Abstract
Progeny from diseased Arabidopsis shows enhanced resistance, which is associated with priming of defense genes.1 This transgenerational systemic acquired resistance (SAR) is effective against biotrophic pathogens, such as the downy mildew pathogen Hyaloperonospora arabidopsidis. In this study, we have examined mutants in RNA-directed DNA methylation (RdDM) for transgenerational SAR. Our analysis suggests that transgenerational SAR is regulated by the RdDM pathway and transmitted by hypomethylation at CpNpG sites.
Keywords: DNA methylation, RNA-directed DNA methylation, chromatin remodelling, priming of defence, transgenerational systemic acquired resistance
Priming of defense is an adjustment of the plant’s immune system, which results in a faster and stronger activation of inducible defense mechanisms after exposure to environmental stress.2-4 A well-characterized form of defense priming takes place during expression of systemic acquired resistance (SAR). This resistance response is effective against biotrophic pathogens, requires regulation by the defense regulatory gene NON EXPRESSOR OF PR GENES (NPR1), and is associated with priming of salicylic acid (SA)-dependent genes.5,6 Recent evidence suggests that priming of SA-inducible genes involves epigenetic regulatory mechanisms, such as post-translational modifications of histone proteins and the RNA Polymerase V.7,8
We recently demonstrated that progeny from Pseudomonas syringae pv Tomato (Pst) DC3000-infected Arabidopsis (P1 progeny) are primed for SA-dependent defense compared with progeny from control-treated healthy plants (C1 progeny). We named this phenomenon “transgenerational SAR,” since the resistance in P1 progeny is effective against (hemi)-biotrophic pathogens, it requires NPR1, and it is associated with priming of SA-inducible defense genes.1 In the same journal issue, two complementary publications demonstrated transgenerational defense priming upon exposure to herbivory and the chemical priming agent β-aminobutyric acid.9,10 Transgenerational SAR in P1 progeny from diseased plants was associated with increased levels of acetylated histone 3 at lysine 9 (H3K9) at SA-inducible gene promoters1, a chromatin mark that is associated with a permissive state of transcription.11 Moreover, the drm1drm2cmt3 (ddc) triple mutant, which is affected in non-CpG DNA methylation,12 mimicked the transgenerational SAR phenotype.1 Since infection by PstDC3000 induces DNA hypomethylation in Arabidopsis,13 we hypothesized that transgenerational SAR is transmitted through DNA hypomethylation at non-CpG sites.
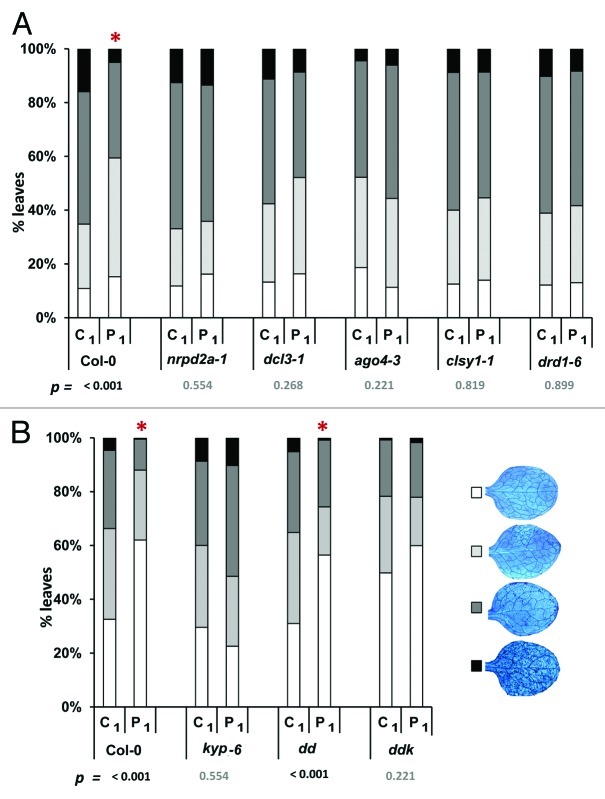
In this study, we have tested transgenerational SAR phenotypes of various Arabidopsis mutants in the RNA-directed DNA methylation (RdDM) pathway (Figs. 1 and 2). Parental plants were repeatedly inoculated with either mock solution, or a suspension containing PstDC3000 bacteria, and allowed to set seeds. At least three independent C1 and P1 progenies of each line were tested for resistance against the downy mildew pathogen Hyaloperonospora arabidopsidis, as described before.1 The results of these bioassays are summarized in Figure 2.
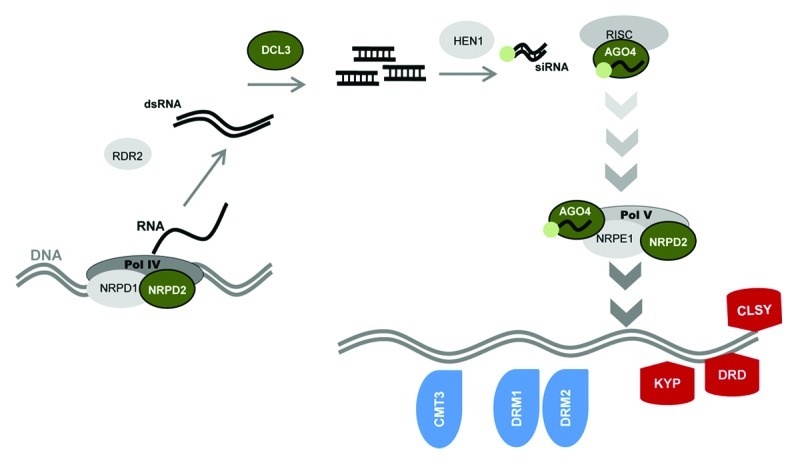
Figure 1. Model of the RNA-directed DNA methylation (RdDM) pathway, adapted from.25,26 Multi-subunit DNA-dependent RNA Polymerase IV (Pol IV) generates single stranded RNAs, which are used as a template by RNA-dependent RNA polymerase 2 (RDR2) to generate double-stranded RNAs (dsRNAs). DICER-LIKE 3 (DCL3) processes dsRNAs into 24 nucleotide RNAs that become methylated by the RNA-methyltransferase HEN1 and subsequently recruited by the AGO4-RISC complex to targeted genomic sites via interaction with the DNA-dependent RNA Polymerase V (Pol V). Pol V is required for DNA methyltransferase activity by DRM1, DRM2 and CMT3 and chromatin remodelling enzymes DRD, CLSY and KYP. The RdDM pathway targets cytosine (C) methylation at non-CpG sites. Signaling components affected in the mutants tested for transgenerational SAR are shown in color; green: enzymes in the siRNA machinery; red: chromatin remodelling enzymes; blue: DNA methyltransferase enzymes.
Figure 2. Transgenerational SAR against Hyaloperonospora arabidopsidis in mutants of Arabidopsis that are impaired in RNA-directed DNA methylation: nrpd2a-1, dcl3–1, ago4–3, clsy1–1 and drd1–6 (experiment A), and kyp-6, drm1drm2 (dd) and drm1drm2kyp (ddk) (experiment B). Induction of transgenerational SAR and challenge inoculation with H. arabidopsidis were performed as described.1 At 6 d after conidiospore inoculation, stained leaves were microscopically examined and assigned to different classes, as described.1 Asterisks and P values at the bottom of the graphs indicate statistical differences in class distributions between C1 and P1 progenies of each genotype (χ2 Test).
P1 progeny from the RdDM pathway mutants ago4-3,14 clsy1-1,15 nrpd2a-1,16 drd1-617 and dcl3-118 failed to express increased resistance in comparison to their C1 progenies. Furthermore, C1 progeny of the ago4-3 mutant showed constitutively enhanced resistance in comparison to C1 progeny of wild-type plants (χ2 = 14.2; p = 0.002). Since mutations in AGO4 cause reduced levels of non-CpG DNA methylation,19 the phenotype of the ago4-3 mutant supports our hypothesis that hypomethylation at non-CpG sites transmits SAR. Surprisingly, however, C1 progeny from the other RdDM mutants displayed similar levels of susceptibility as C1 progeny from wild-type plants. Considering their inability to express transgenerational SAR, these phenotypes suggest positive regulation by the corresponding RdDM components. In a second experiment, we tested additional Arabidopsis mutants impaired in non-CpG DNA methyltransferase activity. As observed for the ago4-3 mutant and ddc triple mutant,1 C1 and P1 progenies from the drm1drm2kyp (ddk) triple mutant12 showed no difference in resistance to H. arabidopsidis, while the C1 progeny of this triple mutant expressed elevated levels of basal resistance in comparison to C1 progeny from wild-type plants (χ2 = 17.31; p = 0.001). By contrast, the drm1drm2 (dd) double mutant, which is specifically impaired in asymmetric CpHpH DNA methylation,12 displayed a wild-type phenotype and was unaffected in transgenerational SAR and basal resistance. Finally, the kyp-612 single mutant, which is specifically affected in CpNpG methylation,20 resembled the defense phenotype of clsy1-1, nrpd2a-1, drd1-6, and dcl3-1: there was no significant difference in resistance between C1 and P1 progenies, and C1 progeny of this mutant displayed similar levels of susceptibility as C1 progeny from wild-type plants.
It is clear from the data presented in this communication that transgenerational SAR in Arabidopsis involves regulation by the RdDM pathway. The exact role of each individual RdDM component remains difficult to decipher on the basis of the presented disease phenotypes. Rasmann et al., (2012) used two RdDM pathway Arabidopsis mutants to assess the contribution of siRNAs in transgenerational priming of jasmonic-acid (JA)-dependent defense against herbivory9: the nrpd2a nrpd2b double mutant21 and the dcl2dcl3dcl4 triple mutant.22 Progenies from healthy, herbivore-exposed and jasmonic acid-treated plants of these mutants failed to show differences in resistance against the specialist herbivore Pieris rapae, suggesting a critical role for the RdDM pathway in transgenerational priming of JA-dependent defenses. Hence, the RdDM pathway controls trans-generational priming of both JA- and SA-dependent defenses. We therefore propose that the RdDM pathway provides the machinery for different transgenerational defense responses, while the specificity of the response is determined by the 24-nucleotide sequence of the siRNAs.
Our findings that the dd double mutant expressed a wild-type phenotype (Fig. 2), whereas C1 and P1 progenies of the ddk and ddc triple mutant display similar levels of constitutively enhanced resistance (Fig. 2;1), points to a critical role for KYP- and CMT3-dependent DNA methylation. KYP directs CMT3 activity through methylation of the lysine 9 residue of histone H3.23 Its involvement in transgenerational SAR supports our previous observation that transgenerational SAR is marked by increased acetylation of H3K9 at SA-inducible gene promoters.1 Since KYP and CMT3 predominantly mediate cytosine methylation at symmetrical CpNpG sites,24 we conclude that transgenerational SAR is transmitted by DNA hypomethylation at CpNpG sites.
Acknowledgments
We thank D. Baulcombe for providing ago4-3, nrpd2a-1, dcl3-1, clsy1-1 and drd1-6 mutant lines and helpful comments. This work was supported by a Biotechnology and Biological Sciences Research Council-Institute Career Path Fellowship to J.T (grant no. BB/E023959/1) and a grant from the Felix Thornley Cobbold Trust to J.T. and E.L.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20155
References
- 1.Luna E, Bruce TJA, Roberts MR, Flors V, Ton J. Next-generation systemic acquired resistance. Plant Physiol. 2012;158:844–53. doi: 10.1104/pp.111.187468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad S, Gordon-Weeks R, Pickett J, Ton J. Natural variation in priming of basal resistance: from evolutionary origin to agricultural exploitation. Mol Plant Pathol. 2010;11:817–27. doi: 10.1111/j.1364-3703.2010.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conrath U. Molecular aspects of defence priming. Trends Plant Sci. 2011;16:524–31. doi: 10.1016/j.tplants.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Conrath U, Beckers GJ, Flors V, García-Agustín P, Jakab G, Mauch F, et al. Prime-A-Plant Group Priming: getting ready for battle. Mol Plant Microbe Interact. 2006;19:1062–71. doi: 10.1094/MPMI-19-1062. [DOI] [PubMed] [Google Scholar]
- 5.Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT. Priming in systemic plant immunity. Science. 2009;324:89–91. doi: 10.1126/science.1170025. [DOI] [PubMed] [Google Scholar]
- 6.Kohler A, Schwindling S, Conrath U. Benzothiadiazole-induced priming for potentiated responses to pathogen infection, wounding, and infiltration of water into leaves requires the NPR1/NIM1 gene in Arabidopsis. Plant Physiol. 2002;128:1046–56. doi: 10.1104/pp.010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaskiewicz M, Conrath U, Peterhänsel C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 2011;12:50–5. doi: 10.1038/embor.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López A, Ramírez V, García-Andrade J, Flors V, Vera P. The RNA silencing enzyme RNA polymerase v is required for plant immunity. PLoS Genet. 2011;7:e1002434. doi: 10.1371/journal.pgen.1002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmann S, De Vos M, Casteel CL, Tian D, Halitschke R, Sun JY, et al. Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol. 2012;158:854–63. doi: 10.1104/pp.111.187831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slaughter A, Daniel X, Flors V, Luna E, Hohn B, Mauch-Mani B. Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol. 2012;158:835–43. doi: 10.1104/pp.111.191593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, Wang X, He K, Charron JB, Elling AA, Deng XW. Genome-wide profiling of histone H3 lysine 9 acetylation and dimethylation in Arabidopsis reveals correlation between multiple histone marks and gene expression. Plant Mol Biol. 2010;72:585–95. doi: 10.1007/s11103-009-9594-7. [DOI] [PubMed] [Google Scholar]
- 12.Henderson IR, Jacobsen SE. Tandem repeats upstream of the Arabidopsis endogene SDC recruit non-CG DNA methylation and initiate siRNA spreading. Genes Dev. 2008;22:1597–606. doi: 10.1101/gad.1667808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavet V, Quintero C, Cecchini NM, Rosa AL, Alvarez ME. Arabidopsis displays centromeric DNA hypomethylation and cytological alterations of heterochromatin upon attack by pseudomonas syringae. Mol Plant Microbe Interact. 2006;19:577–87. doi: 10.1094/MPMI-19-0577. [DOI] [PubMed] [Google Scholar]
- 14.Havecker ER, Wallbridge LM, Hardcastle TJ, Bush MS, Kelly KA, Dunn RM, et al. The Arabidopsis RNA-directed DNA methylation argonautes functionally diverge based on their expression and interaction with target loci. Plant Cell. 2010;22:321–34. doi: 10.1105/tpc.109.072199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith LM, Pontes O, Searle I, Yelina N, Yousafzai FK, Herr AJ, et al. An SNF2 protein associated with nuclear RNA silencing and the spread of a silencing signal between cells in Arabidopsis. Plant Cell. 2007;19:1507–21. doi: 10.1105/tpc.107.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onodera Y, Haag JR, Ream T, Costa Nunes P, Pontes O, Pikaard CS. Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell. 2005;120:613–22. doi: 10.1016/j.cell.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Kanno T, Huettel B, Mette MF, Aufsatz W, Jaligot E, Daxinger L, et al. Atypical RNA polymerase subunits required for RNA-directed DNA methylation. Nat Genet. 2005;37:761–5. doi: 10.1038/ng1580. [DOI] [PubMed] [Google Scholar]
- 18.Xie Z, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, et al. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004;2:E104. doi: 10.1371/journal.pbio.0020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zilberman D, Cao X, Jacobsen SE. ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science. 2003;299:716–9. doi: 10.1126/science.1079695. [DOI] [PubMed] [Google Scholar]
- 20.Jackson JP, Lindroth AM, Cao X, Jacobsen SE. Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature. 2002;416:556–60. doi: 10.1038/nature731. [DOI] [PubMed] [Google Scholar]
- 21.Pontes O, Li CF, Costa Nunes P, Haag J, Ream T, Vitins A, et al. The Arabidopsis chromatin-modifying nuclear siRNA pathway involves a nucleolar RNA processing center. Cell. 2006;126:79–92. doi: 10.1016/j.cell.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Henderson IR, Zhang X, Lu C, Johnson L, Meyers BC, Green PJ, et al. Dissecting Arabidopsis thaliana DICER function in small RNA processing, gene silencing and DNA methylation patterning. Nat Genet. 2006;38:721–5. doi: 10.1038/ng1804. [DOI] [PubMed] [Google Scholar]
- 23.Chan SWL, Henderson IR, Zhang X, Shah G, Chien JSC, Jacobsen SE. RNAi, DRD1, and histone methylation actively target developmentally important non-CG DNA methylation in arabidopsis. PLoS Genet. 2006;2:e83. doi: 10.1371/journal.pgen.0020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindroth AM, Cao X, Jackson JP, Zilberman D, McCallum CM, Henikoff S, et al. Requirement of CHROMOMETHYLASE3 for maintenance of CpXpG methylation. Science. 2001;292:2077–80. doi: 10.1126/science.1059745. [DOI] [PubMed] [Google Scholar]
- 25.Agorio A, Vera P. ARGONAUTE4 is required for resistance to Pseudomonas syringae in Arabidopsis. Plant Cell. 2007;19:3778–90. doi: 10.1105/tpc.107.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haag JR, Pikaard CS. Multisubunit RNA polymerases IV and V: purveyors of non-coding RNA for plant gene silencing. Nat Rev Mol Cell Biol. 2011;12:483–92. doi: 10.1038/nrm3152. [DOI] [PubMed] [Google Scholar]