Abstract
Escherichia coli is one of the most frequent bacteria implicated in biofilm formation, which is a dynamic process whose first step consists in bacteria adhesion to surfaces through type 1 fimbriae. Salicylate induces a number of morphological and physiological alterations in bacteria including the activation of the transcriptional regulator MarA. In this report the effects of salicylate on biofilm formation and their relationship with MarA were studied. An inverse relationship was observed between in vitro biofilm formation and salicylate concentration added to the culture medium. Salicylate increases the expression of marA and decreases the expression of fimA and fimB genes in the wild-type strain. In addition, the fimA and fimB expression was decreased in a MarR mutant in which marA was also overexpressed. In conclusion, the expression of type 1 fimbriae in presence of salicylate may be regulated by the level of marA expression through fimB regulator, albeit through neither the ompX nor the tolC genes.
Keywords: Escherichia coli, MarA, biofilm, salicylate, type 1 fimbriae
Introduction
Biofilms are currently defined as structured bacterial communities embedded in a self-produced exopolysaccharide matrix adherent to any abiotic or biological surface.1 Biofilms are ubiquitous, with almost all material coming into contact with naturally occurring fluids being susceptible to this form of bacterial colonization. These communities may be involved in the development of serious human health problems and are also of concern in environmental and industrial settings. Bacterial biofilm infections are particularly problematic because sessile bacteria can withstand host immune responses and are drastically more resistant to antibiotics, biocides and hydrodynamic shear forces than their planktonic counterparts.2 Escherichia coli is one of the most frequent bacteria forming biofilm which is a dynamic process whose first step consists of bacterial fimbrial adherence to surfaces. This step is crucial for uropathogenic E. coli (UPEC) to colonize the bladder and to cause urinary tract infections. E. coli express several adhesins which recognize specific molecules on target surfaces.
UPEC strains may express a variety of fimbrial adherence factors, such as P, S, Dr and type 1 fimbriae.3 The last factor is encoded by the chromosomally located fim gene cluster which encodes the major structural subunit (FimA) and several minor components: two adaptor proteins (FimG and FimF), the adhesion (FimH), two chaperon proteins (FimC and FimD), two site-specific recombinases (FimB and FimE) and the regulator FimI.4 In addition to their contribution to virulence, they facilitate adherence to mucosal surfaces and inflammatory cells in vitro.5
In a previous study performed in our laboratory in which 151 UPEC clinical isolates collected from patients with cystitis, pyelonephritis and prostatitis were analyzed, it was observed that isolates forming in vitro biofilm presented a significantly higher frequency of type 1 fimbriae expression.6 Uropathogenic E. coli can form intracellular bacterial communities with many biofilm-like properties within the bladder epithelium. These communities may allow bacteria to subvert host defenses and form a persistent reservoir in the bladder.7 Biofilm formation could play an important role in the persistence of bacteria. In fact, the study of UPEC strains causing relapses and re-infection in women showed that those involved in relapses had greater capacity to form in vitro biofilm than those implicated in re-infection.8
Salicylate is a member of a large group of pharmaceuticals referred to as non-steroidal anti-inflammatories and is the active component of the analgesic aspirin. Salicylate induces a number of morphological and physiological alterations in bacteria.9 Growth of bacteria in the presence of salicylate can induce an intrinsic multiple antibiotic resistance phenotype, increasing the resistance to some antibiotics. These effects are induced by concentrations of salicylate that do not affect bacterial growth rates suggesting that they are specifically induced by salicylate.9 One of these effects is the activation of MarA, which is an AraC/XylS transcriptional regulator of E. coli that directly activates or represses multiple chromosomal genes. Thus, strains constitutively expressing MarA showed altered expression of more than 60 chromosomal genes including the TolC outer membrane channel and the outer membrane protein OmpX. These genes belong to a variety of functional groups some of which have been associated with iron transport and metabolism. Other genes have also been reported to be part of the soxRS regulon of E. coli oxidative stress response.10 Thus, overexpression of MarA affects many functions including multiple-antibiotic resistance (Mar), virulence and survival.11
In this report, we studied the effects of salicylate on biofilm formation and its relationship with MarA.
Results
The UPEC HC91255 clinical strain produced in vitro biofilm in minimal culture medium, as confirmed by confocal image (not shown). An inverse relationship was observed between in vitro biofilm formation and salicylate concentration when different salicylate concentrations were added to the medium. Thus, the mean and deviation of optical density at 600 nm values obtained were 0.47 ± 0.021, 0.438 ± 0.066, 0.137 ± 0.020, 0.1 ± 0.024, 0.075 ± 0.005 and 0.055 ± 0.007, corresponding to salicylate concentrations of 0 mM, 0.1 mM, 0.5 mM, 1 mM, 5 mM and the negative control, respectively (Table 1). To rule out the possibility that the results obtained were due to differences in the growth rate, a count of viable bacteria was made and no differences were found in the number of colonies from bacteria growth with or without the different salicylate concentrations.
Table 1. Relationship between uropathogenic E. coli HC91255 strain biofilm formation and salicylate concentrations.
| Salicylate concentration | OD620nm | Χ | δ |
|---|---|---|---|
| 0 mM |
0.485–0.455 |
0.47 |
0.021 |
| 0.1 mM |
0.485–0.391 |
0.438 |
0.066 |
| 0.5 mM |
0.151–0.122 |
0.137 |
0.020 |
| 1 mM |
0.117–0.083 |
0.100 |
0.024 |
| 5 mM |
0.079–0.071 |
0.075 |
0.005 |
|
marR mutant |
0.271–0.169 |
0.220 |
0.020 |
|
marR mutant + 1 mM salicylate |
0.120–0.150 |
0.136 |
0.01 |
| negative control | 0.06–0.05 | 0.055 | 0.007 |
Χ, mean of three different experiments; δ, standard deviation.
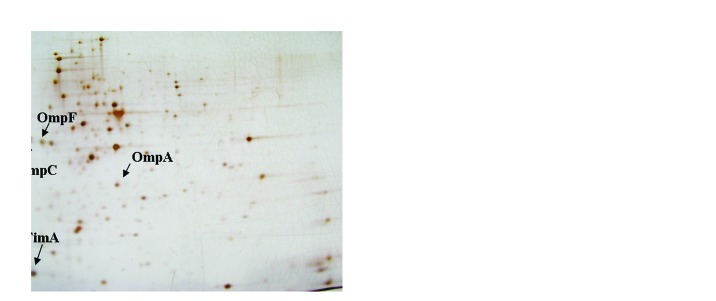
HC91255 was also cultured in Nutrient Broth with and without 1 mM of salicylate and grown until an OD620nm of 0.6. When proteins in cell-free extracts were run in 2D gels, several differences were detected. The greatest change was observed for a protein with an apparent molecular weight of 16 KDa whose expression when decreased when the strain was grown in medium with 1 mM salicylate (Fig. 1). This protein was further characterized by MALDI-TOF and identified as the type 1 fimbriae major subunit (FimA). Other proteins that also decreased their expression in presence of salicylate were OmpF, OmpA and NmpC (Fig. 1).
Figure 1. 2D-gel total proteins of the uropathogenic E. coli HC91255 strain. (A) Strain grown in LB without salicylate. (B) Strain grown in LB with 1 mM salicylate. The arrows indicate the characterized spots.
By amplification-restriction of the fimA promoter no differences in the orientation of this promoter was observed between the strains being observed, a mixture of all four fragments (not shown).
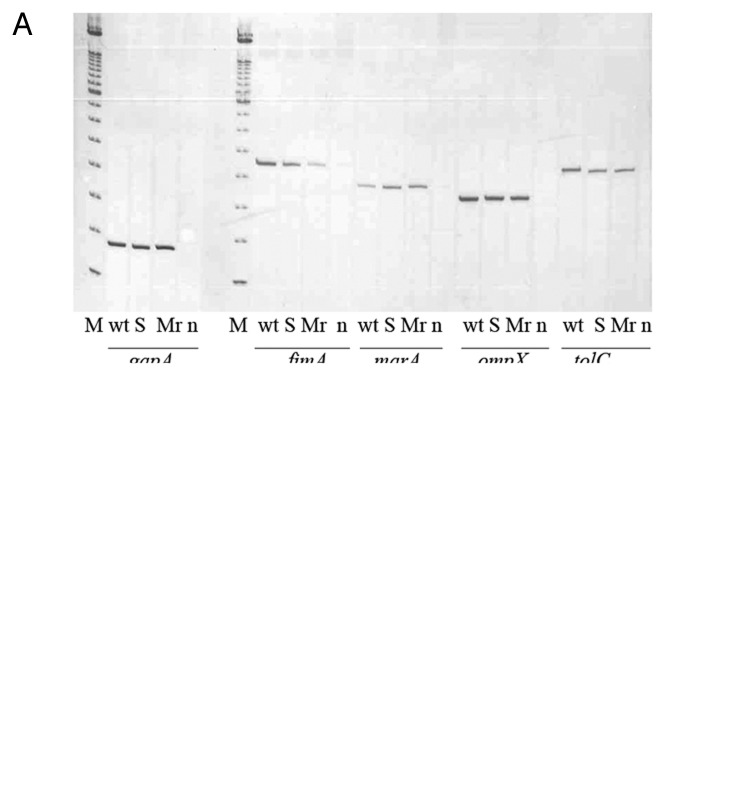
The results obtained by RT-PCR showed that in the presence of salicylate the strain presented an overexpression of the marA gene, as well as a reduction in fimA gene expression confirming the result obtained in the protein analysis. No significant changes were observed in the expression of ompX and tolC genes (Fig. 2A). In addition, a decrease in fimB but not fimE expression in the strain growth in presence of 1 mM salicylate was observed (Fig. 2B).
Figure 2. RT-PCR of the uropathogenic strain HC91255 and its marR mutant. M, size marker 100 bp (Invitrogen, Spain); wt, wild-type strain HC91255; S, wild-type strain grown with 1 mM salicylate; Mr, marR mutant; n, negative control.
A marR mutant from the HC91255 strain showing a deletion of 13 bp between positions 268 and 281 in this gene was obtained and studied. This mutation changed the amino acid sequence after position 90 (Glu to Ala). The mutant selected had a MIC of ciprofloxacin of 0.128 μg/ml. This mutant presented overexpression of the marA gene whereas the fimA and fimB genes but not fimE gene showed a decrease in its expression (Fig. 2A and B) as also occurred when salicylate was added to the culture media. These results were in accordance with the lower capacity of this mutant to form in vitro biofilm in comparison with the wild-type strain (0.47 vs. 0.220). The MarR mutant strain and the strain grown in LB with salicylate had in common that both strains overexpressed the marA gene.
Discussion
Fimbriae-mediated adherence is important for the virulence and persistence of E. coli causing urinary tract infection. Adherence is considered the first and most important step in biofilm formation and is mediated by type 1 fimbriae in E. coli.2
It is well known that salicylate induces a number of morphological and physiological alterations in bacteria. Growth of bacteria in the presence of salicylate can induce a multiple antimicrobial resistance phenotype and can affect the production of various factors involved with bacterial virulence.9 Thus, E. coli may exhibit increased resistance to quinolones, cephalosporins, ampicillin, nalidixic acid, tetracyclines and chloramphenicol due, in part, to increased transcription of the marRAB operon. Salicylate inhibits the binding of MarR to mar operator region of the mar operon which then leads to increased production of MarA and a decrease in antibiotic accumulation associated with a reduction in the production of the outer membrane porins OmpF and OmpC and to a concomitant increase in the production of the multidrug efflux pump AcrAB.12,13 MarA expression is also involved in the virulence of E. coli. Using a murine model of pyelonephritis, Casaz et al.14 observed that strains lacking this gene were unable to maintain colonization of the kidney. NmpC is an outer membrane protein, which functions as a porin. It has four regions with strong homologies to OmpF and both proteins present conserved organization of the functional sequence in their promoters.15 This fact opens the possibility that NmpC could be affected by salicylate in the same way that OmpF. Thus, NmpC could be affected by salicylate in the same way as OmpF. Further studies are needed to elucidate this relationship.
Moreover, salicylate affects the production of bacterial virulence factors. It has also been shown that the synthesis of some types of fimbriae in E. coli, such as colonizing factor antigen, P fimbriae and type 1 fimbriae, are reduced following growth in the presence of salicylate. Kunin et al.16 analyzed whether hypertonic media, salicylates, and tetracycline resistance, which are known to reduce OmpF expression, might also affect the expression of fimbriae and found a reduction of hemoagglutination by P-fimbriated strains using a salicylate concentration of 2.5 mM. They proposed that OmpF may have a role in susceptibility to salicylate-induced effects on fimbrial expression but did not relate MarA to this effect. However, recent studies proposed that the effect of salicylate in OmpF expression is due to the overexpression of the antisense RNA MicF caused by the overexpression of MarA.17 Similarly, type 1 fimbriae expression was affected using a salicylate concentration of 1 mM. Salicylate also appear to inhibit adherence mediated by fimbrial adhesines of EAggEC to Hep-2 cells, although production of outer membrane proteins was not affected.18
The effect on biofilm formation caused by the presence of a mutation in the marR gene observed is in accordance with the results obtained when the strain was grown with salicylate. In E. coli the mar locus is organized into two divergently positioned transcriptional units, marC and marRAB, whose expression is under the control of a centrally collated promoter and mar operator region.19 In the absence of an appropriate stimulus, MarR negatively regulates the marRAB operon by binding to two regions, sites I and II, within marO.19,20 The deletions found in the mutant strain seemed to avoid the binding between MarR and MarO and, therefore, the expression of the marA gene is not regulated, causing an overexpression of this gene similar to what occurred when the salicylate was added to the culture media provoking a decrease in fimA expression and biofilm formation.
The phase variation of type 1 fimbriae is correlated with the inversion of 314-bp DNA sequence immediately upstream of fimA.21 Two elements, FimB and FimE, mapping adjacent to the fim invertible element, are required for the inversion event. It was reported that the relative concentration of these two proteins determine both orientation of the fim invertible element and frequence of inversion, mediating DNA inversion from the off to the on orientation in the case of FimB, and from the on to the off orientation in the case of FimE.22 The decrease in the expression of the fimB gene on growth of the strain in LB with salicylate and in the MarR mutant strain could be the cause of a decrease in the DNA inversion from off to on thereby leading to a decrease in fimA expression. The lack of differences observed with the amplification-restriction of fimA promoter method could be due to the presence of a mixed population of bacteria some of which, the leats, have not affected by salicylate presence at the moment of the experiment.
Another two genes belonging to the mar regulon, ompX and tolC, were studied. OmpX is one of the outer membrane proteins that are present at significantly lower levels in attached cells than in planktonic cells.23 Moreover, ompX belongs to the mar regulon, which is also expressed at a lower level in attached E. coli cells than in planktonic cells.24 Deletion of ompX affected cell-surface interactions of fimbriated and nonfimbriated cells in opposite ways. Indeed, the production of type 1 fimbriae is upregulated in an ompX deficient strain. However, it is not known if the contrary, that is, overexpression of OmpX downregulates type 1 fimbriae, is also true. On the other hand, ompX expression also influences motility and exopolysaccharide production, thereby controlling phenotypes typically altered in surface-associated bacteria.24 Tenorio et al.25 created deletions in several outer membrane proteins including ompX, finding that all of these mutants were able to form biofilm in LB medium. In the present study it was observed that the presence of salicylate in the culture media did not affect ompX expression, although biofilm formation decreased.
TolC is also a member of the mar regulon and has been shown to be repressed in attached cells compared with levels in plantonic cells as in the ompX gene.23 As in the ompX gene, tolC expression was not significantly changed with the presence of salicylate in the culture media neither in the marR mutant. Therefore, the reduction in biofilm formation is due to marA overexpression induced by salicylate causing a decrease in type 1 fimbriae expression and affecting the adhesion step, the first and most important step in biofilm formation.
Barbosa and Levy10 found an increase in the expression levels of the ompX and tolC genes when marA was overexpressed. The lack of differences in the expression of these two genes between the wild-type strain and its marR mutant in our study may be explained by the RNA of the bacteria in the above mentioned study being obtained in the mid-logarithmic phase (0.35 to 0.40) while in our case, the RNA was obtained when the bacteria was close to the stationary phase (0.5 to 0.6).
OmpA is the major outer membrane protein of Gram-negative Enterobacteriaceae and is highly conserved among species.26 It has been reported that OmpA is overexpressed during biofilm formation facilitating the transport of polymeric substances required for the formation of the extracellular matrix which is produced in the biofilm maturation step.27 The fact that salicylate decrease the expression of OmpA provoking a decrease in extracellular matrix production contributes to avoid biofilm formation.
In conclusion, adherence to surfaces through type 1 fimbriae is the most important step in biofilm formation. The expression of these fimbriae is mainly regulated by the level of marA expression through FimB. The effect of salicylate in biofilm reduction could be intensified by the decrease of OmpA expression that causes a decrease in extracellular matrix.
Materials and Methods
Bacterial strain
A biofilm forming UPEC HC91255 clinical isolate was selected. This clinical isolate was collected from a patient with cystitis and presented several virulence factors such as type 1 fimbriae, P-fimbriae, antigen-43, aerobactin and yersiniobactin.
In vitro biofilm assay
Biofilm assay was performed using minimal glucose medium (M63).28 The strains were grown overnight in LB medium at 37°C without shaking. An aliquot (1.25 μl) of the overnight culture was subcultured in 125 μl of M63 medium with 1% of LB in each well of a polystyrene microtiter plate and incubated at 30°C overnight without shaking. Then 1.25 μl of each culture was subcultured again in 125 μl of M63 medium in a new polystyrene microtiter plate and incubated as cited above. After 24 h, the culture was removed from the plate and the biofilm was stained with 175 μl of violet crystal for one minute, washed with 1x PBS, and air-dried for about 1 h. The stain was solubilized in dimethyl sulfoxide (DMSO, D5879-100ML, Sigma) to measure the absorbance at A550 in an automatic spectrophotometer (Anthos Reader 2001, Innogenetics). The result was considered positive when the absorbance was > 4-fold the value obtained in the well containing bacteria free medium. All the assays were performed in duplicate using positive and negative controls.
To analyze the effect of salicylate in biofilm formation, different salicylate concentrations were added to the medium and biofilm was analyzed following the previous protocol.
Protein extraction and analyses
Total protein was extracted by sonication and treatment with 3% SDS (L3771, Sigma), RNAsa and DNAsa. Briefly, 1 ml of a culture with an OD600nm of 0.6 was centrifuged ten minutes and the pellet was homogenized in 300 μl of microccocal nuclease (stock at 10 μg/ml). The sample was sonicated in ice and 30 μl of 3% SDS and RNAsa/DNAsa were added and maintained 15 min at 4°C. After that, rehydratation solution was added (288 mg of urea plus 300 μl of 2x SDS). 2-DE protein analyses were performed following the conditions described elsewhere.29 The protein spot of interest was recovered, digested and sequenced by MALDI-TOF-TOF analysis.
Mutant obtention
A marR mutant of the HC91255 strain was obtained by a multi-step selection process in the presence of ciprofloxacin. The strain was grown at 37°C on McConkey plates with different ciprofloxacin concentrations starting with half of the MIC for the HC91255 strain (MIC to ciprofloxacin 0.004 μg/ml) and increasing 2-fold each step. marA and marR of each mutant obtained in each step were amplified by PCR using the primers marA-f1893 (5′-AGCTTATGACGATGTCCAGACG-3′), marA-r2281 (5′-CTAGAACTAGCTGTTGTAATG-3′), marR-f1452 (5′-TCGTACCAGCGATCTGTTCAATG-3′) and marR-r2229 (5′-GTGGTCATCCGGTATTTATGC-3′).30 The PCR reactions were performed under the following conditions: one cycle of 3 min at 94°C, followed by 30 cycles of 1 min at 94°C, 1 min at 50°C, and 1 min at 72°C. The PCR-products were sequenced and compared with the HC91255 wild-type strain.
Determination of fimA promoter segment orientation
The orientation of the DNA segment containing the fimA promoter was performed using a PCR-based assay.31 Briefly, the switch region was amplified with oligonucleotides OL4 and OL20 to generate a 726 bp DNA product. PCR product were digested with 10 units of BstUI and resolved on 2% agarose gels. The obtaining of two DNA fragments (433 and 293 bp) indicated phase ON whereas two fragments of about 539 and 187 bp indicated phase OFF populations of bacteria.31
RT-PCR
RT-PCR was performed to study the effect of salycilate on expression of marA and other genes belonging (fimA, fimB and fimE, ompX and tolC) or not (gapA) to the MarA regulon. For this purpose, the HC91255 and the marR mutant strain were grown to an OD620nm of 0.5 in Luria-Bertani medium with or without 1 mM salicylate. One milliliter was centrifuged and RNA from the pellet was extracted with TriReagent solution (AM9738, Ambion) following the manufacturer’s instructions, and treated with 1 μl of DNA-free DNase (AM1906, Ambion). RT-PCR was performed using the AccessQuick RT-PCR System (A1702, Promega). Five hundred nanograms of RNA were taken as template. Specific primers were used for the gap gene included as expression control (5′-GTATCAACGGTTTTGGCCG-3′/5′-AGCTTTAGCAGCACCGGTA-3′, 140 bp amplicon), the fimA gene (5′-GGACAGGTTCGTACCGCATC-3′/5′-ACGTTGGTATGACCCGCATC-3′, 250 bp amplicon), the ompX gene (5′-CAGGGCCAAATGAACAAAATG-3′/5′-AGCACCGTAGGAGAAACCGTAGTC-3′, 200 bp amplicon), the marA gene (5′-CATTCATAGCTTTTGGACTGGAT-3′/5′-GTGTAAAAAGCGCGATTCGCC-3′, 220 bp amplicon), the tolC gene (5′-GGGTCAGAACAAAATTGGCGTG-3′/5′-TGATCAGATAGTTATAACGCG-3′, 250 bp amplicon), and the fimB and fimE promoters.32 The PCR reactions were performed under the following conditions: one cycle of 45 min at 45°C and 3 min at 94°C, followed by 30–35 cycles (fimA, ompX, and marA genes) or 16 cycles (gap gene) of 1 min at 94°C, 1 min at 56°C, and 1 min at 72°C. The PCR products were run in commercial acrylamide gels (GeneGel Excel, 17-6000-14, GE Healthcare) and stained with the Plus One DNA Silver staining kit (17-6000-30, GE Healthcare).
Acknowledgments
This work was supported by the projects FIS05/0068 of Fondo de Investigaciones Sanitarias from Ministry of Health of Spain. Thanks to Javier Śnchez-Céspedes for the protein-related protocols. This work has also been supported by funding from the European Community (TROCAR contract HEALTH-F3-2008-223031). S.M.S. is the recipient of a contract from the “Sistema Nacional de Salud-Miguel Servet” (CP05/00140) from Fondo de Investigaciones Sanitarias from Ministry of Health of Spain.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/19205
References
- 1.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–45. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 2.Schembri MA, Kjaergaard K, Klemm P. Global gene expression in Escherichia coli biofilms. Mol Microbiol. 2003;48:253–67. doi: 10.1046/j.1365-2958.2003.03432.x. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomfield IC. The regulation of pap and type 1 fimbriation in Escherichia coli. Adv Microb Physiol. 2001;45:1–49. doi: 10.1016/S0065-2911(01)45001-6. [DOI] [PubMed] [Google Scholar]
- 5.Connell H, Agace W, Hedlund M, Klemm P, Shembri M, Svanborg C. Fimbriae-mediated adherence induces mucosal inflammation and bacterial clearance. Consequences for anti-adhesion therapy. Adv Exp Med Biol. 1996;408:73–80. doi: 10.1007/978-1-4613-0415-9_9. [DOI] [PubMed] [Google Scholar]
- 6.Soto SM, Smithson A, Horcajada JP, Martinez JA, Mensa JP, Vila J. Implication of biofilm formation in the persistence of urinary tract infection caused by uropathogenic Escherichia coli. Clin Microbiol Infect. 2006;12:1034–6. doi: 10.1111/j.1469-0691.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 7.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–7. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 8.Soto SM, Smithson A, Martinez JA, Horcajada JP, Mensa J, Vila J. Biofilm formation in uropathogenic Escherichia coli strains: relationship with prostatitis, urovirulence factors and antimicrobial resistance. J Urol. 2007;177:365–8. doi: 10.1016/j.juro.2006.08.081. [DOI] [PubMed] [Google Scholar]
- 9.Price CT, Lee IR, Gustafson JE. The effects of salicylate on bacteria. Int J Biochem Cell Biol. 2000;32:1029–43. doi: 10.1016/S1357-2725(00)00042-X. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa TM, Levy SB. Differential expression of over 60 chromosomal genes in Escherichia coli by constitutive expression of MarA. J Bacteriol. 2000;182:3467–74. doi: 10.1128/JB.182.12.3467-3474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alekshun MN, Levy SB. The mar regulon: multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 1999;7:410–3. doi: 10.1016/S0966-842X(99)01589-9. [DOI] [PubMed] [Google Scholar]
- 12.Rosner JL, Chai TJ, Foulds J. Regulation of ompF porin expression by salicylate in Escherichia coli. J Bacteriol. 1991;173:5631–8. doi: 10.1128/jb.173.18.5631-5638.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma D, Cook DN, Alberti M, Pon NG, Nikaido H, Hearst JE. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 14.Casaz P, Garrity-Ryan LK, McKenney D, Jackson C, Levy SB, Tanaka SK, et al. MarA, SoxS and Rob function as virulence factors in an Escherichia coli murine model of ascending pyelonephritis. Microbiology. 2006;152:3643–50. doi: 10.1099/mic.0.2006/000604-0. [DOI] [PubMed] [Google Scholar]
- 15.Coll JL, Heyde M, Portalier R. Expression of the nmpC gene of Escherichia coli K-12 is modulated by external pH. Identification of cis-acting regulatory sequences involved in this regulation. Mol Microbiol. 1994;12:83–93. doi: 10.1111/j.1365-2958.1994.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 16.Kunin CM, Hua TH, Guerrant RL, Bakaletz LO. Effect of salicylate, bismuth, osmolytes, and tetracycline resistance on expression of fimbriae by Escherichia coli. Infect Immun. 1994;62:2178–86. doi: 10.1128/iai.62.6.2178-2186.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chubiz LM, Rao CV. Role of the mar-sox-rob regulon in regulating outer membrane porin expression. J Bacteriol. 2011;193:2252–60. doi: 10.1128/JB.01382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang G, Balasubramanian KA, Koshi R, Mathan MM, Mathan VI. Salicylate inhibits fimbriae mediated HEp-2 cell adherence of and haemagglutination by enteroaggregative Escherichia coli. FEMS Microbiol Lett. 1998;166:257–65. doi: 10.1111/j.1574-6968.1998.tb13899.x. [DOI] [PubMed] [Google Scholar]
- 19.Cohen SP, Hächler H, Levy SB. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J Bacteriol. 1993;175:1484–92. doi: 10.1128/jb.175.5.1484-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin RG, Rosner JL. Binding of purified multiple antibiotic-resistance repressor protein (MarR) to mar operator sequences. Proc Natl Acad Sci U S A. 1995;92:5456–60. doi: 10.1073/pnas.92.12.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClain MS, Blomfield IC, Eisenstein BI. Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli. J Bacteriol. 1991;173:5308–14. doi: 10.1128/jb.173.17.5308-5314.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pallesen L, Madsen O, Klemm P. Regulation of the phase switch controlling expression of type 1 fimbriae in Escherichia coli. Mol Microbiol. 1989;3:925–31. doi: 10.1111/j.1365-2958.1989.tb00242.x. [DOI] [PubMed] [Google Scholar]
- 23.Otto K, Norbeck J, Larsson T, Karlsson KA, Hermansson M. Adhesion of type 1-fimbriated Escherichia coli to abiotic surfaces leads to altered composition of outer membrane proteins. J Bacteriol. 2001;183:2445–53. doi: 10.1128/JB.183.8.2445-2453.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otto K, Hermansson M. Inactivation of ompX causes increased interactions of type 1 fimbriated Escherichia coli with abiotic surfaces. J Bacteriol. 2004;186:226–34. doi: 10.1128/JB.186.1.226-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tenorio E, Saeki T, Fujita K, Kitakawa M, Baba T, Mori H, et al. Systematic characterization of Escherichia coli genes/ORFs affecting biofilm formation. FEMS Microbiol Lett. 2003;225:107–14. doi: 10.1016/S0378-1097(03)00507-X. [DOI] [PubMed] [Google Scholar]
- 26.Beher MG, Schnaitman CA, Pugsley AP. Major heat-modifiable outer membrane protein in gram-negative bacteria: comparison with the ompA protein of Escherichia coli. J Bacteriol. 1980;143:906–13. doi: 10.1128/jb.143.2.906-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orme R, Douglas CW, Rimmer S, Webb M. Proteomic analysis of Escherichia coli biofilms reveals the overexpression of the outer membrane protein OmpA. Proteomics. 2006;6:4269–77. doi: 10.1002/pmic.200600193. [DOI] [PubMed] [Google Scholar]
- 28.Danese PN, Pratt LA, Dove SL, Kolter R. The outer membrane protein, antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol Microbiol. 2000;37:424–32. doi: 10.1046/j.1365-2958.2000.02008.x. [DOI] [PubMed] [Google Scholar]
- 29.Martí S, Śnchez-Céspedes J, Oliveira E, Bellido D, Giralt E, Vila J. Proteomic analysis of a fraction enriched in cell envelope proteins of Acinetobacter baumannii. Proteomics. 2006;6(Suppl 1):S82–7. doi: 10.1002/pmic.200500323. [DOI] [PubMed] [Google Scholar]
- 30.Linde HJ, Notka F, Metz M, Kochanowski B, Heisig P, Lehn N. In vivo increase in resistance to ciprofloxacin in Escherichia coli associated with deletion of the C-terminal part of MarR. Antimicrob Agents Chemother. 2000;44:1865–8. doi: 10.1128/AAC.44.7.1865-1868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SG, Dorman CJ. Functional analysis of the FimE integrase of Escherichia coli K-12: isolation of mutant derivatives with altered DNA inversion preferences. Mol Microbiol. 1999;34:965–79. doi: 10.1046/j.1365-2958.1999.01657.x. [DOI] [PubMed] [Google Scholar]
- 32.Xia Y, Gally D, Forsman-Semb K, Uhlin BE. Regulatory cross-talk between adhesin operons in Escherichia coli: inhibition of type 1 fimbriae expression by the PapB protein. EMBO J. 2000;19:1450–7. doi: 10.1093/emboj/19.7.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]