Abstract
Human induced pluripotent stem (iPS) cells obtained from patients are expected to be a useful source for cell transplantation therapy, because many patients (including those with type 1 diabetes and severe type 2 diabetes) are on waiting lists for transplantation for a long time due to the shortage of donors. At present, many concerns related to clinical application of human iPS cells have been raised, but rapid development of methods for the establishment, culture, and standardization of iPS cells will lead autologous cell therapy to be realistic sooner or later. However, establishment of a method for preparing some of desired cell types is still challenging. Regarding pancreatic β-cells, there have been many reports about differentiation of these cells from human embryonic stem (ES)/iPS cells, but a protocol for clinical application has still not been established. Since there is clear proof that cell transplantation therapy is effective for diabetes based on the results of clinical islet transplantation, pancreatic β-cells prepared from human iPS cells are considered likely to be effective for reducing the burden on patients. In this article, the current status of procedures for preparing pancreatic β-cells from human ES/iPS cells, including effective use of small molecules, is summarized, and some of the problems that still need to be overcome are discussed.
Keywords: diabetes cell therapy, differentiation, human iPS cells, pancreatic β-cells, small molecules
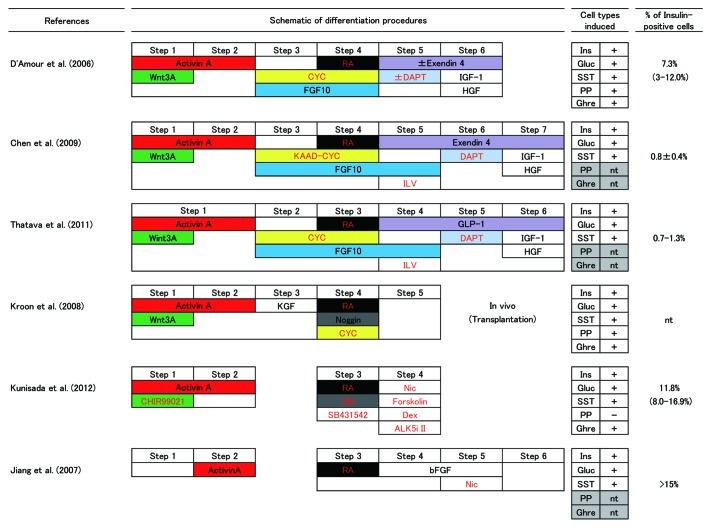
Numerous methods for differentiating insulin-producing cells from human ES/iPS cells have been reported so far. Figure 1 is a schematic representation of the protocols that complete cell treatments in monolayer culture.1-6 All of the protocols shown can induce insulin-positive cells as well as glucagon- and somatostatin-positive cells. Pancreatic polypeptide- and ghrelin-positive cells were also induced in some protocols. The percentage of insulin-positive cells in culture varied among protocols, but precise comparison must be done by controlled studies with standardized cells. Formation of embryoid bodies, which mimic germ-layer specification during early embryogenesis, has also been applied to pancreatic differentiation of human ES/iPS cells.7,8 However, specific growth of endoderm in monolayer culture would be preferable because of its simplicity and greater efficiency. The important common factors in the methods shown in Figure 1 are the treatment of undifferentiated ES/iPS cells with activin A to achieve differentiation into endoderm and subsequent induction of pancreatic differentiation by exposure to retinoic acid (Fig. 1). Differentiation of glucagon-producing cells by a similar method has also been reported.9 Based on information from embryological studies, each protocol has been designed to involve sequential use of cytokines or their signaling modulators at specific times. Addition of Wnt3a or CHIR99021 (an activator of Wnt signaling through inhibition of glycogen synthase kinase 3) during activin treatment is done in many protocols to enhance endodermal differentiation in vitro, mimicking the coordinated expression and action of both activin/Nodal and Wnt during primitive streak formation. Kunisada et al., have compared Wnt3a and CHIR99021 in a same condition, and it was shown that CHIR99021 was more efficient than Wnt3a in inducing Sox17- and Foxa2-positive endodermal cells.6 Since pancreatic differentiation is known to be regulated by the fibroblast growth factor (FGF) and bone morphogenetic protein (BMP) pathways during embryonic development, basic FGF (bFGF; also known as FGF2), keratinocyte growth factor (KGF; also known as FGF7), FGF10, and Noggin (an endogenous protein that inhibits BMP by binding to its receptor) are used in some methods. Other growth factors and incretins, such as insulin-like growth factor-1 (IGF-1), hepatocyte growth factor (HGF), and glucagon-like peptide-1 (GLP-1)/exendin-4 (a peptide analog of GLP-1), have also been used to facilitate differentiation. Hedgehog expression is suppressed in the pancreatic primordium compared with that in surrounding organs, so low molecular weight Hedgehog signaling inhibitors (cyclopamine or KADD-cyclopamine) are used in many methods.1,3-5,8,9 Notch signaling is known to control multiple steps of pancreatic differentiation. Since persistent expression of FGF10 in embryonic pancreas activates Notch signaling and blocks endocrine differentiation, Notch signal activation has been implicated in the self-renewal of Pdx1-expressing pancreatic progenitors. On the other hand, Ngn3 expression in the pancreas occurs as a result of decreased Notch signaling, so the sequential use of FGF10 and DAPT, a gamma-secretase inhibitor that blocks Notch signaling, were included in some protocols.1,4,5 It was consistent well with organogenetic process of pancreas, however, D’Amour et al., have observed minor differences in the differentiation when DAPT, as well as exendin-4, IGF-1 and HGF, were omitted.1
Figure 1. A schematic representation of the protocols for pancreatic β-cell differentiation from human ES/iPS cells. All the protocol shown was featured by treating the cells on wholly in monolayer culture. The steps of each protocol were aligned and colored based on the usage of cytokines (black letters) and small molecules (red letters). Pancreatic endocrine cell types induced and percent of the insulin-positive cells generated by each approach were also shown on the right. RA, retinoic acid; CYC, cyclopamine; DM, dorsomorphin; ILV, (-)-indolactam V; Nic, nicotinamide; Dex, dexamethasone; ALK5i II, ALK5 inhibitor II; Ins, insulin; Gluc, glucagon; SST, somatostatin; PP, pancreatic polypeptide; Ghre, ghrelin; nt, not tested.
Various classes of small molecules other than cyclopamine and DAPT have also been reported to be effective for differentiating human ES cells and iPS cells into insulin-producing cells. The general advantages of small molecules compared with proteins are as follows: i) effective and uniform access to cultured cells based on membrane permeability, ii) specific stimulation or inhibition of target signaling pathways, iii) diversity of structure and properties, iv) low cost, and v) convenient to use when constructing a library for screening. Nicotinamide, a poly(ADP-ribose) synthetase inhibitor, has been shown to induce endocrine pancreatic differentiation and maturation,10 and it is used in some protocols to improve the yield of pancreatic endocrine cells.2,6 Chen et al. reported that (-)-indolactam V is a compound that efficiently induces the differentiation of human ES cells into Pdx1-expressing cells.4 In the method reported by Kunisada et al., Pdx1 expression is induced when Sox17-positive endodermal cells are exposed to retinoic acid and dorsomorphin (a BMP type I receptor inhibitor), while differentiation only proceeds as far as Ngn3-positive pancreatic endocrine precursor cells when SB431542, an inhibitor of the transforming growth factor-β (TGF-β) type I receptor, is added simultaneously.6 Induction of Ngn3 expression by SB431542 in Pdx1-expressing pancreatic progenitors was reported in rhesus monkey iPS cells,11 however, these two marker genes can be induced in single step.6 Use of another TGF-β type I receptor inhibitor, activin receptor-like kinase 5 (ALK5) inhibitor II, in pancreatic-α and β-cell differentiation systems has also been reported.6,9 Forskolin (an activator of adenylyl cyclase) and dexamethasone (a synthetic adrenocortical steroid) have also been shown to enhance cellular maturation, these agents could be combined with other small molecules to obtain synergistic effects.6 Currently, all of the steps in differentiation have been achieved with small molecules, except that activin A is needed for endodermal differentiation.6 The chemical structures of the small molecules mentioned here are listed in Table 1.
Because of differences in the properties of various human ES cell lines, it has been pointed out that it is necessary to find and employ lines that easily differentiate into target lineages such as pancreatic cells.12 Abnormalities of karyotype and variations in the techniques used to obtain or maintain these cells have been excluded as major cause of differences in differentiation potential, but epigenetic differences among human ES cell lines could be a factor. Epigenetic variations are more pronounced in iPS cells than ES cells. Problems with reprogramming and the need for improved iPS cell generation technologies before clinical application can be achieved have been discussed elsewhere.13 It is possible that the efficient use of small molecules will provide us with a solution to such problems. Insulin-producing cells were obtained with a similar efficiency by the method of Kunisada et al., which mainly employs small molecule treatment, when different human iPS cell lines were used.6 The versatility of this method has been confirmed by repeated experiments with multiple iPS cell lines differing in terms of age, gender, and reprogramming method, but unfortunately no studies have been performed with human ES cells so far. Use of selected cell lines that preferentially differentiate into pancreatic cells would be convenient for exploring the optimum experimental conditions. Both effective quality control and standardization of human iPS cells are important before clinical application. In addition, the success rate could be increased by using pancreatic β-cells prepared from tailor-made iPS cells in individual patients.
The functionality and maturity of pancreatic β-cells obtained by differentiation from human ES/iPS cells remain controversial. Detailed analysis of pancreatic β-cells derived from human ES cells has revealed the expression of genes related to β-cell functions, such as glucose sensing, exocytosis, and transcriptional factors involved in the pancreatic endocrine system.14 However, many of the insulin-producing cells differentiated so far have also expressed glucagon simultaneously and little glucose responsiveness had been acquired. This profile resembles that of the immature endocrine cells which emerge during embryonic development. Nevertheless, insulin-producing cells differentiated in vitro are not homogeneous, so it might be possible to obtain cells that are useful for cell therapy by purification based on differences of their properties.14 Moreover, long-term survival and physiological functioning in vivo have been reported when pancreatic islet-like structures were prepared from insulin-producing cells that had been differentiated from human ES cells and transplanted into an animal model of diabetes.15
The fact that human ES cells and iPS cells can undergo differentiation into insulin-producing cells has already been verified by many studies, as mentioned above. On the other hand, there are still many points to be confirmed and problems to be overcome, including the optimum number of cells and transplantation site, the efficacy of treatment and duration of action, immunogenicity, safety issues (including tumorigenicity), before clinically achieving cell therapy with iPS cells for diabetes. Since such studies should be performed from several perspectives, in addition to that of the stem cell field, it is very important to share basic technology and materials. A simple, reproducible, and versatile protocol for the differentiation of β-cells from human iPS cells that employs small molecules is expected to make a great contribution in the future.
Table 1. Chemical formulae of the small molecules appeared in this addendum.
| Name |
Chemical formula |
Description |
| Retinoic acid |
all-trans-3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid |
Metabolite of vitamin A. Retinoic acid receptor agonist. |
| CHIR99021 |
6-[[2-[[4-(2,4-dichlorophenyl)-5-(5-methyl-1H-imidazol-2-yl)-2-pyrimidinyl]amino] ethyl]amino]-3-pyridinecarbonitrile |
Selective inhibitor of glycogen synthase kinase 3β. Activates Wnt signaling. |
| cyclopamine |
(3S,3'R,3'aS,6'S,6aS,6bS,7'aR,9R,11aS,11bR)-3',6',10,11b tetramethylspiro[2,3,4,6,6a,6b, 7,8,11,11a-decahydro-1H-benzo[a]fluorene-9,2'-3a,4,5,6,7,7a-hexahydro-3H-furo[3,2-b]pyridine]-3-ol |
Hedgehog signaling inhibitor. |
| KADD-cyclopamine |
3-Keto-N-(aminoethyl-aminocaproyl-dihydrocinnamoyl)cyclopamine |
Hedgehog signaling inhibitor. |
| DAPT |
N-[(3,5-Difluorophenyl)acetyl]-L-alanyl-2- phenylglycine-1,1-dimethylethyl ester |
Potent and specific inhibitor of γ-secretase. Blocks Notch signaling. |
| Nicotinamide |
Pyridine-3-carboxamide |
Inhibitor of poly(ADP-ribose) polymerase. |
| (-)-indolactam V |
(2S,5S)-1,2,4,5,6,8-Hexahydro-5-(hydroxymethyl)-1-methyl-2-(1-methylethyl)-3H- pyrrolo[4,3,2-gh]-1,4-benzodiazonin-3-one |
Protein kinase C activator. |
| Dorsomorphin |
6-[4-(2-piperidin-1-ylethoxy)phenyl]-3-pyridin-4-ylpyrazolo[1,5-a]pyrimidine |
Inhibitor of AMP-activated protein kinase and BMP signaling. |
| SB431542 |
4-[4-(1,3-benzodioxol -5-yl)-5-pyridin-2-yl- 1H-imidazol-2-yl]benzamide |
Inhibitor of TGF-βtype I receptor. |
| ALK5 inhibitor II |
2-(3-(6-Methylpyridin-2-yl)-1H-pyrazol-4-yl)-1,5-naphthyridine |
Inhibitor of TGF-βtype I receptor. |
| Forskolin |
(3R,4aR,5S,6S,6aS,10S,10aR,10bS)-Dodecahydro-6,10,10b-trihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-3-vinyl-1H-benzo[f]chromen-5-yl acetate |
Activator of Adenylyl cyclase. |
| Dexamethasone | (8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one | Synthetic adrenocortical steroid. Glucocorticoid receptor agonist. |
All of the small molecules in the list are available from commercial suppliers.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/20856
References
- 1.D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 2.Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–53. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 3.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–52. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5:258–65. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 5.Thatava T, Nelson TJ, Edukulla R, Sakuma T, Ohmine S, Tonne JM, et al. Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secreting progeny. Gene Ther. 2011;18:283–93. doi: 10.1038/gt.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunisada Y, Tsubooka-Yamazoe N, Shoji M, Hosoya M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 2012;8:274–84. doi: 10.1016/j.scr.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Shim JH, Kim SE, Woo DH, Kim SK, Oh CH, McKay R, et al. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50:1228–38. doi: 10.1007/s00125-007-0634-z. [DOI] [PubMed] [Google Scholar]
- 8.Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X, et al. Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861–71. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rezania A, Riedel MJ, Wideman RD, Karanu F, Ao Z, Warnock GL, et al. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes. 2011;60:239–47. doi: 10.2337/db10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otonkoski T, Beattie GM, Mally MI, Ricordi C, Hayek A. Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells. J Clin Invest. 1993;92:1459–66. doi: 10.1172/JCI116723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu FF, Zhang PB, Zhang DH, Sui X, Yin M, Xiang TT, et al. Generation of pancreatic insulin-producing cells from rhesus monkey induced pluripotent stem cells. Diabetologia. 2011;54:2325–36. doi: 10.1007/s00125-011-2246-x. [DOI] [PubMed] [Google Scholar]
- 12.Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313–5. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- 13.Okita K, Yamanaka S. Induced pluripotent stem cells: opportunities and challenges. Philos Trans R Soc Lond B Biol Sci. 2011;366:2198–2207. doi: 10.1098/rstb.2011.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basford CL, Prentice KJ, Hardy AB, Sarangi F, Micallef SJ, Li X, et al. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia. 2012;55:358–71. doi: 10.1007/s00125-011-2335-x. [DOI] [PubMed] [Google Scholar]
- 15.Eshpeter A, Jiang J, Au M, Rajotte RV, Lu K, Lebkowski JS, et al. In vivo characterization of transplanted human embryonic stem cell-derived pancreatic endocrine islet cells. Cell Prolif. 2008;41:843–58. doi: 10.1111/j.1365-2184.2008.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]