Abstract
Reactive oxygen species (ROS) are produced when plants are exposed to environmental stresses, such as drought and heat conditions. Oxidative stress imposed by ROS under drought conditions profoundly affects plant growth and development. However, ROS production and scavenging mechanisms under adverse environmental conditions are largely unknown. We have recently reported that a NAM/ATAF1/2/CUC2 (NAC) transcription factor NTL4 is required for generation of ROS under drought conditions in Arabidopsis. 35S:4ΔC transgenic plants overexpressing a truncated NTL4 form (4ΔC) lacking the C‑terminal transmembrane (TM) motif were hypersensitive to drought stress, and ROS accumulated to a high level in the transgenic plants. In contrast, NTL4-deficient ntl4 mutants were less sensitive to drought stress and contained reduced levels of ROS. Furthermore, the plasma membrane-associated NTL4 transcription factor is proteolytically activated by treatments with drought and abscisic acid (ABA) and nuclear-localized, where it induces expression of NADPH oxidase genes involved in ROS biosynthesis. Notably, the 35S:4ΔC transgenic plants showed accelerated leaf senescence and cell death under drought conditions. Taken together, these observations indicate that NTL4 regulation of ROS generation underlies the drought-induced leaf senescence.
Keywords: ABA, Arabidopsis, Drought stress, NAM/ATAF1/2/CUC2 (NAC), ROS, leaf senescence
Responses to adverse environmental conditions are regulated by gene regulatory networks, in which transcription factors play a central role in plants. The NAC transcription factors constitute a representative transcription factor family that mediate diverse aspects of biotic and abiotic stress responses in plants.1 Notably, some NAC transcription factors are integrated into the intracellular membranes and proteolytically activated upon exposure to developmental and external stimuli.2,3 At least 13 membrane-bound NAC transcription factors, collectively designated ‘NAC with transmembrane motif 1 (NTM1)-like (NTL)’ members, have been identified in Arabidopsis.4 Several NTL genes, such as NTL4, NTL6, and NTL8, have been shown to mediate diverse environmental stress responses.4
A truncated NTL4 form (4ΔC) lacking the TM motif possesses transcriptional activation activity, and 35S:4ΔC transgenic plants exhibit distinct phenotypes, such as reduced growth and alteration of leaf morphology.5 These observations suggest that the 4ΔC form is closely related with a biologically functional NTL4 form. Notably, the NTL4 gene is significantly induced by drought and heat stresses.5 Diverse plant developmental processes, such as germination and leaf senescence, are profoundly affected by environmental stresses.6,7 We therefore examined the potential role of the NTL4 gene in drought stress responses. Whereas the 35S:4ΔC transgenic plants were susceptible to drought stress, the ntl4 mutants showed enhanced drought resistance, suggesting that NTL4 gene is involved in drought stress responses.5
ROS are toxic materials that cause oxidative damage to DNA, proteins, and lipids. Recent studies have reported that ROS also function as signaling molecules that mediate various physiological processes, including programmed cell death, and defense to biotic and abiotic stresses.8,9 Drought stress stimulates ROS production, inducing programmed cell death in the root tips and enhancing drought tolerance through modification of the root system.10 Increased ROS levels also induce leaf senescence, contributing to the maintenance of water balance within the whole plant and nutrient remobilization to the youngest leaves or sink organs.11
We found that the NTL4 gene is induced in the presence of H2O2, suggesting that the NTL4 gene would be related with ROS response and/or signaling.5 In addition, endogenous contents of ROS were altered in the 35S:4ΔC transgenic plants and ntl4–1 mutants. Under drought conditions, whereas the ROS levels were drastically elevated in the 35S:4ΔC transgenic leaves, they were reduced in the ntl4 mutant leaves.5 These observations support that the NTL4 gene is related with accumulation of ROS under drought conditions.
It has been known that drought stress disrupts the balance between ROS and antioxidant levels, causing ROS accumulation and inducing leaf senescence.11 However, it is unknown how ROS metabolism is linked with the drought-induced leaf senescence. We found that when the pNTL4‑GUS transgenic plants were exposed to water deficit, GUS activity was significantly elevated in the aerial plant parts, primarily in the distal leaf area where leaf senescing process initiates.5,12 In addition, whereas leaf senescence was accelerated in the 35S:4ΔC transgenic plants, it was significantly delayed in the ntl4 mutants under drought conditions.5 Measurements of chlorophyll contents showed that whereas the chlorophyll contents were drastically reduced in the 35S:4ΔC transgenic leaves, they were elevated in the ntl4 mutant leaves compared with those in Col-0 leaves,5 indicating that NTL4 gene is intimately related with leaf senescence occurring under drought conditions.
During the hypersensitive response (HR), including pathogen infection and exposure to high light and water deficit, production of ROS is rapidly elevated in plants.8,10,13 Disruption of ROS metabolism alters a variety of physiological processes, including cell death, leaf senescence, and organ development.8,11 Measurements of cell death by electrolyte leakage assays and trypan blue staining showed that after drought stress treatments, electrolyte leakage was slightly higher in the 35S:4ΔC plants but lower in the ntl4–1 mutants than that in Col-0 plants.5 Consistent with this, the 35S:4ΔC transgenic leaves were strongly stained with trypan blue. In contrast, the ntl4–1 leaves exhibited reduced degree of trypan blue staining. Together, these observations suggest that NTL4 gene is related with cell death during the HR.
A critical question is how NTL4 modulates the ROS accumulation. We examined the expression patterns of genes encoding antioxidant metabolic enzymes, such as VITAMIN C DEFECTIVE 1 (VTC1), VTC2, and CADMIUM SENSITIVE 2 (CAD2). We also employed paraquat that is known to induce ROS generation in plant cells.14,15 Both the 35S:4ΔC transgenic plants and ntl4 mutants exhibited no discernible differences compared with Col-0 plants in the gene expression levels, indicating that regulation of ROS accumulation by NTL4 is not mediated by antioxidant metabolism.5
NADPH oxidase-mediated ROS biosynthesis is important in ROS metabolism.8,16 We found that several NADPH oxidase genes, such as RESPIRATORY BURST OXIDASE HOMOLOG A (AtrbohA), AtrbohC, and AtrbohE, were drastically induced in the 35S:4ΔC transgenic plants but repressed in the ntl4 mutants.5 Chromatin immunoprecipitation (ChIP) assays and transient GUS expression assays showed that the NTL4 transcription factor regulates the Atrboh genes by binding directly to the gene promoters in planta,5 demonstrating that NTL4 regulates ROS production.
ABA is a crucial stress signaling molecule that mediates plant responses to biotic and abiotic stresses and regulates diverse aspects of plant developmental processes, including seed germination, root elongation, and stomatal opening.17-19 It has been known that ABA also plays an important role in ROS metabolism.20 We observed that NTL4 gene is significantly induced by ABA in the aerial plant parts (Fig. 1A). In addition, germination and seedling growth of the 35S:4ΔC transgenic plants are hypersensitive to ABA. In contrast, those of the ntl4 mutants were influenced to a lesser degree by ABA.5 Moreover, the inductive effects of drought on the NTL4 and Atrboh gene expression were lessened in the ABA‑deficient aba3–1 mutants, indicating that the NTL4-mediated drought stress signals depend on ABA.5
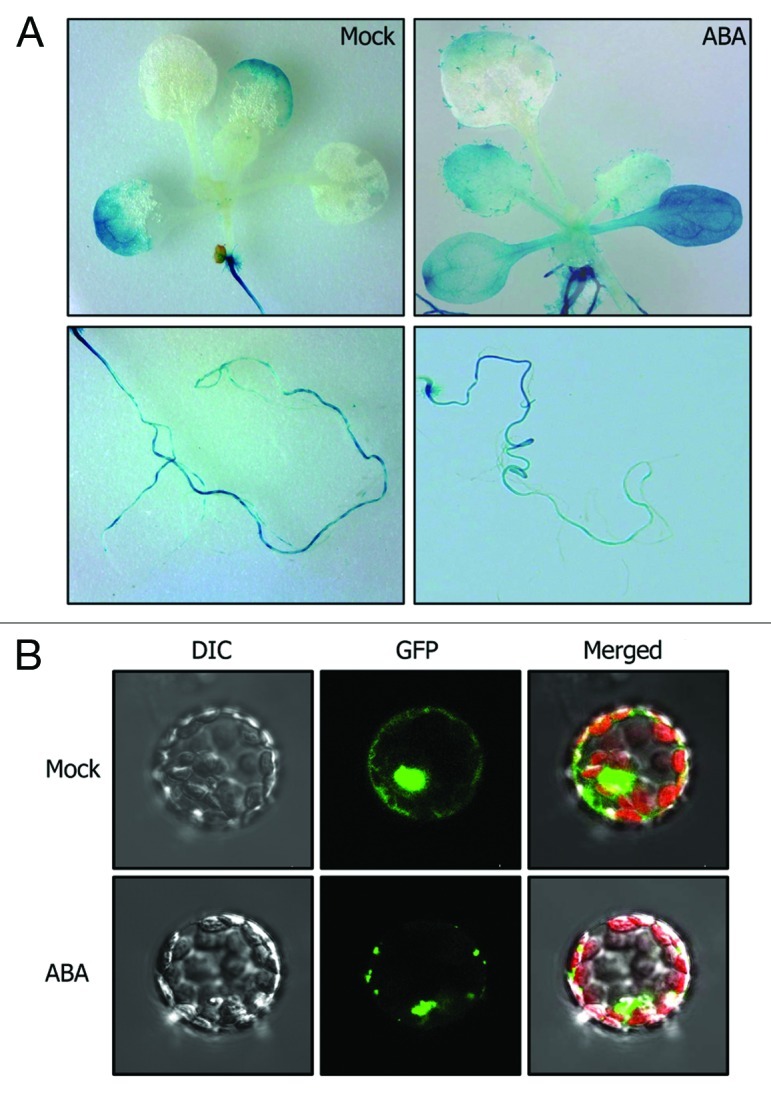
Figure 1. Effects of ABA on NTL4 function. (A) Effects of ABA on NTL4 expression. The GUS-coding sequence was translationally fused to the NTL4 gene with its own promoter consisting of approximately 1 kbp upstream of the transcription start site, and the fusion construct was transformed into Col-0 plants. Two‑week‑old transgenic plants expressing the pNTL4-GUS fusion were incubated in MS liquid cultures supplemented with 20 μM ABA for 24 h and subject to GUS staining. Transgenic plants were fixed in 90% acetone for 20 min on ice and washed twice with rinsing solution [50 mM sodium phosphate, pH 7.2, 0.5 mM K3Fe(CN)6, and 0.5 mM K4Fe(CN)6]. The plant materials were subsequently incubated at 37°C for 24 h in rinsing solution containing 2 mM 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (Duchefa). They were then dehydrated through a series of ethanol dilutions, ranging from 15 to 80%, mounted on slide glasses, and visualized using a DIMIS-M digital camera (JMTECH, Seoul, Korea). (B) ABA-induced nuclear localization of NTL4. The green fluorescence protein (GFP)-coding sequence was fused in-frame to the 5′ end of the NTL4 gene, and the GFP-NTL4 fusion was transiently expressed in Arabidopsis protoplasts prepared as previously described.22 After incubation in WI solution (0.5 M mannitol, 4 mM MES, pH 5.7, 20 mM KCl) with or without 20 μM ABA at room temperature for 24 h, the protoplasts were observed using the Multi-photon Confocal Laser Scanning microscope (LSM510 NLO, Carl Zeiss). The cells were examined by differential interference contrast microscopy (DIC, left panel) and fluorescence microscopy (middle panel). The right panels are merged images.
The NTL4 protein is mainly detected in the plasma membranes and nucleus.21 It has been found that drought stress and ABA stimulated the NTL4 processing.5 Consistent with the ABA activation of the NTL4 processing, the NTL4 protein was detected mostly in the nucleus in ABA-treated Arabidopsis protoplasts transiently expressing the GFP-NTL4 fusion (Fig. 1B). These observations depict that NTL4 function is regulated at the protein processing step by ABA-mediated drought stress signals as well as at the gene transcriptional level.
Interestingly, analysis of NTL4 gene expression and responses of the 35S:4ΔC transgenic plants and ntl4 mutants to pathogen infection suggest that the NTL4 gene may also play a role in disease resistance. The HR to pathogen infection is one of the most extensively studied forms of cell death triggered by ROS in plants. Upon pathogen infection, plants rapidly induce defense mechanisms, such as necrosis and ROS generation at the site of attempted invasion.13 We observed that cell viability and endogenous ROS levels are altered in the 35S:4ΔC transgenic plants and ntl4 mutants, similar to what observed during the HR in infected plants,5 supporting the role of the NTL4 gene in disease resistance response. Heat stress also induces ROS accumulation, and the NTL4 gene is induced under high temperature conditions.5,8 Based on the previous and our own data, we propose that the NTL4-mediated modulation of ROS accumulation is involved in a broad spectrum of plant responses to pathogen infection and environmental stresses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Leaping Research Program (20110016440) provided by the National Research Foundation of Korea, the Next-Generation BioGreen 21 program (Plant Molecular Breeding Center No. PJ008103) provided by the Rural Development Administration, and by grants from the Plant Signaling Network Research Center (20110001099), the National Research Foundation of Korea (20110027355), and the Agricultural R and D Promotion Center (309017-03), Korea Ministry for Food, Agriculture, Forestry and Fisheries. Sangmin Lee is grateful for the Hi Seoul Science Fellowship from Seoul Scholarship Foundation.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19940
References
- 1.Ooka H, Satoh K, Doi K, Nagata T, Otomo Y, Murakami K, et al. Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 2003;10:239–47. doi: 10.1093/dnares/10.6.239. [DOI] [PubMed] [Google Scholar]
- 2.Chen YN, Slabaugh E, Brandizzi F. Membrane-tethered transcription factors in Arabidopsis thaliana: novel regulators in stress response and development. Curr Opin Plant Biol. 2008;11:695–701. doi: 10.1016/j.pbi.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Seo PJ, Kim SG, Park CM. Membrane-bound transcription factors in plants. Trends Plant Sci. 2008;13:550–6. doi: 10.1016/j.tplants.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Kim SY, Kim SG, Kim YS, Seo PJ, Bae M, Yoon HK, et al. Exploring membrane-associated NAC transcription factors in Arabidopsis: implications for membrane biology in genome regulation. Nucleic Acids Res. 2007;35:203–13. doi: 10.1093/nar/gkl1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Seo PJ, Lee HJ, Park CM. A NAC transcription factor NTL4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. Plant J. 2012 doi: 10.1111/j.1365-313X.2012.04932.x. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005;42:567–85. doi: 10.1111/j.1365-313X.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- 7.Park SJ, Kwak KJ, Oh TR, Kim YO, Kang H. Cold shock domain proteins affect seed germination and growth of Arabidopsis thaliana under abiotic stress conditions. Plant Cell Physiol. 2009;50:869–78. doi: 10.1093/pcp/pcp037. [DOI] [PubMed] [Google Scholar]
- 8.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 9.Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, et al. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–9. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Duan Y, Zhang W, Li B, Wang Y, Li K, Sodmergen, et al. An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytol. 2010;186:681–95. doi: 10.1111/j.1469-8137.2010.03207.x. [DOI] [PubMed] [Google Scholar]
- 11.Munné-Bosch S, Alegre L. Die and let live: leaf senescence contributes to plant survival under drought stress. Funct Plant Biol. 2004;31:203–16. doi: 10.1071/FP03236. [DOI] [PubMed] [Google Scholar]
- 12.Lim PO, Kim HJ, Nam HG. Leaf senescence. Annu Rev Plant Biol. 2007;58:115–36. doi: 10.1146/annurev.arplant.57.032905.105316. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–84. doi: 10.1016/S0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- 14.Larkindale J, Hall JD, Knight MR, Vierling E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005;138:882–97. doi: 10.1104/pp.105.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu SH, Noh HN, Kim S, Kim KH, Hong SW, Lee H. Enhanced drought tolerance in Arabidopsis via genetic manipulation aimed at the reduction of glucosamine-induced ROS generation. Plant Mol Biol. 2010;74:493–502. doi: 10.1007/s11103-010-9691-7. [DOI] [PubMed] [Google Scholar]
- 16.Sagi M, Fluhr R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006;141:336–40. doi: 10.1104/pp.106.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharp RE, LeNoble ME. ABA, ethylene and the control of shoot and root growth under water stress. J Exp Bot. 2002;53:33–7. doi: 10.1093/jexbot/53.366.33. [DOI] [PubMed] [Google Scholar]
- 18.Himmelbach A, Yang Y, Grill E. Relay and control of abscisic acid signaling. Curr Opin Plant Biol. 2003;6:470–9. doi: 10.1016/S1369-5266(03)00090-6. [DOI] [PubMed] [Google Scholar]
- 19.Acharya BR, Assmann SM. Hormone interactions in stomatal function. Plant Mol Biol. 2009;69:451–62. doi: 10.1007/s11103-008-9427-0. [DOI] [PubMed] [Google Scholar]
- 20.Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, et al. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–33. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SG, Lee S, Ryu J, Park CM. Probing protein structural requirements for activation of membrane-bound NAC transcription factors in Arabidopsis and rice. Plant Sci. 2010;178:239–44. doi: 10.1016/j.plantsci.2009.12.007. [DOI] [Google Scholar]
- 22.Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2:1565–72. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]


