic tissues from type 2 diabetic subjects by systematically immunostaining for insulin, glucagon, somatostatin (SRIF) and IAPP compared with controls. Sizes of islets were measured by 1 cm scale, mounted in 10X eye piece.
Conclusions/Interpretation: α cells were major islet cells in majority of diabetic pancreas (83%) and all diabetic islets contained less IAPP-positive cells than controls, indicating that IAPP deficiency in pancreatic islets is responsible for decreased IAPP in blood. In diabetic islets, water-soluble IAPP disappeared in β-cell granules, which transformed to water-insoluble amyloid deposits. Amyloid deposits were not readily immunostained using IAPP 1: 800 diluted antibody but were stronger immunostained for IAPP in early stages of amyloid deposited islets using less diluted solutions after formic acid treatment. In early islet amyloidogenesis, dying β-cell cytoplasm was adjacently located to fine amyloid fibrils, supporting that IAPP in secretary granules from dying β cells served as nidus for islet β-sheet formation.
Keywords: amyloid deposit, immunocytochemistry, islet amyloid polypeptide, pancreatic islets, type 2 diabetes
Introduction
Amyloid deposit was originally referred to as hyaline1 and later demonstrated to consist of amyloid,2 which is a characteristic histopathological finding for type 2 diabetic islets,1 found in about 90% of the pancreas from type 2 diabetics.3, 4 The chief constituent of amyloid deposit is islet amyloid polypeptide (IAPP).5–8 IAPP is a 37 amino acid polypeptide, that is originally isolated as the chief constituent of islets from type 2 diabetics.4, 5 IAPP is concomitantly co-secreted with insulin into the blood stream in response to glucose- and amino acid-stimulated insulin secretion.7 IAPP hyposecretion in the blood is well established in type 1 diabetics and insulin-requiring type 2 diabetics,8, 9 and decreased IAPP in pancreatic islets has been recently recognized in islets from type 1 diabetics by immunocytochemical staining.10 A synthetic IAPP, Pranlintide28-30 (pro-hIAPP) has been used for treating both type 1 and insulin-requiring type 2 diabetics with insulin for a better glycemic control.11–13 This study aimed to unfold disappearing water-soluble IAPP in secretary granules from dying β-cells to refold water- insoluble polymerized amyloid fibrils in transforming β-sheet conformation in IAPP-containing islet deposits8, 14–17 by immunocytochemical staining using different dilutions of rabbit antihuman IAPP antibody.
Results
Control islets
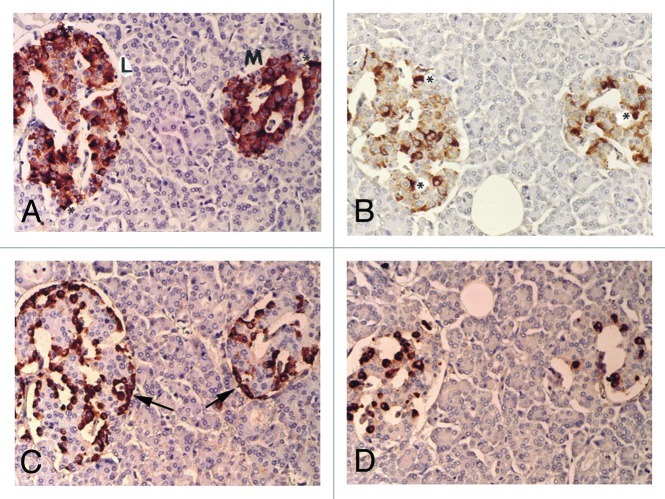
The mean islet cell numbers of extra-large, large and medium-sized islets were 120, 71 and 34 cells, respectively, representing 8%, 44% and 48% in a total of 225 islets examined for 9 age-matched control cases (Table 1). The relative percentages of β-cells for insulin, α-cells for glucagon and δ-cells for somatostatin (SARIF) were about 60%, 30% and 15% respectively, among all three sizes of islets (Table 1). By immunocytochemical staining, all three pancreatic hormone and IAPP staining was granular in the cytoplasm, in which insulin and IAPP staining was of variable staining intensity from moderately to strongly granular in the plump and polygonal cytoplasm whereas glucagon staining was strong in the smaller, compact and round cytoplasm and SRIF staining was also strong in the relatively small cytoplasm between the sizes of β- and α-cells (Fig. 1). β cells and IAPP positive cells were located mostly in mid portions of islets whereas α-cells were in the outer margins of islets and outer margins of islet lobules, and δ-cells were mostly in the mid portions of islets adjacent to β-cells (Fig. 1C). We used anti-IAPP antibody at 1: 800 dilution to avoid excessive cytoplasmic staining, resulting in much less staining than β-cells, at about 40–50% of that of β-cells in all three sizes of islets (Fig. 1 and Table 1).
Table 1. Immunocytochemical staining for Insulin, Glucagon, SRIF and IAPP.
|
Diabetic subjects no. |
Age/sex/ history |
Large islets |
Medium-sized islets |
Extra-large islets |
|||||||||||||||
| Total % |
β % |
σ % |
δ % |
IAPP/β |
(n) |
Total % |
β % |
σ % |
δ % |
IAPP/β |
(n) |
Total % |
β % |
σ % |
δ % |
IAPP/β |
(n) |
||
| Case 1 |
38/M/1 |
64 |
54 |
35 |
9 |
24 |
(8) |
39 |
49 |
40 |
13 |
27 |
(17) |
|
|
|
|
|
|
| Case 2 |
62/F/2 |
73 |
43 |
46 |
11 |
21 |
(11) |
42 |
48 |
42 |
13 |
20 |
(9) |
151 |
46 |
46 |
8 |
24 |
(5) |
| Case 3 |
50/F/1 |
77 |
45 |
49 |
8 |
29 |
(6) |
37 |
54 |
34 |
12 |
30 |
(5) |
183 |
38 |
53 |
7 |
21 |
(14) |
| Case 4 |
62/F/3 |
68 |
51 |
43 |
10 |
40 |
(6) |
40 |
40 |
50 |
10 |
25 |
(6) |
146 |
38 |
50 |
10 |
24 |
(13) |
| Case 5 |
50/F/1 |
74 |
40 |
51 |
9 |
23 |
(14) |
37 |
40 |
48 |
12 |
33 |
(5) |
122 |
36 |
52 |
8 |
22 |
(6) |
| Case 6 |
77/M/3 |
67 |
36 |
53 |
14 |
27 |
(6) |
33 |
37 |
48 |
20 |
39 |
(19) |
|
|
|
|
|
|
| Case 7 |
71/F/4 |
83 |
45 |
43 |
11 |
24 |
(7) |
37 |
36 |
50 |
14 |
22 |
(6) |
158 |
38 |
50 |
17 |
20 |
(12) |
| Case 8 |
52/F/1 |
68 |
38 |
54 |
8 |
25 |
(10) |
41 |
34 |
55 |
10 |
29 |
(11) |
130 |
43 |
53 |
6 |
35 |
(4) |
| Case 9 |
53/M/1 |
82 |
38 |
57 |
5 |
20 |
(10) |
13 |
41 |
44 |
12 |
35 |
(5) |
140 |
39 |
55 |
4 |
23 |
(10) |
| Case 10 |
49/M/1 |
77 |
31 |
53 |
16 |
27 |
(7) |
37 |
30 |
51 |
14 |
19 |
(8) |
144 |
39 |
47 |
14 |
16 |
(10) |
| Case 11 |
54/M/2 |
73 |
27 |
55 |
18 |
24 |
(12) |
38 |
35 |
46 |
19 |
24 |
(5) |
127 |
31 |
51 |
18 |
27 |
(8) |
| Case 12 |
62/F/3 |
77 |
40 |
52 |
9 |
18 |
(9) |
39 |
33 |
53 |
13 |
23 |
(9) |
116 |
33 |
56 |
7 |
21 |
(7) |
| Case 13 |
63/M/3 |
71 |
26 |
65 |
9 |
26 |
(10) |
34 |
39 |
49 |
9 |
27 |
(9) |
150 |
21 |
72 |
7 |
24 |
(6) |
| Case 14 |
64/M/2 |
76 |
31 |
53 |
16 |
29 |
(11) |
39 |
38 |
48 |
13 |
21 |
(6) |
144 |
30 |
54 |
15 |
30 |
(8) |
| Case 15 |
75/M/4 |
77 |
38 |
52 |
11 |
17 |
(8) |
37 |
32 |
54 |
13 |
21 |
(6) |
140 |
32 |
58 |
10 |
16 |
(11) |
| Case 16 |
65/M/2 |
72 |
32 |
56 |
16 |
30 |
(11) |
38 |
35 |
51 |
16 |
25 |
(9) |
129 |
33 |
53 |
12 |
20 |
(5) |
| Case 17 |
66/M/3 |
67 |
28 |
53 |
16 |
24 |
(9) |
40 |
31 |
50 |
18 |
24 |
(11) |
143 |
30 |
57 |
11 |
22 |
(5) |
| Case 18 |
67/F/3 |
71 |
33 |
59 |
75 |
30 |
(12) |
34 |
40 |
53 |
13 |
33 |
(8) |
122 |
32 |
64 |
7 |
27 |
(5) |
| Mean |
|
77 |
37 |
51 |
11 |
25 |
(167) |
38 |
38 |
48 |
13 |
26 |
(154) |
140 |
35 |
54 |
10 |
23 |
|
| SE |
|
1.2 |
1.8 |
1.5 |
0.9 |
1.2 |
|
0.6 |
1.5 |
1.2 |
0.7 |
1.3 |
|
4 |
1.5 |
1.6 |
1 |
1.2 |
|
| Controls |
(n = 9) |
71 |
59 |
27 |
13 |
43 |
(99) |
34 |
58 |
28 |
14 |
49 |
(109) |
120 |
61 |
30 |
12 |
41 |
(17) |
| SE | 2 | 3 | 1 | 1 | 3 | 2 | 1 | 1 | 0.4 | 3 | 7 | 5 | 5 | 2 | 3 | ||||
Clinical history of diabetes: 1, < 5 years; 2, 6–10 years; 3, 11–15 years; 4, > 15 years. β:Insulin, σ:Glucagon, δ:SRIF cells, (n): numbers of islets examined, % in β-, σ- and δ-cells were calculated the hormone-positive cells by the total islet cell numbers. % IAPP/β-cells was clculated dividing IAPP-positive cells by β-cell numbers.
Figure 1. Control islets. β cells were the most abundant major islet cells (about 60% of total islet cells) with plump and polygonal cytoplasm of variable staining intensity, from moderate to strong staining, followed by α-cells (arrow, about 30%) with strongly stained, round smaller cytoplasm. δ cells accounted for about 15% of islet cells, containing plump or small cytoplasm. β cells and IAPP-positive cells were mostly located in the middle of islets and so were δ-cells whereas strongly immunostained α-cells (arrow) were located at the outer margin of islets and islet lobules. There were globular to sickle-shaped strongly immunostained cytoplasms for insulin and IAPP, which appeared to be dying β-cells (*). L, Large islet; M, Medium-sized islet; Original magnification X 400; (A) Insulin; (B) IAPP; (C) Glucagon; (D) SRIF immunostained.
Type 2 diabetic islets
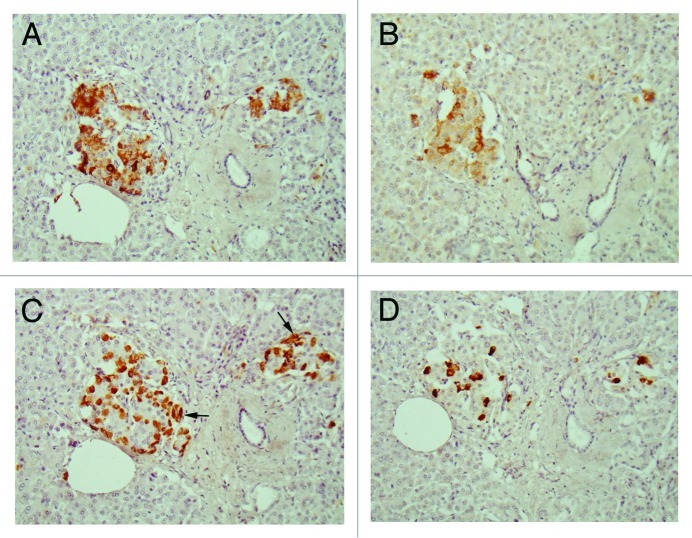
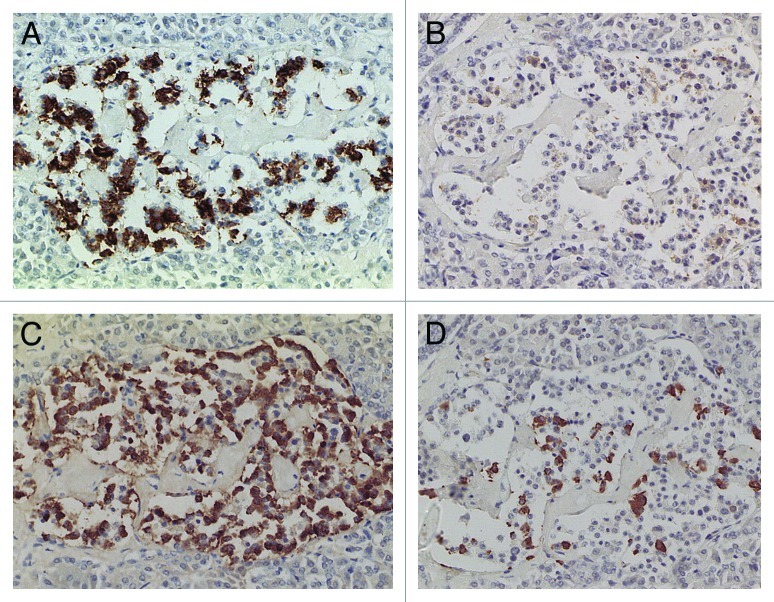
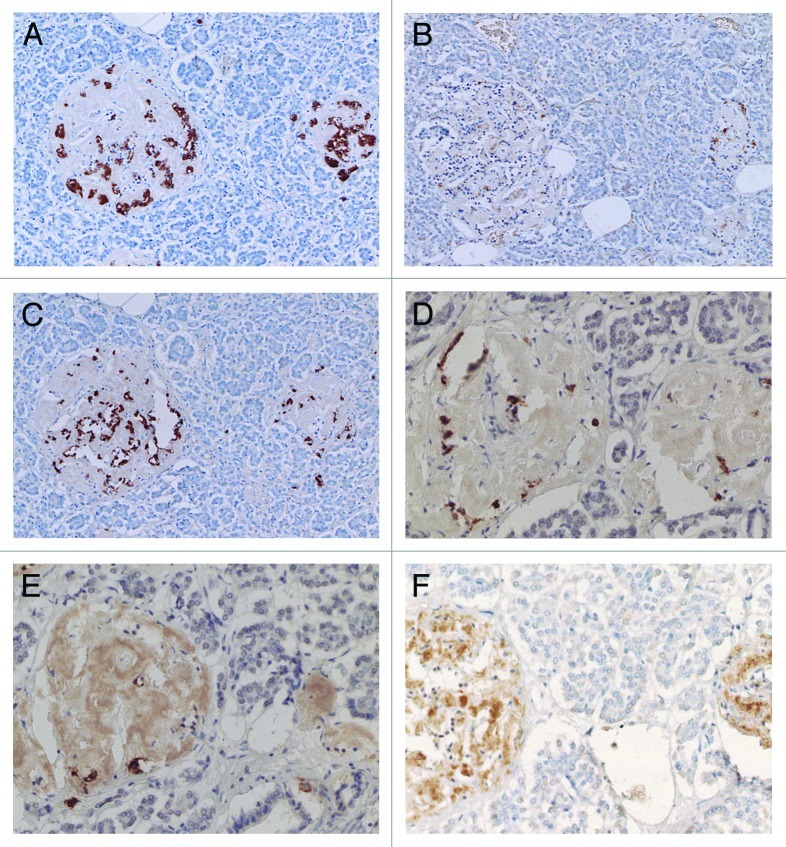
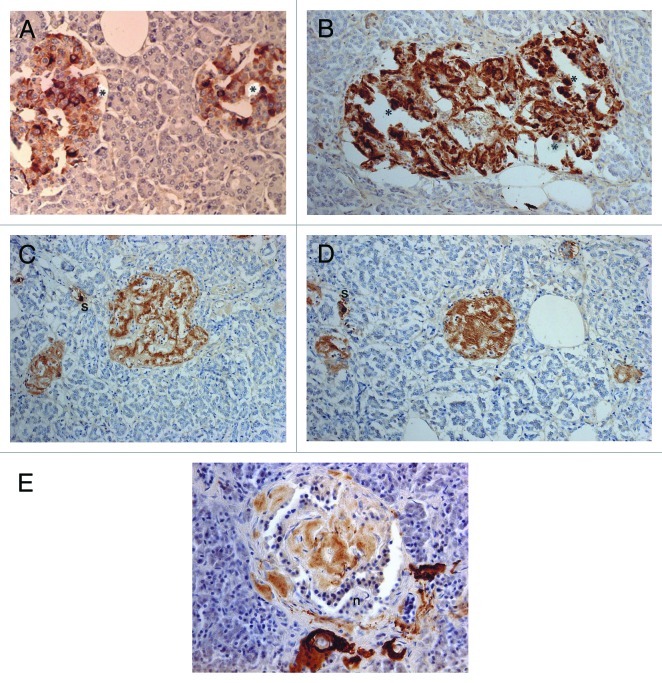
Compared with control islets, which consisted of β-, α- and δ-cells at a ratio of 4: 2: 1, 15 out of 18 (15/18, 83%) type 2 diabetic cases, consisted of mostly α-cells as the major islet cells (Table 1). Two cases (Cases 2 and 3) had about the same ratio of β- and α-cells and Case 1 had slightly more β-cells than α-cells (Fig. 2, Table 1). Extra-large islets containing more than 100 islet cells were observed in 16 diabetic cases (16/18, 89%) excluding Cases 1 and 6, the latter case consisted of 24% large islets and 76% medium-sized islets (Table 1) and islets were generally smaller than other type 2 diabetic islets with medium-sized islets as the major islet and α-cells were more than β-cells by 30–50% (Table 1). The relative percentage of IAPP-positive cells against β-cells was about 30% for large and medium islets, less than the control values of 40–50% (Table 1). About one half of β- and δ-cells revealed plump cytoplasm with strong immunostaining for their hormones whereas α-cells revealed uniformly small, round compact cytoplasm with strong immunostaining (Fig. 2C). There was no obvious amyloid deposit in the islets from Case 1 (Fig. 2). In Case 1, β-cells were 20 to 50% more than α-cells in large islets with a relative percentage of IAPP-positive cells against β- cells being about 25%, less than control values of 41 to 49% (Table 1) and stromal amyloid deposits ranged 0 to 25% of the islet area. Two cases (Cases 4 and 5) revealed about 20% more α-cells than β-cells and four cases (Cases 6, 7, 8 and 9) having more than 30% α-cells than β-cells (Table 1). Among nine cases with α-cells more than β-cells by 50% (Cases 10, 11, 12,13,14, 15, 16, 17 and 18), three cases (Cases 16,17 and 18) showed 70% more α-cells than β-cells, in whom relative percentages of IAPP-positive cells against β-cells ranged from 20 to 30% (Table 1). Case 15 was unique, which revealed a vast variety of islet cell percentages and amyloid deposits in a single case: β-: α-: δ- cell percentage was 40%, 50% and 11% for large islets in 77 islet cells whereas extra-large islets containing 140 islet cells revealed 30%, 60% and 10% of β-, α- and δ- cells, respectively (Fig. 3, Table 1). In these islets, β-cells were strongly and granularly immunostained with irregular, fuzzy cell membrane (Fig. 3A). Relative percentages of IAPP-positive cells against β-cells were about 16 to 21%, however, there were some β-cells weakly IAPP-immunostained and there were strongly immunostained α-cells (Fig. 3). δ cells were between the sizes of β-cells and α-cells (Fig. Three A, C, D). Case 10 presented with the most advanced stromal islet amyloid deposits with a mean deposit occupying 75% of the islets, ranging from 8% of islets occupied by < 25% amyloid deposits, 20% of islets occupied by 25 to 49% deposits, 28% of islets occupied by 50 to 74% deposits, 20% of islets occupied by 75 to 94% deposits and 16% of islets occupied by > 95% amyloid deposits (Fig. 4), whereas islet cells were weakly positive for IAPP in large islets and several β-cells in medium-sized islets were moderately positive for IAPP (Fig. 4B). α cells revealed strong immunostaining in the relatively plump cytoplasm, with all the islet cells surrounded by amyloid deposits (Fig. 4C). IAPP immunostaining for amyloid deposits was weak or almost negative using a 1: 800 antibody solution (Figs. 2B and 3B). Using 1: 400 diluted antibody after the sections were treated with 100% formic acid, major β-cells and the minor α- and δ-cells were located in amyloid occupied islets (> 99%), consisting of a few IAPP-positive islet cells and moderately IAPP-positive amyloid deposits (Fig. 4E). In the islets from the same case occupying less amyloid deposits about 30% of the islet area, more viable islet cells containing plump cytoplasm adjacent to fine amyloid fibrils were moderately positive for IAPP, showing dying β-islet cells adjacent to the IAPP-positive amyloid fibrils (Fig. 4F). Using a 1: 200 diluted IAPP antibody solution, the majority of control islet cells were stronger positive for the cytoplasm with markedly dense staining in the several irregular sickle-shaped cytoplasm, suggesting a few dying islet cells even in control islets (Fig. 5A). Diabetic islets from Case 10 showed about 20% amyloid deposits with strong IAPP-staining in the round dying islet cell cytoplasm adjacent to the fine amyloid fibrils (Fig. 5B). The islet with 95% amyloid deposits in Case 10 showed moderate IAPP staining for amyloid deposits but residual islet cells were completely IAPP-negative (Fig. 5C). Two single islet cells forming small single islet cell islets were strongly positive for IAPP, representing scattered viable β-cells (Fig. 5C and D). The end-stage islet with > 99% islet occupied by amyloid deposits were strongly immunopositive for stromal amyloid deposits in the presence of a few IAPP-negative islet cells (Fig. 5D). One strongly IAPP-positive single cell islet was also present (Fig. 5D). In three cases of advanced islet amyloidosis with more than 95% islet occupied (Cases 10, 11 and 14), moderately IAPP-positive amyloid deposits were the major islet component, containing no viable islet cells, and further resulted in totally amyloid-occupied shrunken islets using 1: 200 diluted antibody, corresponding to the very end-stage of islet amyloidosis by Hayden (Fig. 5C and D, ref.18). These almost completely amyloid occupied islets were moderately lamellar stained for amyloid p whereas peri-islet blood vessel walls were strongly stained for amyloid p (Fig. 5E). Sizes of pancreatic islets: The length and width of control islets in three different sizes of islets were: in large islets—103 +/− 4 µm (length) and 66 +/− 3 µm (width), in medium-sized islets—66 +/− 3 µm (length) and 45 +/− 2 µm (width) and in extra-large islets—141 +/− 7 µm (length) and 94 +/− 6 µm (width), respectively (Table 2), as these numbers were within the sizes of normal islets, which were reported as 50–150 µm in diameter.19 In control islets, large and medium-sized islets were the major components with only 7% of extra-large islets (Table 2). In the total diabetic islets, large, medium-sized and extra-large islets accounted for about 1/3 each with relatively more extra-large islets than the controls (Table 2). Despite relatively small sizes of diabetic extra-large islets compared with that of control islets, diabetic islets consisted of more islet cells of predominantly small compact σ-cells as compared with β-cells with plumper cytoplasm (Figs. 1–3, Table 1 and 2).
Figure 2. Disbetic islets, Case 1. β cells and α-cells (arrow) were about equal in number in large islet (Left), and α-cells were slightly more in medium-sized islet (Right). β cells were moderately to strongly immunopositive with plump cytoplasm whereas α-cells (arrows) and δ-cells were slightly smaller in the cytoplasm and were strongly immunostained. IAPP-positive cells were about ¼ that of β-cells in large islet, but medium-sized islet had only a few positive cells. (A) Insulin; (B) IAPP; (C) Glucagon; (D) SRIF immunostained; Original magnification X 470.
Figure 3. Extra-large diabetic islet, Case 15. This extra-large islet showed less β-cells (A) than α-cells (C). β cells were strongly and granularly immunostained with irregular, fuzzy cell membrane whereas α-cells contained dense positive compact cytoplasm. δ cells consisted of a few large cytoplasm and mostly compact cytoplasm (D). IAPP staining was almost completely negative with only weak residual granular positive staining (B). Stromal amyloid deposits occupied about 20% of the islet area, which was negatively stained for IAPP using a 1: 800 antibody solution (B). (A) Insulin; (B) IAPP; (C) Glucagon; (D) SRIF immunostained; Original magnification X 470.
Figure 4. Diabetic islets Case 10, Islets occupied by amyloid deposits in 95% (A, B and C) and Islets occupied by amyloid deposits in > 99% (D, E and F). Islets occupied by amyloid deposits in 95%, A,B and C: Both large islet (Left) and medium-sized islet (Right) consisted of more than 95% amyloid deposits, within which β-cells with partly plump cytoplasm and α cells with dense small cytoplasm were located. IAPP-positive cells were weakly stained in the large islet but were moderately stained in the medium-sized islet. δ cells showed mostly small cytoplasm mixed with a few large cytoplasms. Islets occupied by amyloid deposits > 99%, D, E and F: Both large (Left) and medium-sized islet (Right) contained more than 99% amyloid deposits. Residual β-cells and δ-were minor cells and α-cells were major cells (D). IAPP immunostaing was performed using a 1: 400 diluted antibody solution, revealing moderately positive staining in amyloid deposits (E). In islets containing viable islet cells, residual islets cells with plump cytoplasm and amyloid deposits were stronger stained for IAPP than in Figure 3B. (A and D) Insulin; (B, E and F) IAPP by 1: 400 diluted solution; (C) Glucagon immunostained; Original magnification (A-C) X 320; (D-F) X 420.
Figure 5. Control (A) and Diabetic islets, Cases 10 (B-D) and Case 14 (E) IAPP immunostaining was performed using a 1: 200 diluted antibody solution. Control islets were strongly immunostained for IAPP in the majority of islet cell cytoplasm (A). Diabetic islets of plump cytoplasm (*) were densely immunostained for IAPP in the cytoplasm, continuous to the moderately immunostained amyloid deposits (B). Diabetic islets occupying 95% amyloid deposits were immunostained moderately to strongly positive whereas viable islet cells surrounded by amyloid deposits were negative for IAPP. Two single cell islets were strongly positive for IAPP (s) (C, D). The end-stage small islets occupied by > 99% amyloid deposits revealed only a few IAPP negative residual islet cells. One strongly IAPP positive, single cell islet was localized (s) (D). Amyloid p immunostaining for the end-stage amyloid deposited islet was moderately positive in lamellar amyloid deposits and peri-islet blood vessel walls were strongly immunostained for amyloid p. Stromal amyloid deposits were moderately positive for amyloid p (E). (A) Control islet; (B-D) Case 10; (E) Case 14. (A-D) IAPP by 1: 200 diluted solution; Case 10; (E) amyloid p; Case 14 immunostained Original magnification X 350.
Table 2. Size (length and width) of pancreatic islets.
| Diabetic groups |
Large islets |
Medium-sized islets |
Extra-large islets |
||||||
| |
Length µm |
Width µm |
Percent* |
Length µm |
Width µm |
Percent* |
Length µm |
Width µm |
Percent* |
| 1,Cases 1-3 |
80 +/- 3 |
56 +/- 5 |
33% |
68+/- 5 |
44+/-4 |
41% |
112+/-9 |
81+/17 µm |
25% |
| 2,Cases 4-10 |
106+/-7 |
83+/- 5 |
31% |
70+/- 5 |
49+/-4 |
34% |
127+/-10 |
83+/-8 |
34% |
| 3,Cases11-18 |
110+/-4 |
76+/-4 |
41% |
67+/-3 |
44+/-2 |
32% |
140+/-4 |
85+/-4 |
28% |
| 1-3,Cases 1-18 |
103+/-3 |
75+/-2 |
37% |
68+/-0.3 |
46+/-1 |
34% |
130+/-2 |
83+/-0.4 |
29% |
| | |||||||||
| Controls (n=9) | 103+/-4 | 66+/-3 | 44% | 66+/-3 | 45+/-2 | 48% | 141+/-7 | 94+/-6 | 7% |
Percentages were calculated for the relative percentages of the sizes of the islets in each group.
Discussion
The main etiology of type 2 diabetes is characterized as insulin resistance by deficient insulin actions through relative insulin deficiency due to insufficient insulin receptor sites on the target organs.20 Thus, islet cells in type 2 diabetes must show different islet cell components from the control islets with loss of β-cell mass and α-cell hyperplasia using immunocytochemical staining for insulin, glucagon, SRIF and IAPP as the results of long remodeling process for islet cells. Type 1 diabetes is characterized by an absolute insulin deficiency as shown by absent or markedly decreased β-cells in the islets,10 but type 2 diabetes is more heterogeneous in islet histopathology by relatively decreased β-cells after long time sequences of islet cell remodeling. Our cases of type 2 diabetes had 5 to 20 y of history of diabetes and all succumbed to diabetic complications including coronary heart disease, renal failure and multiple organ failures.21 We were unable to directly correlate diabetic complications with exact history of diabetes since many type 2 diabetics did not present typical symptoms of diabetes at the time of diagnosis and when type 2 diabetes was diagnosed, practically all type 2 diabetics already had some on-going diabetic complications. Compared with type 1 diabetic islet histopathology, which presented with an absolute β-cell deficiency and α-cell hyperplasia,10 type 2 diabetic islet histopathology revealed several stages as follows: As seen in type 1 diabetic islets, the majority of type 2 diabetic pancreas (15/18, 83%) showed α-cell hyperplasia of lesser degree than type 1 diabetic pancreas (Table 1, ref.10). Although three cases (Cases 1, 2 and 3, 3/18, 17%) showed slightly more β-cells or about equal numbers of β- and α-cells, those three cases revealed much less β-cells than in non-diabetic control pancreas at a 2: 1 ratio of β-: α- cells (Table 1). In two cases (Cases 1 and 6), islets were generally and uniformly small, consisting of minor large islets and major medium-sized islets without extra-large islets, similar to type 1 diabetic islets (Fig. 2, ref.10). However, islet cell percentages in five cases (Cases 1 -5) were that of less severe type 2 diabetes, containing relatively less β-cells than in control islets (Table 1). In control islets, extra-large islets were minor components, representing only 7% of the total islets, whereas extra-large islets were much more often observed in type 2 diabetic islets (16/18, 89%) except Cases 1 and 6, at a mean value of 32% in the total islets, ranging from 16% (Case 8), 20% (Cases 2, 5, 11, 13,16, 17 and 18) to 30 to 56% (Case 3,4,7,9,10,12,14 and 15)(Table 2), suggesting that islet hyperplasia resulted through remodeling in order to produce and secrete more insulin for glucose homeostasis. Decreased IAPP immunostaining in type 2 diabetic islets was anticipated as also observed in type 1 diabetic islets.10 Both type 1 and insulin-requiring type 2 diabetics presented with IAPP hyposecretion into the blood since the source of IAPP in blood was β-islet cells.10 In type 1 diabetic islets, IAPP-positive cells were less than that of β-cells, ranging from 20 to 40% of β- cells as compared with about 40 to 50% in control islets (Table 1, ref.10). Control islets also revealed less stronger staining for IAPP than insulin staining (Fig. 1A, B), corresponding to the fact that IAPP levels of pancreatic tissue extracts are about 10% that of insulin.22 The fasting serum IAPP level in nonobese controls is 2.0 µM/L at 5% that of insulin level of 48 µM/L.22 Two cases of type1 diabetes succumbed to diabetic coma as previously reported, in whom there were insulin-negative β-cells despite the residual IAPP-positive staining, supporting that some IAPP-positive cells were insulin-depleted β-cells.10
In this study, relative percentages of IAPP-positive cells against β- cells in type 2 diabetic islets ranged from 16 to 35%, which was less than control values of 41 to 49% (Table 1). Amyloid deposits increasingly accumulated in islets perivascularly, which formed lamellar layers adjacent to the dying islet cells (Fig. 5C). In the end-stage, islets consisted of circular, lamellar dense amyloid deposits, which were moderately positive for IAPP using 1: 200 diluted antibody solution and 1: 100 diluted amyloid p (Fig. 5E). It is probably significant that amyloid p immunostaining was stronger in extra-islet blood vessels than in islet deposits (Fig. 5E). Amyloid p, a glycoprotein, is distinct from amyloid fibrils and is closely associated with all forms of amyloidosis and is a marker for all types of amyloidosis.23 Both IAPP and amyloid p immunostaining is limited in the pancreas only for type 2 diabetes in contrast to diffuse organ involvement by amyloid p in AL-type amyloidosis including heart, liver, spleen, kidneys, adrenals, thyroid and occasionally bone marrow and pancreatic islets.23
In type 2 diabetic pancreas, different stages of islet amyloidosis were observed in the pancreas from even the same subjects, until all islets ended up with massive diffuse islet amyloidosis containing no viable remaining islet cells (Figs. 4 and 5). IAPP oligomers can form nonselective ion-permeable membrane pores, which lead to increased calcium concentration, endoplasmic reticulum stress and apoptosis.3,24 According to the toxic oligonomer hypothesis, β-cells in type 2 diabetes are somehow killed though IAPP-induced damage of the β-cell membrane.24–28 These toxic oligomers (not monomers or mature amyloid fibrils) formed by different amylodogenic proteins including IAPP, Aβ, synuclein, transthyrenin and prion protein, share a common epitope.29 Antibodies generated to this epitope using toxic oligomers of Aβ 1–40 also bind toxic oligomers generated from the other amyloidogenic proteins in cell culture, block the cytotoxic effects of each of these diverse oligomers.30 In early stages of islet amyloidosis and β-cell death, sickle-shaped β-cell cytoplasm without nucleus was strongly immunopostive for IAPP and insulin as observed in a few control islet cells (Fig. 1) and more in diabetic islets (Fig. 4). This cytoplasm probably represents an early fibrillar form of amyloidogenic β-cell cytotoxic proteins, which subsequently form extracellular amyloid β-sheets since these IAPP-rich cytoplasm was adjacently located to the thin extracellular amyloid fibrils (Figs. 4 and 5). The above finding is supported by the fact that amyloid fibrils were oriented perpendicular to the membrane of β-cells, with some thin fibrils bundles sticking into membrane invaginations in a tissue culture study.31 Matrix metalloproteinases (MMTs) and tissue inhibitors for metalloproteinases (TIMPs) play an important role in tissue remodeling, histogenesis, tumor invasion, inflammation and others.32–36 MMP-2 and -9 were required for islet formation in tissue culture study and were indispensable for islet formation and endocrine cell differentiation in mouse study.37 MMP-2 and -9 and TIMP-1 and-2 were specifically involved in remodeling and apoptosis of islet cells and pancreatic endocrine tumors.38 Normal islet cells and pancreatic endocrine tumors, especially normal β-cells and insulinomas, were specifically equipped with MMPs and TIMPs, which suggested that β-cells and insulinoma cells were special cell lines in order to produce and secrete enough insulin for glucose homeostasis by constant remodeling by MMPs-TIMPs homeostasis through apoptosis.37-39 Every endocrine tissues remodel and reproduce according to an apoptosis process as supported by the presence of MMPs and TIMPs shown in pituitary gland, thyroid C-cells and medullary thyroid tumors,40 and an essential component in apoptosis is played by cleaved caspase-3, a family of cysteine proteases, and activated cleaved caspase-3 was specifically located in β-islet cells and insulinoma cells, which make them distinctly unique from the other non-β islet cells.38 The main goal of this immunocytochemical study was to analyze how dying β-cell secretary granules containing low molecular weight IAPP transform to stromal amyloid β-sheet containing polymerized high molecular weight IAPP, which is characteristic for type 2 diabetes as reported in 90% of type 2 diabetes2,3 and is not seen in type 1 diabetes.10 A dilution of 1: 800 IAPP antibody solution did not show much IAPP immunostaining in amyloid deposits except irregular weak staining, however, 1: 400 and 1: 200 diluted solutions showed more IAPP-staining in amyloid stromal deposits in early islet amylodogenesis after the deparaffinized sections were treated with 100% formic acid for up to 60 min, which was used for immunostaining cerebral and AL-type amyloidosis for amyloid p.41 However, even less diluted 1: 400 and 1: 200 diluted IAPP antibody solutions did not strongly immunostain the very end-stage islets as similarly seen in Figure 4B. Responding to insulin resistance at the target organs, β-islet cells overproduce and oversecrete insulin in an attempt to maintain glucose homeostasis in type 2 diabetes, which causes β-cell exhaustion and eventual cell death through apoptosis. After β-cells started to die, dying β-cells become a nidus or template to form IAPP polymers intracellularly at first shown by the swollen dying IAPP-positive β-cells (Figs. 5B and 6B), adjacent to the newly forming fine extracellular amyloid fibrils. Amyloid deposits then accumulate perivascularly in the blood vessel-rich islets to transform water-insoluble β-sheet conformation containing IAPP polymers, including 20 more proteins such as amyloid p, apolipoprotein E (Apo E) and heparin sulfate-type proteoglycans.7,8, 30,31 IAPP and insulin form heteromolecular complex in vitro,22 which suggests that insulin stabilizes IAPP in β-cells and lack of insulin in β-granules in type 1 and type 2 diabetic islets destabilizes and facilitates more breakdown and consequent disappearance of IAPP from β-granules.22 Freshly prepared intermediate IAPP polymers (25—6000 IAPP molecules) have a toxic effect on β-cells7,8 and these intermediate IAPP polymers further damage β-cells and accelerate apoptosis of β-cells, but in somehow spare α-cells and δ-cells.7,8,42 Water-soluble IAPP with low molecular weight in β-cell granules is readily and densely immunostained whereas water- insoluble amyloid fibrils containing IAPP polymers are only weakly immunostained using antihuman IAPP antibody as also reported by several authors.8,14,22 Many papers had shown only Congo red staining for islet amyloid deposits but had not published immunostaining for IAPP in diabetic islets although these authors had antihuman IAPP antibodies at hand.3-5,14,15,18,30 This lack of IAPP immunostaining in the literature certainly implies technically difficult IAPP immunostaining for amyloid deposits in type 2 diabetic islets. The reasons for lack of strong IAPP immunostaining of islet amyloid deposits are not clear at present, but one likely reason may be due in part to the unexposed epitope of IAPP polymers within the water-insoluble amyloid fibrils with β-sheet conformation. Treating deparaffinized sections with formic acid somehow facilitates immunostaining for IAPP by exposing the IAPP epitope in amyloid deposits as previously shown for amyloid p immunostaining in cerebral and AL-type amyloidosis.41 Formic acid treatment was also used in extracting IAPP from type 2 diabetic pancreas.5,6 Thus, β-cells are special cells in even among all four types of islet cells equipped with an ample capacity to remodel and reproduce for maintaining glucose homeostasis through apoptosis.20,37,38,42- 47 While β-cells die in type 1 and type 2 diabetic islets, mostly α-cells and some σ-cells and PP-cells in lesser degree proliferate from the islet stem cells to form hyperplasia, which causes hyperglucagonemia, leading to more hyperglycemia and exacerbation of clinical diabetes in both type 1 and type 2 diabetics.13,48,49 The major strong immunopositive σ-cells in relatively larger diabetic islets also histopathologically support hyperglucagonemia in diabetes.
Materials and Methods
All cases of pancreatic tissues from type 2 diabetics and control cases were collected by autopsy at the University of Kansas Medical Center between 1975 and 2001 during my tenure. A total of 18 cases of type 2 diabetic pancreas were studied together with 9 cases of age-matched non-diabetic controls. Pancreatic tissues were collected from the mid body portion of the pancreas, not representing PP-cell rich uncinate process or α-cell rich tail portion of pancreas50–52 and at least two tissue sections were studied for control and diabetic cases. Information on age, sex and years after diagnosis of type 2 diabetes was obtained for each case from the chart and is included in Table 1. History of type 2 diabetes was quite variable for each case and information on periods of diabetes after the diagnosis is listed in Table 1 as follows: 1, < 5 y, 2, 5–10 y, 3, 11–15 y and 4, > 15 y after diagnosis. All tissues were routinely fixed in buffered formalin and were embedded in paraffin. Deparaffinized sections were treated with antigen retrieval procedure using citrate buffer pH 6.2. All staining procedures were the same as previously reported10, 38–40 except IAPP immunostaining, in which rabbit antihuman IAPP 1–13 (Peninsula Laboratory, San Carlos, CA) was used to immunostain IAPP-positive islet cytoplasm at 1: 800 dilution, however islet amyloid deposit was not readily immunostained at 1: 800 dilution, and 1: 400 and 1: 200 diluted solutions were used to immunostain islet amyloid deposit after treating the tissue sections in 100% formic acid solution up to 60 min.41 Sections of diabetic and control cases were also immunostained using antihuman amyloid p (Biocare Medical, Walonut Creek, CA) at 1: 100 dilution as reported before.23 All the serial sections were systematically immunostained for insulin in β-cells, glucagon in α-cells, SRIFF in δ-cells and for IAPP. The counting immunostained cytoplasms for β-, α-, δ- cells and IAPP-positive cells was performed by counting positively immunostained cytoplasm at 10 x 20 = x 200 magnification to cumulatively count the total islet cell numbers by adding all hormone positive cells per islet for both diabetic and control islets, with which relative percentages of β-, α- and δ-cells were calculated by dividing the each hormone positive cells by the total islet cell numbers together with relative percentages of IAPP-positive cells against β-cell numbers. The islets were divided into three sizes, extra-large islets containing more than 100 islet cells, large islets containing 50 to 99 islet cells and medium-sized islets containing 20 -49 islet cells, respectively, excluding small islets of less than 20 islet cells as these small islets or parts of large and medium-sized islets provided large variation of islet cell percentages and IAPP-positive cell counts as previously reported.21 For each diabetic case, each case was considered as either α-cells or β-cells as the major cells when two of the three sizes of islets revealed the major islet cells as α-cells or β- cells (Table 1). A total of 25 islets were randomly counted for each type 2 diabetic and control cases. By mounted 1 cm linear scale with 5 µm intervals in the 10x eye piece, the length and width for each islet were measured at 10 x 10 = x 100 magnification. The length and width for extra-large, large and medium-sized islets were calculated for both control and diabetic islets. The type 2 diabetic cases were divided into three groups according to the relative percentages of σ-cells: Group 1 (Cases 1–3) consisted of three cases with slightly more β-cells than σ-cells and about same numbers of β- and α-cells, Group 2 (Cases 4–10) more σ-cells than β-cells by 20 to 50%, and Group 3 (Cases 11 -18) more σ-cells than β-cells by more than 50% as well as the statistical analysis of the entire 18 cases (Table 2).
Acknowledgments
I sincerely thank Dr Ov Slayden for kindly allowing me to use his research laboratory to perform immunocytochemical staining at Reproductive Science Division, Oregon National Primate Center, Beaverton, Oregon. This study was supported in part by ONRRC Core Grant: NIH RR 00063.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/20477
References
- 1.Opie EL. THE RELATION OF DIABETES MELLITUS TO LESIONS OF THE PANCREAS. HYALINE DEGENERATION OF THE ISLANDS OF LANGERHANS. J Exp Med. 1901;5:527–40. doi: 10.1084/jem.5.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrlich JC, Ratner IM. Amyloidosis of the islets of Langerhans. A restudy of islet hyalin in diabetic and non-diabetic individuals. Am J Pathol. 1961;38:49–59. [PMC free article] [PubMed] [Google Scholar]
- 3.Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–9. [PubMed] [Google Scholar]
- 4.Röcken C, Linke RP, Saeger W. Immunohistology of islet amyloid polypeptide in diabetes mellitus: semi-quantitative studies in a post-mortem series. Virchows Arch A Pathol Anat Histopathol. 1992;421:339–44. doi: 10.1007/BF01660981. [DOI] [PubMed] [Google Scholar]
- 5.Westermark P, Wernstedt C, Wilander E, Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun. 1986;140:827–31. doi: 10.1016/0006-291X(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 6.Cooper GJ, Willis AC, Clark A, Tumer RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreas of type 2 diabetic pancreas. Proc Natl Acad Sci U S A. 1987;84:8628–32. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn SE, Andrikopoulos S, Verchere CBN. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–53. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 8.Höppener JW, Ahrén B, Lips CJ. Islet amyloid and type 2 diabetes mellitus. N Engl J Med. 2000;343:411–9. doi: 10.1056/NEJM200008103430607. [DOI] [PubMed] [Google Scholar]
- 9.Kruger DF, Gatcomb PM, Owen SK. Clinical implications of amylin and amylin deficiency. Diabetes Educ. 1999;25:389–97, quiz 398. doi: 10.1177/014572179902500310. [DOI] [PubMed] [Google Scholar]
- 10.Tomita T. Islet amyloid polypeptide in pancreatic islets from type 1 diabetic subjects. Islets. 2011;3:166–74. doi: 10.4161/isl.3.4.15875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weyer C, Maggs DG, Young AA, Kolterman OG. Amylin replacement with pramlintide as an adjunct to insulin therapy in type 1 and type 2 diabetes mellitus: a physiological approach toward improved metabolic control. Curr Pharm Des. 2001;7:1353–73. doi: 10.2174/1381612013397357. [DOI] [PubMed] [Google Scholar]
- 12.Buse JB, Weyer C, Maggis DC. Amylin replacement with Pramlintide in type 1 and type 2 diabetes: a physiological approach to overcome barriers with insulin therapy. Clin Diabetes. 2002;20:137–44. doi: 10.2337/diaclin.20.3.137. [DOI] [Google Scholar]
- 13.Fineman M, Weyer C, Maggs DG, Strobel S, Kolterman OG. The human amylin analog, pramlintide, reduces postprandial hyperglucagonemia in patients with type 2 diabetes mellitus. Horm Metab Res. 2002;34:504–8. doi: 10.1055/s-2002-34790. [DOI] [PubMed] [Google Scholar]
- 14.Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3629–43. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368:756–60. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 16.Jaikaran ET, Clark A. Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology. Biochim Biophys Acta. 2001;1537:179–203. doi: 10.1016/s0925-4439(01)00078-3. [DOI] [PubMed] [Google Scholar]
- 17.Marzban L, Park K, Verchere CB. Islet amyloid polypeptide and type 2 diabetes. Exp Gerontol. 2003;38:347–51. doi: 10.1016/S0531-5565(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 18.Hayden MR. Islet amyloid, metabolic syndrome, and the natural progressive history of type 2 diabetes mellitus. JOP. 2002;3:126–38. [PubMed] [Google Scholar]
- 19.Kloppel G, Lenzen S. Anatomy and physiology of the endocrine pancreas. In: Kloppel, G, Heitz, PU Editors. Pancreatic Pathology. Edinburgh, Churchill-Livingstone: 1984; 133-153. [Google Scholar]
- 20.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835, ix. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Tomita T. Immunocytochemical localization of capase-3 in pancreatic islets from type 2 diabetic subjects. Pathology. 2010;42:432–7. doi: 10.3109/00313025.2010.493863. [DOI] [PubMed] [Google Scholar]
- 22.Clark A, Nilsson MR. Islet amyloid: a complication of islet dysfunction or an aetiological factor in Type 2 diabetes? Diabetologia. 2004;47:157–69. doi: 10.1007/s00125-003-1304-4. [DOI] [PubMed] [Google Scholar]
- 23.Tomita T. Amyloidosis of pancreatic islets in primary amyloidosis (AL type) Pathol Int. 2005;55:223–7. doi: 10.1111/j.1440-1827.2005.01815.x. [DOI] [PubMed] [Google Scholar]
- 24.Mirzabekov TA, Lin MC, Kagan BL. Pore formation by the cytotoxic islet amyloid peptide amylin. J Biol Chem. 1996;271:1988–92. doi: 10.1074/jbc.271.4.1988. [DOI] [PubMed] [Google Scholar]
- 25.Anguiano M, Nowak RJ, Lansbury PT., Jr. Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry. 2002;41:11338–43. doi: 10.1021/bi020314u. [DOI] [PubMed] [Google Scholar]
- 26.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–8. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 27.Engel MFM, Khemtémourian L, Kleijer CC, Meeldijk HJ, Jacobs J, Verkleij AJ, et al. Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane. Proc Natl Acad Sci U S A. 2008;105:6033–8. doi: 10.1073/pnas.0708354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303–16. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–9. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 30.Bonner-Weir S, O’Brien TD. Islets in type 2 diabetes: in honor of Dr. Robert C. Turner. Diabetes. 2008;57:2899–904. doi: 10.2337/db07-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westermark P. Fine structure of islets of Langerhans in insular amyloidosis. Virchows Arch A Pathol Pathol Anat. 1973;359:1–18. doi: 10.1007/BF00549079. [DOI] [PubMed] [Google Scholar]
- 32.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–54. [PubMed] [Google Scholar]
- 33.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 34.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–73. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 35.Nagase H, Woessner JF., Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 36.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 37.Perez SE, Cano DA, Dao-Pick T, Rougier JP, Werb Z, Hebrok M. Matrix metalloproteinases 2 and 9 are dispensable for pancreatic islet formation and function in vivo. Diabetes. 2005;54:694–701. doi: 10.2337/diabetes.54.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomita T. Caspase-3 immunocytochemical staining for pancreatic endocrine tumors. Hum Pathol. 2009;40:1050–2. doi: 10.1016/j.humpath.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Tomita T, Iwata K. Gelatinases and inhibitors of gelatinases in pancreatic islets and islet cell tumors. Mod Pathol. 1997;10:47–54. [PubMed] [Google Scholar]
- 40.Tomita T. New markers for pancreatic islets and islet cell tumors. Pathol Int. 2002;52:425–32. doi: 10.1046/j.1440-1827.2002.01368.x. [DOI] [PubMed] [Google Scholar]
- 41.Kitamoto T, Ogomori K, Tateishi J, Prusiner SB. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987;57:230–6. [PubMed] [Google Scholar]
- 42.Law E, Lu S, Kieffer TJ, Warnock GL, Ao Z, Woo M, et al. Differences between amyloid toxicity in alpha and beta cells in human and mouse islets and the role of caspase-3. Diabetologia. 2010;53:1415–27. doi: 10.1007/s00125-010-1717-9. [DOI] [PubMed] [Google Scholar]
- 43.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guardado-Mendoza R, Davalli AM, Chavez AO, Hubbard GB, Dick E, Majluf-Cruz A. Pancreatic islet amyloidosis, β-cell apoptosis and α-cell proliferation and determinants of islet cell remodeling in type 2 diabetic baboons. Proc Natl Acad Sci U S A. 2009;106:13992–7. doi: 10.1073/pnas.0906471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirata H, Takahashi A, Kobayashi S, Yonehara S, Sawai H, Okazaki T, et al. Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. J Exp Med. 1998;187:587–600. doi: 10.1084/jem.187.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gown AM, Willingham MC. Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J Histochem Cytochem. 2002;50:449–54. doi: 10.1177/002215540205000401. [DOI] [PubMed] [Google Scholar]
- 47.Butler AE, Janson J, Bonner-Weir S, Ritzel RT, Rizza RA, Butler PC. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 48.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic β cell death in type 1 and type 2 diabetes:many differences, few similarities. Diabetes. 2005;54(Suppl):S97–107. doi: 10.2337/diabetes.54.suppl_2.S97. [DOI] [PubMed] [Google Scholar]
- 49.Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1:14–6. doi: 10.1016/S0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- 50.Stefan Y, Grasso S, Perreltm A, Orci L. The pancreatic polypeptide-rich lobe of the human pancreas; definitive identification of its derivation from the ventral pancreatic primordium. Diabetologia. 1982;23:141–2. doi: 10.1007/BF01271177. [DOI] [PubMed] [Google Scholar]
- 51.Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24:366–71. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
- 52.Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4:110–25. doi: 10.1159/000156969. [DOI] [PubMed] [Google Scholar]