Abstract
A non-invasive method to image the mass and/or function of human pancreatic islets is needed to monitor the progression of diabetes, and the effect of therapeutic interventions. As yet, no method is available for this purpose, which could be applied to in situ human islets. Animal and in vitro studies have documented that manganese infusion could improve the magnetic resonance imaging (MRI) of the endocrine pancreas. Here, we have tested whether a similar approach could discriminate diabetic and non-diabetic patients. In vitro, human isolated islets readily incorporated manganese. In vivo, 243 manganese-enhanced magnetic resonance imaging (MEMRI) examinations were reviewed, including 41 examinations which were run on 24 patients with type 2 diabetes and 202 examinations which were run on 119 normoglycemic patients. The results show that MEMRI discriminates type 2 diabetics from non-diabetic patients, based on the signal enhancement of pancreas.
Keywords: MEMRI, MRI, diabetes, imaging, manganese
Introduction
Despite intense effort of prevention and education, diabetes is still rising throughout the world. There are currently no established methods to non-invasively assess in vivo the mass and function of the native insulin-producing β cells,1 which form the bulk of the endocrine pancreatic islets, and are central to diabetes pathogenesis.2 The non-invasive imaging of these cells, under either diabetic conditions or regeneration regimens,3,4 would be of great value in this context. To date, in vivo visualization of pancreatic islets has been demonstrated only after isolation and in vitro labeling with either iron-oxide nanoparticles5,6 or PET isotopes,7,8 prior to their transplantation into the liver or muscle.5-9 However, such methods will not be easily applicable to the imaging of native islets in situ, due to the unavailability of a proper specific ligand that could act in vivo.10 One promising alternative is manganese-enhanced magnetic resonance imaging (MEMRI). Recent studies have shown that the intensity of the MRI pancreas signal can be enhanced by infusion of manganese,10-13 a cation which mimics the behavior of Ca2+, an essential regulator of insulin secretion.14,15
The main hypothesis of this study was that Mn2+ and Ca2+ also compete during secretion of human insulin, enhancing the contrast of MR images in a way dependent on β cell function. Would that be the case, the MRI of β cells from normoglycemic patients would be expected to be more enhanced by manganese than that of β cells from patients with type 2 diabetes.
As yet, however, whether the MEMRI approach may be useful to monitor the human endocrine pancreas in vivo has not yet been shown. To address this question, we evaluated the manganese uptake by islets isolated from normoglycemic individuals. We then compared by MEMRI the in vivo enhancement of pancreas, liver, muscle and spleen signals in type 2 diabetic and normoglycemic patients. Using this approach, we further compared subsets of patients with type 2 diabetes, differing by age, BMI, and therapeutic requirement of insulin. Eventually, we investigated subsets of normoglycemic and diabetic patients that received gadolinium-DTPA, an extra-cellular contrast agent, which allows for evaluation of blood volume/perfusion16 in the pancreas. The data indicate that the MEMRI of pancreas non-invasively distinguishes normoglycemic and type 2 diabetic patients, that this distinction is organ specific, and does not result from a different blood supply of the pancreas.
Results
In vitro experiments
To test whether manganese was uptaken by human islets, in sufficient amounts to enhance the MRI signal, we incubated islets isolated from normoglycemic organ donors in the absence or presence of Mn2+, prior to MRI. MRI showed that the signal to noise ratio (SNR) increased by 159% (n = 3, p = 0.0003) after exposure to manganese (Fig. 1). An increase in SNR was also observed in the islets isolated from the single patient with type 2 diabetes we could access (data not shown). The data show that the MEMRI signal of pancreas is at least partly due to the uptake of manganese by the endocrine islets.
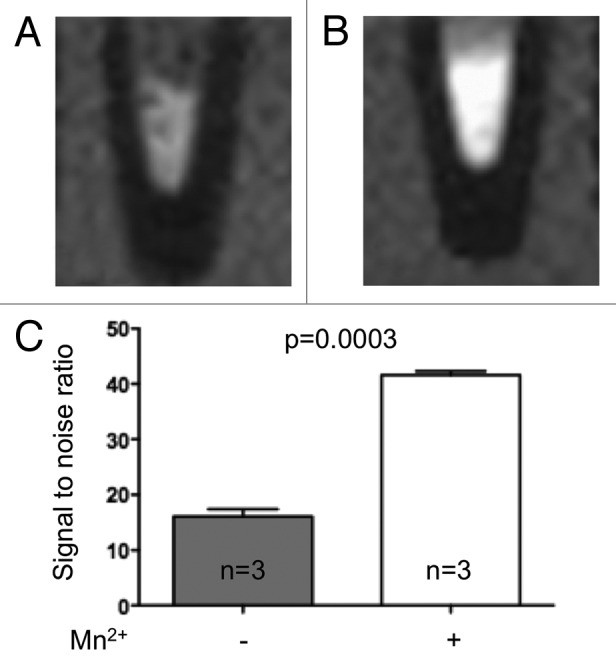
Figure 1. Manganese is uptaken by human islets. T1-weighted magnetic resonance images of pellets of human islets from non-diabetic multi-organ donors before (A) and after a 30 min exposure to MnCl2 (B). C) Manganese significantly enhanced the MRI signal to noise ratio (16.0 ± 1.3 vs. 41.6 ± 0.7, p = 0.0003). Data are mean + SEM signal-to-noise ratio.
In vivo study
To test whether the MEMRI of pancreas can distinguish normoglycemic and type 2 diabetic patients, we retrospectively analyzed the abdominal recording of MEMRI examinations, which had been performed in our institution for a variety of clinical conditions (Table 1). In most cases, these examinations were not purposely tailored for pancreas imaging. Based on the selection criteria which are given in the method section, including the obligatory requirement for each patient of a MRI before and after manganese infusion, we could include in the study 119 normoglycemic patients, who totalized 202 MEMRI examinations and 24 patients with type 2 diabetes, who totalized 41 examinations (some of the patients underwent twice the imaging protocol).
Table 1. MRI indication.
|
Targeted organ |
MRI indication |
No of patients |
|
|
normoglycemic |
Type 2 diabetic |
||
|
liver |
ear, nose and throat tumors |
2 |
0 |
| melanoma |
2 |
0 |
|
| small cell lung carcinoma |
1 |
0 |
|
| non small cell lung carcinoma |
6 |
0 |
|
| breast cancer |
7 |
0 |
|
| hepatocellular carcinoma |
6 |
0 |
|
| hepatic nodule |
0 |
2 |
|
| hepatic abcess |
1 |
0 |
|
| gallbladder carcinoma |
1 |
0 |
|
| cholangiocarcinoma |
6 |
1 |
|
| ampulloma |
2 |
0 |
|
| esophagus tumor |
1 |
0 |
|
| gastric adenocarcinoma |
4 |
0 |
|
| gastrointestinal stromal tumor |
3 |
0 |
|
| duodenal carcinoma |
1 |
0 |
|
| terminal ileon carcinoid |
2 |
0 |
|
| colorectal cancer |
45 |
14 |
|
| pancreatic carcinoma |
4 |
2 |
|
| retroperitoneal sarcoma |
1 |
0 |
|
| nephroblastoma |
3 |
0 |
|
| urothelial tumor |
3 |
1 |
|
| ovarian cancer |
3 |
0 |
|
| endometrial cancer |
1 |
0 |
|
| testicular cancer |
1 |
0 |
|
| diffuse non differentiated cancer |
0 |
1 |
|
| diffuse neuroendocrine tumor |
1 |
0 |
|
| multiple myeloma |
1 |
0 |
|
|
biliary tract |
fistules, leaks, post-traumatic and post-surgery indications |
10 |
2 |
|
pancreas |
carcinoma |
1 |
1 |
|
Total number of patients |
119 | 24 | |
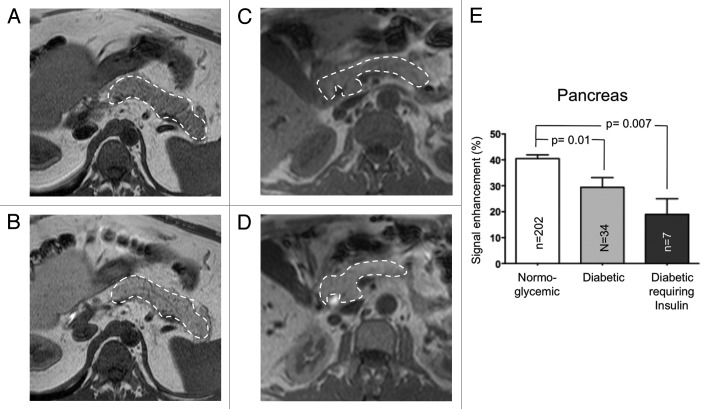
In all cases, the MRI signal of pancreas was enhanced after Mn-DPDP infusion (Fig. 2A–D). However, this enhancement was higher (p = 0.002) in normoglycemic controls (39.7 ± 1.5%) than in patients with type 2 diabetes (Fig. 2E). In the latter group there was no significant difference between the patients without insulin therapy (29.4 ± 3.7%) and those who required it (19.02 ± 6.0%) (Fig. 2E). The MEMRI enhancement of pancreas was similar whichever the age of the diabetic patients (Fig. 3B), and was inversely correlated with their BMI (Fig. 3A). The parallel evaluation of MR images of pancreas obtained after injection of gadolinium-DTPA, revealed a similar enhancement of pancreas in both normoglycemic and type 2 diabetic patients (42 ± 20% vs. 43 ± 15%, p = 0.87).
Figure 2. The MRI signal enhancement of pancreas is enhanced by manganese. T1-weighted magnetic resonance imaging showing the pancreas (area limited by the dashed line) before (A, C) and 20 min after Mn-DPDP infusion (B, D) of a normoglycemic (A, B) and a type 2 diabetic patient (C, D). In both patients, the MRI signal of pancreas was enhanced by the manganese infusion. E) This enhancement was significantly higher in normoglycemic than in type 2 diabetic patients. Data are mean + SEM signal enhancement, expressed as % of the signal evaluated prior to the manganese infusion.
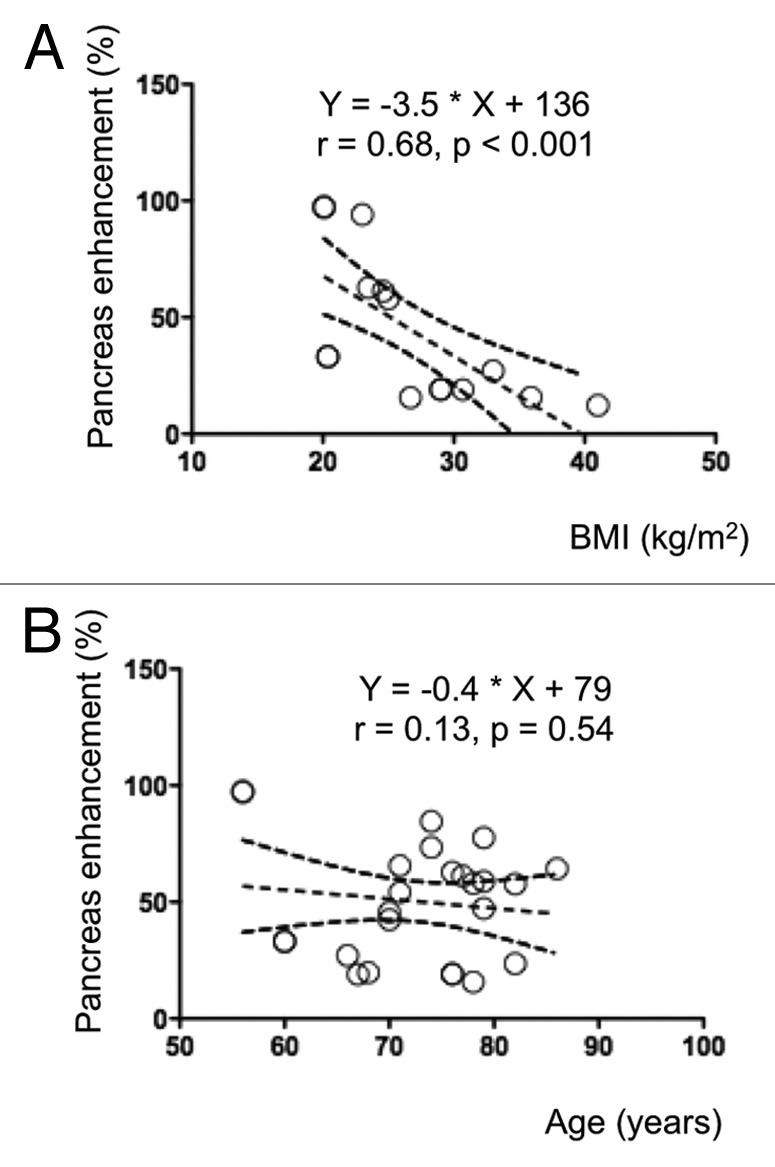
Figure 3. The MEMRI enhancement of pancreas correlated with the BMI of diabetic patients. The pancreas enhancement due to Mn2+ was inversely correlated with the BMI (A), but not the age of the diabetic patients (B), as assessed by Pearson’s correlation.
To assess whether the MEMRI changes were specific to pancreas, we further evaluated the signals of different other insulin-dependent and insulin-independent organs in the very same set of patients. Contrasting with the pancreas data, the Mn2+-induced enhancement of the liver signal was similar (Fig. 4A) in normoglycemic (76.1 ± 2.2) and diabetic patients, whether requiring insulin therapy (53.6 ± 15.1) or not (82.8 ± 11; p = 0.16; Figure 4A). This was also the case for the muscle (6.5 ± 9.0, 7.5 ± 9.1 and 7.5 ± 9.1 in normoglycemic patients, type 2 diabetics with and without insulin therapy, respectively; p = 0.75) and spleen signals (18.4 ± 7.0, 22.4 ± 6.7 and 21.0 ± 4.5 in normoglycemic patients, type 2 diabetics with and without insulin therapy, respectively; p = 0.52) (Fig. 4B and C, respectively).
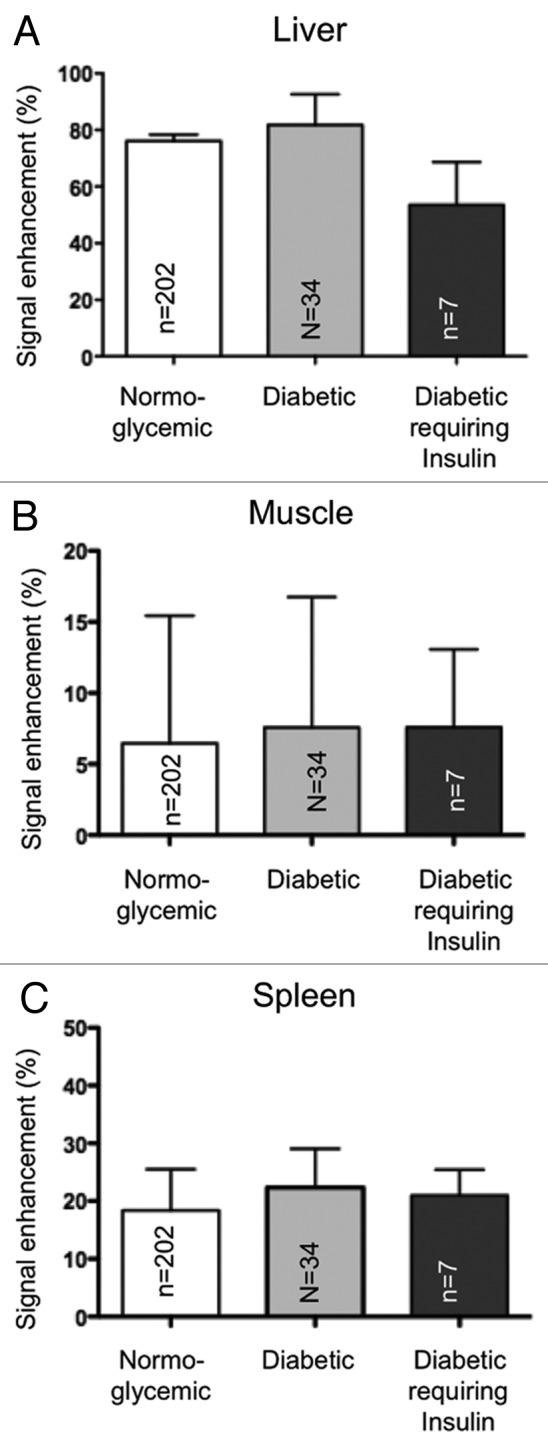
Figure 4. The MEMRI signal of liver (A), muscle (B) and spleen (C) does not differentiate normoglycemic patients from diabetic patients. The signal enhancement of liver (A), muscle (B) and spleen (C) was not statistically different between normoglycemic and type 2 diabetic patients. Data are mean + SEM signal enhancement, expressed as % of the signal evaluated prior to the manganese infusion.
Discussion
This study provides evidence that manganese enhances the MRI signal of pancreas in both non-diabetic and type 2 diabetic patients. It further documents that this enhancement is significantly different in the two groups of patients, and, in the latter group, correlates with their BMI but not their age or insulin requirement. Given that the administration of gadolinium-DTPA similarly enhanced the pancreas signal in non-diabetic and type 2 diabetic patients, the enhancement of the MEMRI signal is unlikely to be significantly affected by differences in the blood perfusion of pancreas of the two groups of patients. Furthermore, the enhancement of the MEMRI signal of liver, muscle and spleen enhancement was also similar in the three very same groups of individuals, stressing the pancreas specificity of the observed changes. The findings indicate that MEMRI may be a helpful imaging strategy for investigating the evolution of diabetic alterations, and their potential modulation during therapy.11-13
The approach has several advantages compared with alternative methods10,18 in which isolated islets are loaded with either iron oxide nanoparticles (for MRI) or positron emitting radioisotopes (for PET/SPECT).18 First, MEMRI can be used for the repeated analysis of the same pancreas, which will be required for the prospective monitoring of patients. This repeated monitoring, which was performed in 25% of the cases studied here, is not presently possible with nanoparticles, and may be unsafe using the high energy positron-emitting radioisotopes that are required to obtain a PET labeling of the small pancreatic islets.19 While each labeling method has its own safety problems,10,18 the fact that manganese is handled by pancreatic islets like Ca2+,15,21 disappears from the plasma in a few hours22 and from most tissues within days,23 and is already frequently used in clinical MRI, makes its repeated use on the same patient more conceivable. Second, in the absence of a β cell- or islet-specific probe suitable for targeted imaging, MEMRI may provide a higher and more specific enhancement of the pancreatic endocrine tissue than of other pancreatic and non-pancreatic compartments. Thus, nanoparticles persist in the pancreas outside the endocrine islet cells, also when inflammation is present6,24,25 and radioisotopic PET labeling has a spatial resolution that largely exceeds the small islet sizes. Furthermore, both nanoparticles and PET ligands substantially label the exocrine pancreas as well as nearby organs18,26 due to the expression of the targeted molecules in multiple cell types. At least experimentally, MEMRI allows for the quantitative detection of individual islets, and their changes in a model of diabetes.13 Third, in the context of type 2 diabetes, in which both the function and likely the mass of β cells is altered, MEMRI may provide information about both events, as manganese is uptaken by β cells as a function of their ability to release insulin in response to glucose.12-15 The inverse correlation observed between BMI and the MEMRI signal of pancreas is consistent with this view, given that BMI relates to residual β cell function.27 In this respect, the 15–30% loss of pancreas signal enhancement we evaluated in type 2 diabetic patients, under MRI conditions, which, for most patients were not optimized for pancreas imaging, is consistent with the changes in β cell mass that are anticipated from pathology studies in the European population.28,29
The retrospective nature of the study allowed for the analysis of only a limited number of patients, under conditions, which most likely, were not ideal for pancreas imaging, and prevented any functional assessment of β cell function. In spite of these limitations, and specifically the lack of direct data about the mass of β cells (some patients are still alive, and the pancreas was not sampled from those that passed away) and their function (beside the very indirect evaluation of blood glucose levels), the data document that MEMRI does non-invasively distinguish, under double blind conditions, the pancreas of diabetic and non-diabetic individuals. Further studies should now test whether the observed changes in the MRI signal specifically reflect an alteration in the mass/function of β cells or are influenced by other concurrent alterations. Nevertheless, the differential uptake of Mn2+ observed between non-diabetic and diabetic patients is in favor of imaging changes of mass/function of β cells.
Moreover, our findings that the enhancement of the liver, spleen and muscle signal was similar in the two groups of patients reinforce the pancreatic specificity of the method. This is further supported by the observation that the non-specific extracellular gadolinium chelate, which also enhanced the pancreas signal, did not differentiate the pancreas signal of non-diabetic and diabetic patients. Together, these findings indicate that MEMRI reveals changes that may be related to altered mass/function of the islet cells. Further developments of the technique, to specifically tailor it to the endocrine pancreas conditions, should now attempt at distinguish these two, likely coincidental β cell changes.
Materials and methods
In vitro islet testing
Human islets from multi-organ donors were obtained thanks to the European Consortium for Islet Transplantation (ECIT). Islets were isolated as per the Edmonton protocol.30 Three thousands islet equivalents isolated from control donors (n = 3) were imaged after a 30 min incubation in RPMI-1640 medium supplemented with 10% fetal calf serum, 100 IU penicillin/ml and 100 μg streptomycin/ml, in the presence or absence of 12.5 μM manganese chloride. After 3 washings in RPMI-1640 medium without manganese chloride, the islets were imaged in a clinical 1.5T Philips MR system (Philips Achieva, Philips Medical System, Best, NL), using a T1-weighted gradient echo sequence, with an inversion time of 300 msec. The MRI signal to noise ratio (SNR) was defined as signal intensity (SI) of the islet pellet divided by the standard deviation of the noise.
Patient study design
After obtaining the approval by the human ethic committee of our institution, we retrospectively analyzed the data from all patients who were addressed for MEMRI at our institution, for a variety of clinical conditions (Table 1). The cohort comprised 24 type 2 diabetics (who provided for 41 MEMRI) and 119 normoglycemic patients (who provided for 202 MEMRI). Patients were included in the diabetic group based on the filed medical history and clinical data (basal fed glycemia, HbA1c levels). The 24 diabetic men were aged 51–85 y (mean 67 ± 7), and featured a mean BMI of 26 ± 6, an average basal fed glycemia of 8.3 ± 2.2 mmol/l, and an HbA1c of 7.0 ± 0.8%. Of the 24 diabetic patients, seven were under insulin therapy, and were handled separately. The control group comprised 41 men and 78 women, aged 14–88 y (mean 60 +/− 14), who featured a mean BMI of 24 ± 5, and a basal fed glycemia of 6.0 ± 1.0 mmol/l. The HbA1c levels of most of these normoglycemic patients were not available.
We also analyzed the data from another group of 15 normoglycemic and 15 type 2 diabetic patients, who were studied by MRI 1 min after injection of 0.1 mmol/kg gadolinium-DTPA.
MRI protocols
243 MRI sessions involving a T1-weighted gradient echo sequence (GRE), acquired before and 20 min after a 2–3 ml/min infusion of 0.5 ml/kg b.w. manganese dipyridoxal diphosphate (Mn-DPDP, Teslascan®, GE-Amersham), were analyzed. All MRI sessions were realized at 1.5T, using either a Philips MR system (Philips Achieva, Philips Medical System) or a Siemens MR system (Siemens Magnetom Espree, Siemens AG.
For imaging the isolated islets, we used a surface coil of 47 mm diameter, and a 3D T1-weighted GRE with the following parameters: TR = 15.9 ms, TE = 9 ms, TI = 300 ms, flip angle = 45°, number of averages = 4, field of view = 6 cm, matrix of 204 x 204, slice thickness = 0.3 mm.
For imaging of the patients, we used a body coil, and a T1-weighted GRE with the following parameters: TR = ~4 msec, TE = ~2 msec, flip angle = 10°, number of averages = 1, field of view = ~375 x 297 mm (depending on the size of the abdomen), matrix of 188 x 147, slice thickness = 4 mm.
The analysis of the recorded images was performed by two readers (XM and DB), who were blinded to all clinical information concerning the patients. To this end, a region of interest, which avoided major vessels, ducts, image inhomogeneities and artifacts, was defined in the pancreas, liver, spleen and muscle for evaluation of the MR signal. The signal enhancement (SI) was calculated as follows: SI = SIpost – SIpre / SIpre, where SIpre and SIpost are the signal intensities before and 20 min after the infusion of Mn-DPDP, respectively.
Statistical analysis
Statistical analysis was performed with Prism (Prism, version 5.0d, 2010; GraphPad Software, San Diego, CA, USA) and Statistica (Statistica version 8, Statsoft Inc.). Results are presented as mean + standard error. In vitro islets enhancement was compared using a two-side, unpaired t-test. The data sets which were non-normally distributed (established from Kolmogorov-Smirnov tests) were compared using analysis of variance with a Dunn’s post hoc test. Differences were considered significant at p < 0.05.
Acknowledgments
Our teams are supported by grants from the Swiss National Science Foundation (310030-141162, 310000-109402, CR32I3_129987), the Juvenile Diabetes Research Foundation (31-2008-416, 40-2011-11), the European Union (BETAIMAGE 222980; IMIDIA C2008-T7, BETATRAIN 289932) and the Boninchi Foundation.
Disclosure of PotentialConflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/20857
References
- 1.Leibiger IB, Caicedo A, Berggren PO. Non-invasive in vivo imaging of pancreatic beta-cell function and survival - a perspective. Acta Physiol (Oxf) 2011 doi: 10.1111/j.1748-1716.2011.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillespie KM. Type 1 diabetes: pathogenesis and prevention. CMAJ. 2006;175:165–70. doi: 10.1503/cmaj.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cernea S, Herold KC. Drug insight: New immunomodulatory therapies in type 1 diabetes. Nat Clin Pract Endocrinol Metab. 2006;2:89–98. doi: 10.1038/ncpendmet0082. [DOI] [PubMed] [Google Scholar]
- 4.Yamaoka T. Regeneration therapy of pancreatic beta cells: towards a cure for diabetes? Biochem Biophys Res Commun. 2002;296:1039–43. doi: 10.1016/S0006-291X(02)02000-4. [DOI] [PubMed] [Google Scholar]
- 5.Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A. In vivo imaging of islet transplantation. Nat Med. 2006;12:144–8. doi: 10.1038/nm1316. [DOI] [PubMed] [Google Scholar]
- 6.Ris F, Lepetit-Coiffe M, Meda P, Crowe LA, Toso C, Armanet M, et al. Assessment of human islet labeling with clinical grade iron nanoparticles prior to transplantation for graft monitoring by MRI. Cell Transplant. 2010;19:1573–85. doi: 10.3727/096368910X515863. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y, Dang H, Middleton B, Zhang Z, Washburn L, Stout DB, et al. Noninvasive imaging of islet grafts using positron-emission tomography. Proc Natl Acad Sci U S A. 2006;103:11294–9. doi: 10.1073/pnas.0603909103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souza F, Simpson N, Raffo A, Saxena C, Maffei A, Hardy M, et al. Longitudinal noninvasive PET-based beta cell mass estimates in a spontaneous diabetes rat model. J Clin Invest. 2006;116:1506–13. doi: 10.1172/JCI27645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pattou F, Kerr-Conte J, Wild D. GLP-1-receptor scanning for imaging of human beta cells transplanted in muscle. N Engl J Med. 2010;363:1289–90. doi: 10.1056/NEJMc1004547. [DOI] [PubMed] [Google Scholar]
- 10.Andralojc K, Srinivas M, Brom M, Joosten L, de Vries IJ, Eizirik DL, et al. Obstacles on the way to the clinical visualisation of beta cells: looking for the Aeneas of molecular imaging to navigate between Scylla and Charybdis. Diabetologia. 2012;55:1247–57. doi: 10.1007/s00125-012-2491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antkowiak PF, Tersey SA, Carter JD, Vandsburger MH, Nadler JL, Epstein FH, et al. Noninvasive assessment of pancreatic beta-cell function in vivo with manganese-enhanced magnetic resonance imaging. Am J Physiol Endocrinol Metab. 2009;296:E573–8. doi: 10.1152/ajpendo.90336.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gimi B, Leoni L, Oberholzer J, Braun M, Avila J, Wang Y, et al. Functional MR microimaging of pancreatic beta-cell activation. Cell Transplant. 2006;15:195–203. doi: 10.3727/000000006783982151. [DOI] [PubMed] [Google Scholar]
- 13.Lamprianou S, Immonen R, Nabuurs C, Gjinovci A, Vinet L, Montet XC, et al. High-resolution magnetic resonance imaging quantitatively detects individual pancreatic islets. Diabetes. 2011;60:2853–60. doi: 10.2337/db11-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun M. The αβδ of ion channels in human islet cells. Islets. 2009;1:160–2. doi: 10.4161/isl.1.2.9405. [DOI] [PubMed] [Google Scholar]
- 15.Leoni L, Dhyani A, La Riviere P, Vogt S, Lai B, Roman BB. β-Cell subcellular localization of glucose-stimulated Mn uptake by X-ray fluorescence microscopy: implications for pancreatic MRI. Contrast Media Mol Imaging. 2011;6:474–81. doi: 10.1002/cmmi.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikulis DJ, Roberts TP. Neuro MR: protocols. J Magn Reson Imaging. 2007;26:838–47. doi: 10.1002/jmri.21041. [DOI] [PubMed] [Google Scholar]
- 17.Meda P. 2012. Symposium on Beta-Cell Imaging at the 2011 EANM meeting. Imaging Med 4: 17-23. [Google Scholar]
- 18.Vinet L, Lamprianou S, Goulley J, Nabuurs C, Immonen R, Babic A, et al. Towards an In Vivo portrait of pancreatic beta-cells: The bare essentials. Treatment Strategies Diabetes. 2011;3:43–7. [Google Scholar]
- 19.Brom M, Andrałojć K, Oyen WJ, Boerman OC, Gotthardt M. Development of radiotracers for the determination of the beta-cell mass in vivo. Curr Pharm Des. 2010;16:1561–7. doi: 10.2174/138161210791164126. [DOI] [PubMed] [Google Scholar]
- 20.Medarova Z, Moore A. Non-invasive detection of transplanted pancreatic islets. Diabetes Obes Metab. 2008;10(Suppl 4):88–97. doi: 10.1111/j.1463-1326.2008.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dryselius S, Grapengiesser E, Hellman B, Gylfe E. Voltage-dependent entry and generation of slow Ca2+ oscillations in glucose-stimulated pancreatic beta-cells. Am J Physiol. 1999;276:E512–8. doi: 10.1152/ajpendo.1999.276.3.E512. [DOI] [PubMed] [Google Scholar]
- 22.Toft KG, Hustvedt SO, Grant D, Martinsen I, Gordon PB, Friisk GA, et al. Metabolism and pharmacokinetics of MnDPDP in man. Acta Radiol. 1997;38:677–89. doi: 10.1080/02841859709172400. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Gordon PB, Hustvedt SO, Grant D, Sterud AT, Martinsen I, et al. MR imaging properties and pharmacokinetics of MnDPDP in healthy volunteers. Acta Radiol. 1997;38:665–76. doi: 10.1080/02841859709172399. [DOI] [PubMed] [Google Scholar]
- 24.Denis MC, Mahmood U, Benoist C, Mathis D, Weissleder R. Imaging inflammation of the pancreatic islets in type 1 diabetes. Proc Natl Acad Sci U S A. 2004;101:12634–9. doi: 10.1073/pnas.0404307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaglia JL, Guimaraes AR, Harisinghani M, Turvey SE, Jackson R, Benoist C, et al. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest. 2011;121:442–5. doi: 10.1172/JCI44339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweet IR, Cook DL, Lernmark A, Greenbaum CJ, Wallen AR, Marcum ES, et al. Systematic screening of potential beta-cell imaging agents. Biochem Biophys Res Commun. 2004;314:976–83. doi: 10.1016/j.bbrc.2003.12.182. [DOI] [PubMed] [Google Scholar]
- 27.Mihic M, Modi P. Metabolic syndrome--risk factors for atherosclerosis and diabetes. Curr Diabetes Rev. 2008;4:122–8. doi: 10.2174/157339908784220750. [DOI] [PubMed] [Google Scholar]
- 28.Marchetti, P., Bugliani, M., Boggi, U., Masini, M., and Marselli, L. The pancreatic beta cells in human type 2 diabetes Austin, TX: Landes Bioscence. [DOI] [PubMed]
- 29.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–30. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]