Abstract
In this paper we demonstrate how peroxidase (PO) activities and their heat stability correlate with the availability of free Ca2+ ions. Calcium ions work as a molecular switch for PO activity and exert a protective function, rendering POs heat stable. The concentration ranges of these two activities differ markedly. POs are activated by µM Ca2+ concentration ranges, whereas heat stabilization is observed in the nM range. This suggests the existence of different Ca2+ binding sites. The heat stability of POs depends on the source plant species. Terrestrial plants have POs that exhibit higher temperature stability than those POs from limnic and marine plants. Different POs from a single species can differ in terms of heat stability. The abundance of different POs within a plant is dependent on age and developmental stage. The heat stability of a PO does not necessarily correlate with the maximum temperature the source species is usually exposed to in its natural habitat. This raises questions on the role of POs in the heat tolerance of plants. Consequently, detailed investigations are needed to identify and characterize individual POs, with regard to their genetic origin, subcellular expression, tissue abundance, developmental emergence and their functions in innate and acquired heat tolerance.
Keywords: Apoplast, calcium, heat stability, peroxidase
Introduction
Peroxidase activities in plants
Peroxidases are widely distributed among living organisms. In plants, peroxidases fulfil many different physiological functions.1-3 In particular, class III peroxidases are assumed to enable plants to withstand biotic stress situations and pathogen attack.4 However, the diversity of their functions is not yet completely clear,5 as genetic approaches using either knock down or overexpression often do not lead to distinct phenotypes.6 Furthermore the recombinant expression and purification of individual peroxidases has proved to be challenging.7-9 Therefore, the isolation, quantification, and characterization of expressed and active peroxidases from source plant material may help to clarify the diversity of their functions.
Many peroxidases transfer electrons (Figs. 1A and B) from organic donor substrates to hydrogen peroxide (H2O2) which is then converted into water.6,10 Consequently, a pivotal task of many peroxidases is to catalyze the removal of H2O2.11,12 Thus, peroxidases are considered part of the antioxidative system.13 Some peroxidases accept a broad spectrum of electron donor substrates11 including artificial compounds. Luminol, for instance, gives off chemiluminescence when converted by peroxidases and can therefore be used to quantify peroxidase activity in vitro (Fig. 1C).
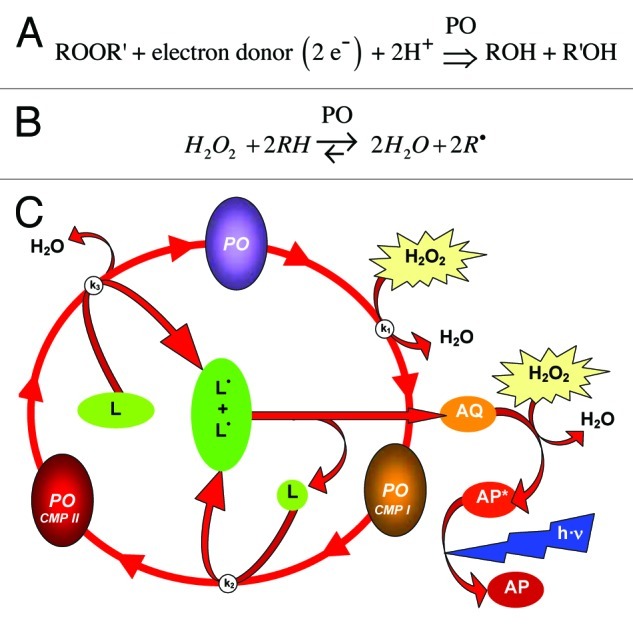
Figure 1. The peroxidase (PO) reaction and chemiluminescence produced by luminol converting peroxidases (LUPOs). (A) The general peroxidase reaction is a transfer of electrons from an organic electron donor substrate to a peroxide ROOR'. (B) Usually H2O2 serves as specific electron acceptor and the organic substrate is converted into its radical form. (C) Luminol (L) is accepted as a substrate by many plant peroxidases (PO) and converted to its radical L•. A di-aza-quinone (AQ) is formed as intermediate from luminol radical (L•). This in turn is oxidised by hydrogen peroxide (H2O2) to form an excited state of aminophtalate (AP*). The final step is the emission of blue (420 nm) light (h·ν) when the excited AP* relaxes to its ground state. This light generating process is used to quantify the peroxidase activity. B is adapted from69 and C is from supplements of31
A direct dependence of peroxidase activity on free Ca2+-ion concentration and divalent buffers has not yet been demonstrated. However, it is well known that peroxidase activity is strongly dependent on the presence of calcium ions14,15 and consequently on cation binding by the divalent buffer. Calcium is essential for horseraddish peroxidase (HRP) activity16,17 and other peroxidases have been shown to bind Ca2+ ions.14,18 Some peroxidases even have a calmodulin binding domain14,19 and calcium may switch a peroxidase between different modes of action.20 In addition, apoplastic peroxidases are anchored in the cell wall matrix by calcium-dependent binding to pectate (i.e., polygalacturonic acid).7,8,21,22
Peroxidases and their innate heat tolerance
Peroxidases are among the most heat stable plant enzymes.23-26 The date palm (Phoeniyx dactylifera), for instance, which grows in the summer under extremely high day temperatures, was shown to possess very heat stable peroxidases.27 The activity-temperature curve in this latter study suggests an effective inhibition temperature (ET50) of above 75°C. The peroxidase from soybean (SBP), also a species well adapted to high temperatures, is only slightly impaired at 80°C.28 From these data an ET50 > 90°C can be assumed for SBP. This correlates with circular dichroism data which show that the SBP-protein denatures at about 90°C.
Class III peroxidases have evolved within the land plants since their separation from aquatic plants more than 500 MY ago29 and evolution has maintained and improved their heat stability. This suggests that peroxidases constitute elements of plant heat tolerance and possibly of drought resistance. Since these peroxidases are dependent on calcium ions as well as on H2O2, they may function as indispensable nodes of a signaling network which employs these two known signaling molecules for stress defense.19,30
In this study we have extracted peroxidases from a range of different plant sources and investigated their heat stability and dependency on calcium using a previously established in vitro assay.31 We demonstrate that enzymes of similar function in plant species from natural habitats with varying maximum day temperatures exhibit different thermostability and different calcium responsiveness.
Results
Peroxidase activity strongly depends on calcium
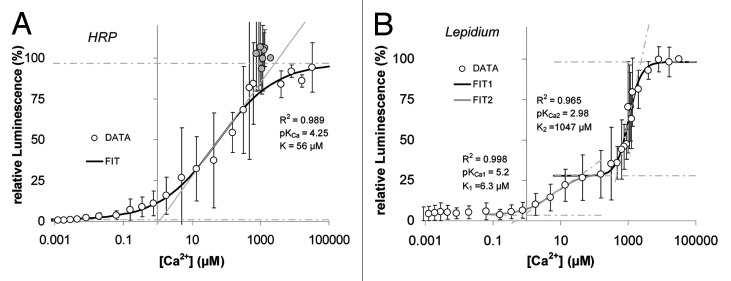
As mentioned above, peroxidases have calcium ions bound. In the absence of calcium, peroxidases are effectively inactive. This is shown in Figure 2. The titration of calcium-free peroxidases with increasing concentrations of calcium enhances their activity. Horseradish peroxidase (HRP) shifts its activity at [Ca2+] of 56 µM. (Fig. 2A). For Lepidium POs the maximum increase is at a calcium concentration of 1 mM (Fig. 2B).
Figure 2. Influence of calcium ions on the activity of peroxidases. Peroxidase activity was measured by luminol luminescence in the presence of different free calcium ion concentrations. (A) For Horseradish peroxidase (HRP) the maximum increase of activity was observed in the range of 10 µM < [Ca2+]free < 1000 µM. Here, the activity increases from 2% to 95%. However, there is a maximum activity at 1000µM. Luminescence is expressed relative to luminescence from samples assayed at [Ca2+] = 2 mM. Gray datapoints were disregarded for the sigmoidal curve fit. (B) For Lepidium peroxidase the increase of activity is biphasic. In the range of 1 µM < [Ca2+]free < 100 µM activity increases from to 27% (green shaded area). The maximum increase is in the range 400 µM < [Ca2+]free < 2400 µM (yellow shaded area). Here, the activity increases from 27% to 100%. The relative luminescence refers to samples assayed at [Ca2+] = 32 mM. Free calcium ion concentrations were titrated with a mix of different chelators. MaxChelator68 was employed to calculate the free Ca2+ ion concentrations. Means of n = 6 individual measurements are shown; Error bars represent StDv.
Peroxidase activity is irreversibly inhibited by high temperatures
On a sunny day, plants may be exposed to high temperatures for several hours. A previous study31 demonstrated that peroxidases from Lepidium are heat inactivated in vivo during heat shock (42°C; 6 h).
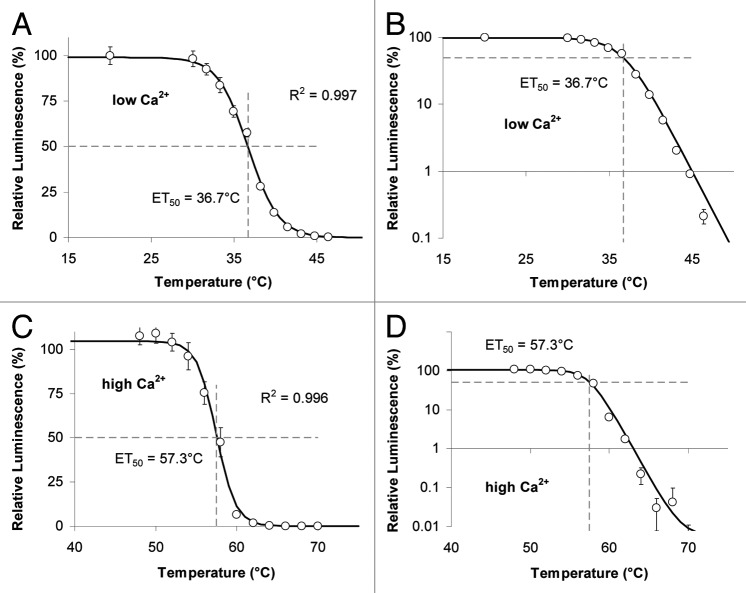
The temperature of 50% peroxidase inhibition (ET50 = 36.7°C) is remarkably low (Fig. 3A and B) with Lepidium. However, in this experiment31 the temperature inhibition of the enzyme was performed in a phosphate buffer, where the free calcium ion concentration is very low. When the same experiment is performed in a buffer with a sufficiently high calcium concentration then the ET50 is drastically increased (Fig. 3C and D). Thus, calcium not only activates (Fig. 2) but also stabilizes the peroxidase protein (Fig. 3 and S1). This phenomenon has also been reported by other workers32 and can be observed for POs from many other higher plant species (Table S1).
Figure 3. Heat sensitivity of LUPOs from Lepidium. (A) Peroxidases from Lepidium dissolved in a phosphate buffer of low [Ca2+] were treated with the temperatures indicated before the light yield of the H2O2-luminol reaction was measured. The temperature of 50% inactivation (ET50 = 36.7°C) is the curve's point of inflexion. (B) Data from A are plotted on a log scale to show that the ET50 is identical to the point of maximal curve bending. Data given are means of n = 5. Data are taken from.31 (C) The same experiment was performed as described in A, however this time peroxidases were kept in TriCaT-buffer (high [Ca2+]) during heat treatment. The temperature of 50% inactivation is 57.4°C. Means of n = 3 individual measurements are shown. (D) Data from C plotted on a log scale. Luminescence is expressed relative to luminescence from samples kept at RT (≈20°C). R2 are correlation coefficients obtained from curve fitting. Error bars represent StDv or are below symbol size.
A more detailed experiment revealed that for luminol converting peroxidases (LUPOs) from Lepidium the dependence of heat stability (ET50) on calcium (Fig. 4) is different from the calcium-dependency of its activity (Fig. 2). Within the range of 0.1 µM < [Ca2+]free < 10 µM Lepidium LUPOs switch from heat sensitive to heat stable, whereas the main shift in activity is around [Ca2+]free = 1000 µM (Fig. 2B).
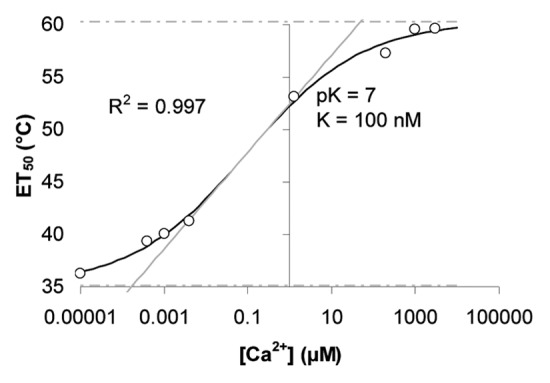
Figure 4. Calcium dependency of heat sensitivity of LUPOs from Lepidium. Peroxidases from Lepidium dissolved in buffers of different [Ca2+]free as indicated on the X-axis were treated with a range of different temperatures (as in Figure 3). The remaining activity after heat treatment was measured and the ET50 was evaluated for each calcium concentration. Here, ET50 is plotted against the corresponding [Ca2+]free and a curve was fitted to the data by sigmoidal curve fitting.
Generally, it can be assumed that mesophilic plants (i.e., plants thriving below 40°C) have low innate heat tolerance compared with thermophilic plants (plants thriving above 40°C). If this assumption is also true for the respective POs, then it can be argued that there may be a relationship between the heat-stability of a PO and the innate heat tolerance of the species harbouring this enzyme. This was tested by investigating the heat stabilities of POs from a range of different plant species.
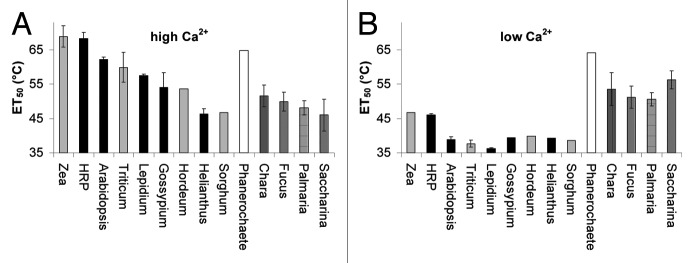
Among vascular plants the results (Fig. 5 and Table S1) appear inconsistent, since species usually well adapted to high temperatures (e.g., Gossypium, Zea, Sorghum) and commonly growing in the plant heat-zones # 8 to # 1233 do not always display POs of higher ET50 compared with plants usually growing in the moderate heat-zones #3 to #7 (e.g., Lepidium, Armoracia, Hordeum). However, when cryptogam species are also considered, a trend becomes obvious. Cryptogams have POs of low heat stability while POs from phanerogams are more heat stable. There is no correlation between heat stability (ET50) at low Ca2+ and high Ca2+ concentrations (data shown in Figure 5 and Table S1 give a correlation coefficient of R2 = 0.014 between ET50 at lowCa2+ and ET50 at highCa2+).
Figure 5. Heat stability of peroxidases from different plant species. The temperatures leading to 50% irreversible LUPO inhibition (ET50) of different plants species under high and under low Ca2+ conditions are given in (A) and (B), respectively. 'High [Ca2+]' designates buffer conditions with a minimum of 1 mM free Ca2+ during heating. 'Low [Ca2+]' designates buffer conditions with free Ca2+ below 0.1 µM during heating. The LUPO activity assays were performed for all samples at RT and with high [Ca2+]. Data are also presented as supplemental Table S1. The means of at least three independent replicates are shown where standard deviations are indicated by errorbars.
Plants harbour a variety of POs with different molecular mass and different heat sensitivity
The heat-inactivation curve of POs from maize (Fig. 6) is biphasic. At ET50(#1) = 48.6°C the activity in the samples decreases to about 63% of the 20°C control and at ET50(#2) = 70.4°C a further reduction is seen. This suggests the presence of at least two different POs in the sample preparation.
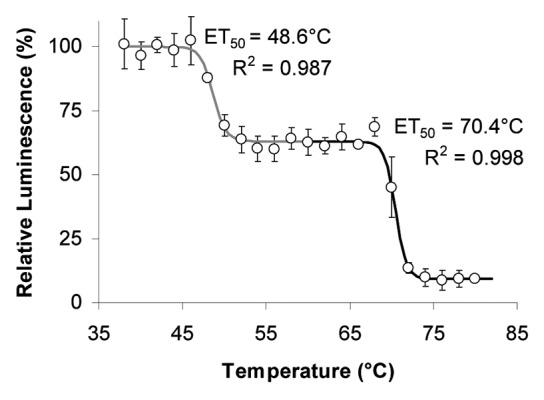
Figure 6. Effect of temperature on LUPOs from maize. LUPOs from 3-week old Zea mays at high [Ca2+]free were treated in vitro with the temperatures indicated by the abscissa before light yield of the H2O2-luminol reaction was measured. The curve is biphasic and thus reveals the presence of two POs with different ET50. Means of n = 3 individual measurements are shown. Error bars represent StDv or are below symbol size.
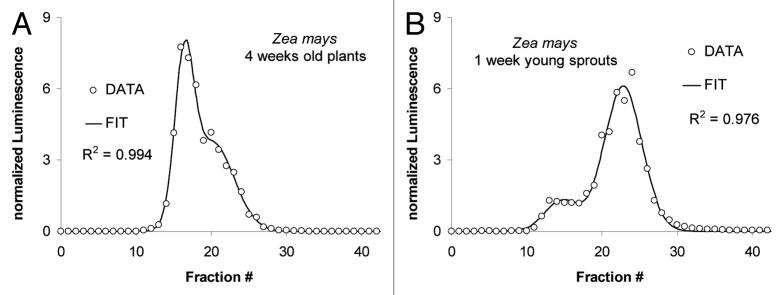
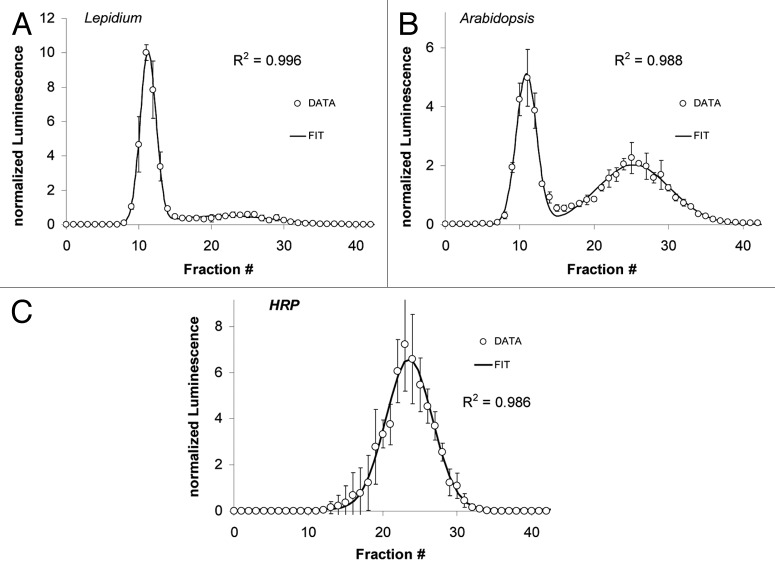
Fractionation of the maize extract by gel filtration reveals two distinct peaks of PO activity (Fig. 7) representing low and high molecular mass species. However, the relative abundance of the two activities depends on the developmental stage. Younger plants have more low molecular-weight peroxidases (Fractions #20 to #30; Figure 7B) relative to the high molecular-weight fractions (#14 to #19) when compared with older plants (Fig. 7A).
Figure 7. Dependence of relative PO size distribution on the developmental stage of maize. (A) LUPOs from 4-weeks-old maize plants. (B) LUPOs from 1-week-young maize sprouts. LUPO activity is plotted against the fraction number. Fraction # '0' is collected during sample application. Luminescence was normalized to the mean luminescence of all fractions (i.e., the area under the curve). A double-peak Gaussian distribution (black curve) was fitted to the data with correlation coefficients indicated in the panels. The light PO (Fractions 18–25 correspond to approx. 40–50kDa as can be estimated from from Figure 8C. The heavy Zea-PO (Fractions 13–17) is about the double size.
Most of the experiments in this study were performed with extracts of garden cress (Lepidium) and in this case (Figs. 2–4) no biphasic temperature inactivation curve was found. This is in line with the finding that in Lepidium seedlings one main peak of PO activity is observed after gel filtration (Fig. 8A). Arabidopsis, in contrast, harbours two PO-species which can be well separated (Fig. 8B). When the separated POs from Arabidopsis are individually studied in terms of heat stability, two different ET50-values are obtained (Table S1). The low molecular weight POs have an ET50 of 62.2°C while the high molecular weight ones are inactivated at ET50 = 58.3°C.
Figure 8. Characterization of peroxidase size distribution in samples from Lepidium and Arabidopsis using gel filtration chromatography. LUPO activity is plotted against the fraction number. Lepidium mainly harbours peroxidases of one molecular size (A), whereas in Arabidopsis two well separating peroxidases are found (B). The elution profile of peroxidase from Armoracia rusticana (Horseradish peroxidase; HRP - Sigma #P6140; MW ≈40kDa) is shown for comparison (C). Luminescence was normalized to the mean luminescence of all fractions. Data (circles) are averages of n ≥ 3 experiments. Gaussian distributions (black curve) have been fitted to the data with the correlation coefficients R2 indicated in the panels. Error bars represent standard deviations.
Nevertheless, samples from Arabidopsis have no biphasic heat-inactivation curve (Fig. S1A) due to the fact that the ET50 of both PO species are too close together and cannot be resolved by a single heat-inactivation experiment. This shows that a heat inactivation curve with a single ET50 does not necessarily indicate a single PO species in the sample.
Discussion
Peroxidase activities in plants under heat stress
Abiotic stress applied under long-term experimental conditions generally causes an increase in peroxidase activity in plants.34 Consequently, peroxidases have been regarded as stress indicators.35 In the case of heat stress the increase in PO activity is dependent on the time period during which the plant can adapt. Moderate heat leads to a PO activity increase.36 However, peroxidase activity decreased when plants were exposed to heat shock during short-term experiments and without pre-conditioning.31 This heat-induced decrease appeared to be caused by direct thermal inactivation of the peroxidases. This finding prompted further investigations on different plant species (Fig. 3–8 and Table S1).
Are peroxidases calcium-regulated molecular switches?
Molecules that are able to change conformation and activity according to redox state or effector binding/unbinding are often regarded as 'molecular switches'. However, for a sensitive response, a 'molecular switch' must have steep on-off characteristics within a very narrow effector concentration range (typically less than one order of magnitude) which should be reflected by the physiological effector concentration of the respective cellular compartment. Class III peroxidases are mostly secreted into the apoplast (peroxibase.toulouse.inra.fr/cellular_localization.php). Here, ionic conditions are very different from the cytosol. The apoplast constitutes an acidic environment of pH in the range of 4 < pHapo < 6 and of low buffer capacity.37,38 In contrast, the cytoplasmic pH is above 7 and strongly buffered.39,40 There is a similar difference between apoplastic and cytosolic calcium. The free Ca2+ ion concentration is high in the apoplast (3 < pCaapo < 5) and weakly buffered37 whereas in the cytoplasm [Ca2+] is in the range 6.5 < pCacyt < 7.5 and strongly buffered.40-42 Consequently, apoplastic peroxidases are typically active at [H+] ≈10 µM and [Ca2+] ≈100 µM. However, H+ and Ca2+ antagonistically influence peroxidase activity.43 Higher calcium [Ca2+] increases the activity (Fig. 1), whereas higher proton concentrations [H+] lower it, possibly due to competitive ion displacement.
Here, (Fig. 2A) HRP shifts its activity in the range 10 µM < [Ca2+]free < 1000 µM. Lepidium peroxidase responds in a biphasic manner to increasing Ca2+ concentrations, with one phase in the range 1 µM to < 100 µM and the other between 400 µM and < 2400 µM (Fig. 2B). This is within the expected range of steady-state apoplastic free calcium ion concentrations [Ca2+]apo in living plants.37 Consequently peroxidases are not fully active in this compartment, and their activity can respond according to changes in [Ca2+]apo..As mentioned above, this is a typical pre-requisite for a molecular switch.
It has long been speculated that calcium has a signaling function in the apoplast, though as yet no conclusive picture could be drawn.38 The finding that [Ca2+]apo controls extracellular enzymes is supported by studies on cell wall phosphatases.44 In addition, apoplastic calmodulin plays an important role in signal transduction.45-47 Here, we show that the calcium response of apoplastic POs matches the apoplastic Ca2+ concentration.
Peroxidase thermostability depends on molecular structure and molecular environment
Generally, heat inactivation in vitro depends on the 'heat dosage', i.e., both, the temperature and its duration.23,25,48 In this work we have shown that thermal inactivation is also dependent on calcium ions (Fig. 4).
PO activities were also measured after long-term exposure to buffer conditions of low free [Ca2+] (Fig. 3A and B). Thus, POs are reversibly inhibited by Ca2+ chelators.
This effect is not due to iron chelation, as removal of iron from the haem group would lead to irreversible inactivation of POs. Obviously here, the POs bind iron firmly despite the presence of chelators, as long as the prevailing temperature is moderate. Very high temperatures can irreversibly inactivate peroxidases irrespective of [Ca2+] (Figs. 2–5). In contrast Mura et al.15 demonstrated for Euphorbia latex peroxidase that peroxidase activity may recover after heat inactivation and restoring [Ca2+] rescues a manganese peroxidase from heat inactivation.18 But, this happens only, when Ca2+ is in excess (i.e., [Ca2+] ≥ 10 mM) and when sufficient time is given for recovery.
Sakharov (2004)49 showed that the African oil palm tree peroxidase is much more sensitive to heat at low pH and this effect has also been reported earlier.50 This is also likely to be a calcium effect, as an increase in the proton concentration causes calcium unbinding.43
In this work we determined peroxidase activities (LUPOs) in all experiments at pH 8.6, since this is optimal for producing luminol chemiluminescence. In the apoplast, where many peroxidases are located, the pH value is around 6.38,51 Thus, the in vitro-ET50 values reported here (Fig. 5 and Table S1) for the different species are likely to lie somewhere between their 'low Ca2+'- and their 'high Ca2+'-value in vivo, due to protonation at lower pH in the apoplast.
Other factors influencing the heat stability of peroxidases are water availability and osmolarity of the molecular environment. Peroxidases were found to be much more heat-stable, when in the dry state.25 Further, the presence of dissolved sugars increases heat stability of plant peroxidases.24,50 Finally, covalently bound neutral carbohydrates may also alter its heat resistance.52,53 In most cases heat stress is accompanied by drought stress. Drought stress leads to a lower cellular water content and hence higher concentrations of osmotically active metabolites, for example sugars. Such a network of factors may improve the heat resistance of essential enzymes like peroxidases during drought periods.
POs bind calcium at different sites
Activation (Fig. 2) or heat stabilization (Fig. 4) of POs are prompted at very different calcium concentrations. The fact suggests two binding sites: (i) a catalytic domain which promotes enzyme activity and (ii) a stabilizing domain which make the protein more heat stable. Since the molecular surface tension has been found to stabilize globular proteins54 one could speculate that Ca2+ binding to the protein surface increases its tension and consequently its heat stability while Ca2+ binding near the catalytic site43 confers activity.
In addition to its signaling functions, calcium seems to protect plants against oxidative damage that occurs during and after heat stress.55 A more direct effect of calcium has now been confirmed by the findings presented here (Figs. 2–4) and this is in line with the many other protective functions that calcium has in plants.56,57
Annexins are soluble multifunctional lipid binding proteins with both Ca2+-dependent lipid binding and peroxidase activity.58-60 The function of this protein family is not completely understood. However, their in vitro properties strongly point to a function within a calcium-peroxide-peroxidase network.
Different peroxidases from a plant species can be characterized individually
It has been estimated that each plant genome encodes approximately 8 to 15 peroxidase families.12 In Arabidopsis 73 different class III peroxidase genes have been found. However, only 12 putative POs have been identified via proteomic approaches in this species.6 Ultimately, a few peroxidase isoenzymes of different molecular mass and different isoelectric points could be isolated and partially purified from plant material.53
Here, we partially separated luminol converting peroxidases (LUPOs) from Arabidopsis, Lepidium and maize by size exclusion chromatography (SEC; Figures 7–8). However, it has been argued that peroxidases separated by SEC may perhaps be identical.61 But this does not apply for the separation of Arabidopsis peroxidases reported here (Fig. 8B) as the two POs separated from Arabidopsis have different ET50 values (Table S1). This is in line with findings from Thongsook and Barrett32 who characterized three peroxidases from broccoli and reported different heat inactivation profiles for each enzyme.
The two peroxidases found here in maize could not be completely separated (Fig. 7) presumably due to a small difference in molecular weights. However, they do have different ET50 values, as shown by the biphasic inhibition curve of the dialysate (Fig. 6). This demonstrates that heat inactivation curves may reveal the presence of different peroxidases even if their molecular masses are similar, provided the ET50 values are reasonably distinct. Conversely, SEC may separate different peroxidases of similar ET50 (Fig. 8B and 5; Table S1).
The abundance of different peroxidases seems to be dependent on the developmental stage (Fig. 7). In addition it could be speculated that from the large arsenal of different possible peroxidases a plant harbours in its genome, only those are expressed, which are appropriate to the prevailing growth temperature. However, this doesn't always seem to apply. Peroxidases isolated from Lepidium germinated and grown at different temperatures (9°C, 21°C, 33°C) had all the same ET50 (data Lepidium_growthtemperature_II.xls not shown).
Evolution generated and maintains heat stability of peroxidases in land plants
In warm regions, the time of day with highest temperatures does not vary much during the growing season. The mean temperature of the habitat is of less importance for survival. The maximum day temperature, in contrast, constitutes a natural selection factor which may have promoted the evolution of peroxidases into heat stable enzymes. Consequently, during evolution the selection pressure on plants in terms of heat stress is most likely to be determined by the maximum temperature they can withstand, rather than by the 'heat dosage', although the 'heat dosage' is effective for in-vitro-inactivation of POs.23,25,48 The data from the species examined in this study (Fig. 5; Table S1) appear to be inconsistent, but within a wider frame, they suggest a tendency for species thriving in habitats with occasional high temperatures (cotton, wheat, barley, maize) to evolve peroxidases with high ET50 compared with species usually growing at moderate or low temperatures (garden cress, sunflower).
Interestingly, kelp species (red dulse, sea belt, toothed wrack) have peroxidases with a relatively low ET50 of around or below 50°C. Stonewort (Charophyceae), an aquatic 'living fossil' on the phylogenetic border between the limnic and the terrestrial way of life,62-64 harbours peroxidases with higher ET50 of above 50°C. Finally, the peroxidase from the white rot fungus, a terrestrial organism, has a high ET50 of well above 60°C.
A remarkable finding here (Fig. 5; Table S1) is that the heat sensitivity of peroxidases from cryptogams does not depend on the presence of calcium, whereas peroxidases from vascular plants require Ca2+ ions in order to be heat stable (Figs. 3–5; Table S1). In terms of phylogeny, these findings indicate that class III peroxidases with their Ca2+-dependent heat stability evolved when green plants adopted a terrestrial way of life and became vascular plants. However, in terms of ontogeny, the abundance of heat-stable peroxidases appears to depend on the developmental stage of a plant. In the experiments reported here, Helianthus and Sorghum were harvested as young sprouts after 10 and 12 d, respectively (Table 1) and low ET50 of peroxidases were measured (Fig. 5; Table S1). Possibly, in a later developmental stage and later in the year, when the plants have to cope with heat stress, the abundance of more heat tolerant peroxidases may increase. Such a shift in PO abundance during development is shown in Figure 7. Evidently, more detailed experiments are needed.
Table 1. Plant material. Species, varieties, sources, and growth period.
| Popular name |
Scientific species name |
Race / variety sub-species |
Source / retailer / location |
Growth period (days) |
|---|---|---|---|---|
| Dicots | ||||
| Garden cress |
Lepidium sativum |
einfache Grüne |
Sperli, #40.9666 www.sperli-samen.de |
6 |
| Cotton |
Gossypium herbaceum |
Levant cotton |
select # SE_120162 www.saemereien.ch |
36 |
| Sunflower |
Helianthus annuus |
|
#618282 Kiepenkerl www.samenshop24.de |
12 |
| Thale cress | Arabidopsis thaliana | Columbia (Col-0) | Lehle www.arabidopsis.com in-house progeny |
30 |
| Monocots | ||||
|---|---|---|---|---|
| Millet |
Sorghum bicolor |
Grain sorghum |
Rapunzel www.rapunzel.de |
10 |
| Wheat |
Triticum aestivum |
#500411 |
Davert www.davert.de |
12 |
| Barley |
Hordeum vulgare |
“Popp-Gerste” |
Dreschflegel, www.dreschflegel-saatgut.de |
12 |
| Maize | Zea mays | Popcorn | Rapunzel www.rapunzel.de |
8, 28 |
| Cryptogams | ||||
|---|---|---|---|---|
| Stonewort |
Chara corallina |
australis |
permanent culture in-house progeny |
∞ |
| Toothed wrack |
Fucus serratus |
North Sea |
offshore collection Scotland |
n.d. |
| Sea belt |
Saccharina latissima |
Baltic |
offshore collection west baltic sea, Kiel Fjord |
n.d. |
| Red dulse | Palmaria palmata | Atlantic | offshore collection atlantic coast, Normandy |
n.d. |
It could be argued that plants lost the calcium-independent heat stability of their POs when they adapted to terrestrial life. However, evolution goes from simple to more complex systems. Simple aquatic plants (algae, kelp) enforcedly live in close contact to an environment of high calcium concentration. Thus, an extracellular calcium dependent molecular switch is neither necessary nor can it be established. This is different in vascular land plants which can control the environment of their cells (i.e., the apoplast). This way, more complex signaling pathways involving ROS and calcium have evolved in higher plants which can trigger systemic responses. Thus, the calcium dependency of enzymatic activities is rather a gain than a loss of function. This hypothesis is discussed in more detail in an addendum paper.
Species and their peroxidase specifics
The geographical distribution of maize cultivation starts at 58°N latitude and reaches 40°S latitude (www.agroatlas.ru). However, maize is a sub-tropical graminaceous C4-plant which is native to Central and South America and is thus expected to harbour peroxidases which can withstand temperatures up to 70°C.
Sorghum is also a heat-loving grass. In this case, the low ET50 of 47°C (Fig. 5; Table S1) needs explanation. It is well known that optimal seed germination and growth temperatures are below 30°C for Sorghum (www.agroatlas.ru) and heat resistance develops during a later stage. Since sprouts were used here as source material, the low ET50 is in line with the natural temperatures at which this species usually germinates.
Both, barley and wheat have high ET50 (Fig. 5; Table S1), which may be indicative of their origin in the warm climate of the mesopotamian region. Nowadays barley is mainly cultivated south of and around the 50° northern latitude (www.agroatlas.ru). The wide geographical distribution of barley can be explained by its large number of ecotypes (http://data.gbif.org/ = > browse Hordeum) and their adaptability to their respective environments. In northern Europe barley can even grow north of the arctic circle, (www.agroatlas.ru/en/content/cultural/Hordeum_vulgare_K/) at 70°N in Norway and 68°N in Finland. Wheat is also a steppe crop and is mainly cultivated between 50° and 60° latitude (www.agroatlas.ru/en/content/cultural/Triticum_aestivum_spring_K/map/) (www.agroatlas.ru/en/content/cultural/Triticum_aestivum_winter_K/map/) and up to 66°N in Sweden. Thus, the ET50 of peroxidases from crops with a wide anthropogenic distribution may reveal something about the species origin, but not necessarily anything about the current needs at the location of tillage.
Implications for breeding projects
If heat tolerant plants produce heat tolerant peroxidases, then it is tempting to assume that the heat sensitivity of a plant is influenced by the sensitivity of its peroxidases. Thus, one aspect of breeding heat tolerant crop cultivars could be to investigate, whether heat stable peroxidases confer heat tolerance. The heat stability (ET50) of plant POs might be employed as a quantitative trait to screen for thermotolerance among novel mutants, breeds, or transgenics.
Implications for the vegetable processing food industry
Peroxidases can change the color, odour, and taste of frozen, dried and otherwise processed vegetables.24 Therefore, in the food industry peroxidases are undesirable and efforts have been made to inactivate these enzymes, e.g., by blanching.23,24 However, with any heat treatment of food, temperatures need to be high enough to inactivate all unwanted enzymes65 and must at the same time as low as possible to preserve desired food ingredients, like vitamins, pigments and other specific metabolites. As shown here, the heat stability of peroxidases from biological samples can easily be measured with the LUPO assay and used to find the optimal temperatures to preserve vegetables that are processed for long periods of storage.
Perspective
Although peroxidases belong to the most investigated enzymes not all properties of this versatile enzyme family have yet been investigated in detail. Much more investigations are needed to identify and characterize individual active POs, with regard to their genetic origin, subcellular expression, tissue abundance, developmental emergence and their functions in innate and acquired stress tolerance. In particular for cryptogams, the separation of cell wall enzymes and intracellular soluble peroxidase may help to understand their evolution and the role of calcium in controlling the activity of peroxidases in living plant cells.
Material and Methods
The LUPO-assay: Probing peroxidase activity with luminol
Most peroxidases accept artificial organic electron donors like luminol (L) as substrates (Fig. 1B). Luminol produces chemiluminescence when oxidised. Luminol converting peroxidases (LUPOs) can thus readily be quantified by the light yield they produce in presence of luminol and H2O2.31
Listing of used chemicals
Calcium chloride (CaCl2 6 H2O; Riedel deHaen #12074; MW = 220 g/mol)
EDTA (EDTA; Aldrich #E2,628, MW = 292 g/Mol)
Glycerol (Roth #3783; MW = 92.1g/Mol, ρ = 1.26 kg/l)
HEDTA (Hydroxyethyl-Ethylenediaminetriacetic acid; Sigma #7154; MW = 278 g/mol)
Horseradish Peroxidase (HRP; Sigma #P6140; ca. 2kU/mL)
Hydrochloric acid (HCl; Roth #4625; 1 M, i.e., 34% 1:10 diluted in H2O)
Hydrogen peroxide solution (H2O2; Merck # 1.08597; 30% = 8.8 M; MW = 34 g/Mol)
Iodophenol (Fluka #58020; MW = 220 g/Mol)
Fungal lignin peroxidase (FLP; Sigma #42603; ≈0.5U/mg)
Luminol (Fluka #09253; MW = 177 g/Mol)
Murashige and Skoog (M&S) medium (Duchefa, #M0222)
NTA (Nitrilotriacetic acid; Aldrich #39,814; MW = 191 g/mol)
Potassium chloride (Roth #6781; MW = 74.5 g/mol)
Sephadex G-200 (Pharmacia, Uppsala, Sweden)
TRIS (Roth #5429; MW = 121 g/Mol)
Triton® X-100 (Sigma #X100; MW = 647, Liquid ρ = 10.7 kg/L ≅ 1.65 M)
Buffers and solutions
Unless stated otherwise, TriCaT-buffer, consisting of 100 mM TRIS/HCl pH 8.6 + 2 mM CaCl2 + 1 mM Triton® X-100 was used as assay buffer.
Luminol (1 mM) solution was prepared by diluting 1 M alkaline luminol stock solution in TriCaT.
A 8.8 mM H2O2 solution was prepared by diluting a 30%-H2O2 solution 1:1000 in TriCaT.
Instrumentation and special consumables
Luminescence assays were performed with a simple chemiluminometer (PMT 9829A, Electron Tubes Ltd. Ruislip, UK) equipped with a custom-made light-tight sample housing to hold vials directly in front of the detector.66
Luminometer plastic vials (# 55.484, Sarstedt, Nümbrecht, FRG);
Fluted paper filters (MN 6151/4 Ø 320mm; #531 032, Macherey-Nagel, FRG);
Dialysis membrane tubing (MWCO = 8–10 kDa; Roth #E668.1);
Chromatography 24 mL columns (16 x 120mm) (#732–1010, BioRad, Munich, Germany)
Plant growth
Seeds were obtained from various sources as indicated in Table 1. For surface sterilization dry seeds were immersed for 10 min in a solution containing 5% bleach (i.e., NaOCl; 0.6% free chlorine) and 40% ethanol and subsequently washed three times in sterile H2O (vortexed for 2 min) and drained on filter paper. Unless stated otherwise, seeds were sown on 2 layers of synthetic capillary matting (medical fleece “Rolta®-soft” # 932048, Hartmann, Germany, http://de.hartmann.info/) soaked with Murashige and Skoog medium (Duchefa #M0222) and sprouts were grown in mini propagators (26 x 11 x 7 cm, Windhager®; Austria; www.windhager.at) at 21°C with a 16 h photoperiod (50 µE, white fluorescent lamps Osram L36 W/77). Young plants were harvested after a growth period as indicated in Table 1, and portions of 4 g fresh weight were snap-frozen in liquid nitrogen and stored at -80°C until processing. Arabidopsis was grown in pots containing a mixture of turf-based substrate (TKS-II) and washed sand. Light and temperature conditions were as above. Arabidopsis plants were harvested at onset of bolting. Chara corallina was grown in permanent culture (100 L plastic container) as described in.40 Kelp (Fucus, Saccharina, Palmaria) was collected offshore during June/July, dried and stored at RT until processing.
Processing plant material
Plant material was ground in liquid nitrogen with a mortar and pestle. Ten volumes (mL/g) of assay buffer were added to the powder, vortexed for 2 min, centrifuged for 20 min at 4000 g and the supernatant was filtered through a fluted paper filter. Since low molecular weight antioxidants and phenolics can interfere with a peroxidase assay, these compounds were removed by dialysing the filtered extract in dialysis membrane tubing (MWCO = 10 kDa) for a minimum of 15 h against a two-hundred-fold volume of ice-cold assay buffer. The dialysed extract was supplemented with the same volume of pure glycerol, mixed and stored at -20°C until further processing.
Temperature treatment of peroxidases
For heat inactivation experiments, glycerol-containing dialysed extracts were treated for 2 h at temperatures indicated in the figures on a polymerase chain reaction gradient cycler (PTC225; MJ Research) before the light yield of the H2O2-luminol reaction was measured.
LUPO assay procedure
Dialysed and heat-treated samples were diluted in assay buffer. A dilution between 5- and 500-fold was chosen, depending on the peroxidase activity in the dialysate. Commercial peroxidases were diluted to approx. 18 mU/mL (FLP) and 6 mU/mL (HRP). 0.5 mL of diluted sample was mixed with 0.5 mL luminol solution in luminometer vials and allowed to stand for at least 1 h at RT in the dark. Background luminescence was recorded and the light reaction was started by adding 0.5 mL of the H2O2 solution. A final H2O2 concentration of between 1 and 25 mM was chosen depending on the H2O2-sensitivity of the enzyme. Counts per second (cps) were recorded for several minutes and light output integrated. For assaying commercial peroxidases (i.e., HRP and FLP) the assay buffer was supplemented with 1 µM iodophenol as an enhancer.67
Gel filtration
G-200 sephadex beads were swollen in assay buffer over night and 20 mL of the slurry filled into chromatography columns. The packed column was capped with a fritted plastic disk. Each column was equilibrated with a minimum flowthrough of five column volumes (i.e., 120 mL) of assay buffer. Dialysed sample (1 mL) was loaded onto the column and eluted by a gravitational buffer flow adjusted to approximately 500 µl/min. Fractions of 500 µl were collected for analysis.
Calcium titration
A constant-volume titration using three calcium chelators of different Kd was performed to titrate the calcium concentration continuously over several orders of magnitude. A buffer 'TriCHT' with a chelator mix consisting of EDTA, HEDTA, and NTA (4 mM each) in 100 mM Tris/HCl (pH = 8.6), 100 mM KCl and 1 mM Triton was prepared. The calcium-containing buffer was TriCaT with 100 mM KCl. Factor 0.9 x dilution series of TriCHT with increasing concentrations of Ca2+ were prepared by mixing TriCHT with TriCaT/KCl. Finally, the buffer mix was supplemented with Ca2+ from a 1 M CaCl2 stock to produce concentrations of [Ca2+] > 2 mM. The ionic strength of the buffers as determined by osmometry was around 0.4 M.
MaxChelator5 (www.stanford.edu/~cpatton/webmaxcS.htm) was used for calculating free calcium ion concentrations ([Ca2+]free) from titrated total buffer/ion mixtures.68
Data processing
Integrated light output correlating with the peroxidase activity in the sample was plotted over the applied temperature. A Boltzmann curve was fitted to the data to identify the temperature of 50% peroxidase inhibition (i.e., ET50%). Multi-peak Gaussian curves were fitted to elution profiles from gel filtration experiments. All fitting routines were performed using Origin 7.0 (OriginLab Corp., Northhampton, MA, USA).
Supplementary Material
Acknowledgments
We thank Levent Piker of CRM Kiel (www.crm-online.de) for providing the kelp. We are indebted to Livia Saleh (ZBM, Kiel) and Lee Shaw (Kiel) for critically reading the manuscript and to Axel Scheidig (ZBM, Kiel) for his generous support. This study profited from access to the core facilities of the ZBM, CAU, Kiel and from financial support by the DFG (Grant No.: PL253/5).
Glossary
Abbreviations:
- Ca2+
calcium ions
- [Ca2+]
calcium ion concentration
- EDTA
Ethylenediaminetetraacetic acid
- ET50
effective temperature of 50% activity inhibition
- FLP
fungal lignin peroxidase
- HEDTA
Hydroxyethyl-Ethylenediaminetriacetic acid
- HRP
horseradish peroxidase
- LUPO
luminol converting peroxidases
- NTA
Nitrilotriacetic acid
- PO
peroxidase
- SBP
soy bean peroxidase
- SEC, size exclusion chromatography (
gel filtration)
- TRIS
Tris(hydroxymethyl)aminomethane
Author Contributions
SV performed the experiments and processed the raw data. CP conceived of the study, analyzed the data and wrote the manuscript. Both authors approved the final version.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20065
References
- 1.Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H. A large family of class III plant peroxidases. Plant Cell Physiol. 2001;42:462–8. doi: 10.1093/pcp/pce061. [DOI] [PubMed] [Google Scholar]
- 2.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–10. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 3.Passardi F, Cosio C, Penel C, Dunand C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005;24:255–65. doi: 10.1007/s00299-005-0972-6. [DOI] [PubMed] [Google Scholar]
- 4.Penel C, Dunand C. Signaling via Plant Peroxidases. In: Baluška F, Vivanco J, eds. Signaling in Plants: Springer Berlin Heidelberg, 2009:155-71. [Google Scholar]
- 5.Bakalovic N, Passardi F, Ioannidis V, Cosio C, Penel C, Falquet L, et al. PeroxiBase: a class III plant peroxidase database. Phytochemistry. 2006;67:534–9. doi: 10.1016/j.phytochem.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Cosio C, Dunand C. Specific functions of individual class III peroxidase genes. J Exp Bot. 2009;60:391–408. doi: 10.1093/jxb/ern318. [DOI] [PubMed] [Google Scholar]
- 7.Carpin S, Crèvecoeur M, de Meyer M, Simon P, Greppin H, Penel C. Identification of a Ca(2+)-pectate binding site on an apoplastic peroxidase. Plant Cell. 2001;13:511–20. doi: 10.1105/tpc.13.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunand C, Tognolli M, Overney S, von Tobel L, de Meyer M, Simon P, et al. Identification and characterisation of Ca2+-pectate binding peroxidases inArabidopsis thaliana. J Plant Physiol. 2002;159:1165–71. doi: 10.1078/0176-1617-00768. [DOI] [Google Scholar]
- 9.de Weert S, Lokman B. Heterologous expression of peroxidases. In: Torres E, Ayala M, eds. Biocatalysis Based on Heme Peroxidases. Berlin Heidelberg: Springer-Verlag 2010:315-33. [Google Scholar]
- 10.Kawano T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 2003;21:829–37. doi: 10.1007/s00299-003-0591-z. [DOI] [PubMed] [Google Scholar]
- 11.Almagro L, Gómez Ros LV, Belchi-Navarro S, Bru R, Ros Barceló A, Pedreño MA. Class III peroxidases in plant defence reactions. J Exp Bot. 2009;60:377–90. doi: 10.1093/jxb/ern277. [DOI] [PubMed] [Google Scholar]
- 12.Welinder KG. Superfamily of plant, fungal and bacterial peroxidases. Curr Opin Struct Biol. 1992;2:388–93. doi: 10.1016/0959-440X(92)90230-5. [DOI] [Google Scholar]
- 13.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–80. [Google Scholar]
- 14.Pintus F, Spanò D, Medda R, Floris G. Calcium ions and a secreted peroxidase in Euphorbia characias latex are made for each other. Protein J. 2011;30:115–23. doi: 10.1007/s10930-011-9310-8. [DOI] [PubMed] [Google Scholar]
- 15.Mura A, Longu S, Padiglia A, Rinaldi AC, Floris G, Medda R. Reversible thermal inactivation and conformational states in denaturant guanidinium of a calcium-dependent peroxidase from Euphorbia characias. Int J Biol Macromol. 2005;37:205–11. doi: 10.1016/j.ijbiomac.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Veitch NC. Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry. 2004;65:249–59. doi: 10.1016/j.phytochem.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Shiro Y, Kurono M, Morishima I. Presence of endogenous calcium ion and its functional and structural regulation in horseradish peroxidase. J Biol Chem. 1986;261:9382–90. [PubMed] [Google Scholar]
- 18.Sutherland GRJ, Aust SD. The effects of calcium on the thermal stability and activity of manganese peroxidase. Arch Biochem Biophys. 1996;332:128–34. doi: 10.1006/abbi.1996.0324. [DOI] [PubMed] [Google Scholar]
- 19.Mura A, Medda R, Longu S, Floris G, Rinaldi AC, Padiglia AA. A Ca2+/calmodulin-binding peroxidase from Euphorbia latex: novel aspects of calcium-hydrogen peroxide cross-talk in the regulation of plant defenses. Biochemistry. 2005;44:14120–30. doi: 10.1021/bi0513251. [DOI] [PubMed] [Google Scholar]
- 20.Mura A, Pintus F, Lai P, Padiglia A, Bellelli A, Floris G, et al. Catalytic pathways of Euphorbia characias peroxidase reacting with hydrogen peroxide. Biol Chem. 2006;387:559–67. doi: 10.1515/BC.2006.072. [DOI] [PubMed] [Google Scholar]
- 21.Penel C, Van Cutsem P, Greppin H. Interactions of a plant peroxidase with oligogalacturonides in the presence of calcium ions. Phytochemistry. 1999;51:193–8. doi: 10.1016/S0031-9422(98)00741-9. [DOI] [Google Scholar]
- 22.Shah K, Penel C, Gagnon J, Dunand C. Purification and identification of a Ca(2+)-pectate binding peroxidase from Arabidopsis leaves. Phytochemistry. 2004;65:307–12. doi: 10.1016/j.phytochem.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Lee CY, Pennesi AP. Isolation and further characterization of a heat resistant peroxidase isoenzyme from Cauliflower. J Food Sci. 1984;49:1616–7. doi: 10.1111/j.1365-2621.1984.tb12859.x. [DOI] [Google Scholar]
- 24.Burnette FS. Peroxidase and its relationship to food flavor and quality: a review. J Food Sci. 1977;42:1–6. doi: 10.1111/j.1365-2621.1977.tb01204.x. [DOI] [Google Scholar]
- 25.Hendrickx M, Saraiva J, Lyssens J, Oliveira J, Tobback P. The influence of water activity on thermal stability of horseradish peroxidase. Int J Food Sci Technol. 1992;27:33–40. doi: 10.1111/j.1365-2621.1992.tb01175.x. [DOI] [Google Scholar]
- 26.Morales-Blancas EF, Chandia VE, Cisneros-Zevallos L. Thermal inactivation kinetics of peroxidase and lipoxygenase from broccoli, green asparagus and carrots. J Food Sci. 2002;67:146–54. doi: 10.1111/j.1365-2621.2002.tb11375.x. [DOI] [Google Scholar]
- 27.Alokail MS, Ismael MA. Thermostable Characteristics of peroxidase from Leaves of Arabian Palm Date (Phoenix dactylifera L.) Saudi Journal of Biological Sciences. 2005;12:25–31. [Google Scholar]
- 28.McEldoon JP, Dordick JS. Unusual thermal stability of soybean peroxidase. Biotechnol Prog. 1996;12:555–8. doi: 10.1021/bp960010x. [DOI] [Google Scholar]
- 29.Oliva M, Theiler G, Zamocky M, Koua D, Margis-Pinheiro M, Passardi F, et al. PeroxiBase: a powerful tool to collect and analyse peroxidase sequences from Viridiplantae. J Exp Bot. 2009;60:453–9. doi: 10.1093/jxb/ern317. [DOI] [PubMed] [Google Scholar]
- 30.Saidi Y, Finka A, Goloubinoff P. Heat perception and signalling in plants: a tortuous path to thermotolerance. New Phytol. 2011;190:556–65. doi: 10.1111/j.1469-8137.2010.03571.x. [DOI] [PubMed] [Google Scholar]
- 31.Saleh L, Plieth C. Fingerprinting antioxidative activities in plants. Plant Methods. 2009;5:2. doi: 10.1186/1746-4811-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thongsook T, Barrett DM. Heat inactivation and reactivation of broccoli peroxidase. J Agric Food Chem. 2005;53:3215–22. doi: 10.1021/jf0481610. [DOI] [PubMed] [Google Scholar]
- 33.AHS AHS. Plant Heat-Zone Map - http://www.ahs.org/pdfs/05_heat_map.pdf - http://www.ahs.org/publications/heat_zone_map.htm Alexandria, VA: American Horticultural Society, 1997.
- 34.Peiris B, Siegel B, Siegel S. The relation of electrolyte-induced peroxidase changes in salt-sensitive and salt-tolerant rice varieties to changes in other physiological parameters In: Lobarzewski J, Greppin H, Penel C, Gaspar T, eds. Biochemical, molecular, and physiological aspects of plant peroxidases. University M. Curie-Sklodowska, Lublin, Poland and University of Geneva, Switzerland, 1991:425-32. [Google Scholar]
- 35.Siegel BZ. Plant peroxidases—an organismic perspective. Plant Growth Regul. 1993;12:303–12. doi: 10.1007/BF00027212. [DOI] [Google Scholar]
- 36.Gulen H, Eris A. Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Sci. 2004;166:739–44. doi: 10.1016/j.plantsci.2003.11.014. [DOI] [Google Scholar]
- 37.Felle HH, Hanstein S, Sattelmacher B, Horst WJ. Probing apoplastic ion relations in Vicia faba as influenced by nutrition and gas exchange. In: Sattelmacher B, Horst W, eds. The Apoplast of higher plants: Compartment of storage, transport and reactions. Dordrecht: Springer Netherlands, 2007:295-306. [Google Scholar]
- 38.Gao D, Knight MR, Trewavas AJ, Sattelmacher B, Plieth C. Self-reporting Arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiol. 2004;134:898–908. doi: 10.1104/pp.103.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulte A, Lorenzen I, Böttcher M, Plieth C. A novel fluorescent pH probe for expression in plants. Plant Methods. 2006;2:7. doi: 10.1186/1746-4811-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plieth C, Sattelmacher B, Hansen UP. Cytoplasmic Ca2+-H+-exchange buffers in green algae. Protoplasma. 1997;198:107–24. doi: 10.1007/BF01282136. [DOI] [Google Scholar]
- 41.Plieth C, Sattelmacher B, Hansen UP, Thiel G. The action potential in Chara: Ca2+ release from internal stores visualized by Mn2+-induced quenching of fura-dextran. Plant J. 1998;13:167–75. doi: 10.1046/j.1365-313X.1998.00019.x. [DOI] [Google Scholar]
- 42.Plieth C, Hansen UP. Cytoplasmic Ca2+ and H+ buffers in green algae: a Reply. Protoplasma. 1998;203:210–3. doi: 10.1007/BF01279478. [DOI] [Google Scholar]
- 43.George SJ, Kvaratskhelia M, Dilworth MJ, Thorneley RN. Reversible alkaline inactivation of lignin peroxidase involves the release of both the distal and proximal site calcium ions and bishistidine co-ordination of the haem. Biochem J. 1999;344:237–44. doi: 10.1042/0264-6021:3440237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeMarty M, Morvan C, Thellier M. Calcium and the Cell Wall. Plant Cell Environ. 1984;7:441–8. doi: 10.1111/j.1365-3040.1984.tb01434.x. [DOI] [Google Scholar]
- 45.Ma L, Sun DY. The effects of extracellular calmodulin on initiation of Hippeastrum rutilum pollen germination and tube growth. Planta. 1997;202:336–40. doi: 10.1007/s004250050135. [DOI] [Google Scholar]
- 46.Ma L, Xu X, Cui S, Sun D. The presence of a heterotrimeric G protein and its role in signal transduction of extracellular calmodulin in pollen germination and tube growth. Plant Cell. 1999;11:1351–64. doi: 10.1105/tpc.11.7.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang J, Wu S, Bai J, Sun DY. Extracellular calmodulin-binding proteins in plants: purification of a 21-kDa calmodulin-binding protein. Planta. 1996;198:510–6. doi: 10.1007/BF00262636. [DOI] [PubMed] [Google Scholar]
- 48.Ling AC, Lund DB. Determining kinetic parameters for thermal inactivation of heatresistant and heat-labile isozymes from thermal destction curves. J Food Sci. 1978;43:1307–10. doi: 10.1111/j.1365-2621.1978.tb15295.x. [DOI] [Google Scholar]
- 49.Sakharov IY. Palm tree peroxidases. Biochemistry (Mosc) 2004;69:823–9. doi: 10.1023/B:BIRY.0000040213.91951.bc. [DOI] [PubMed] [Google Scholar]
- 50.Nebesky EA, Esselen WB, Jr., Kaplan AM, Fellers CR. Thermal destruction and stability of peroxidase in acid foods. Food Res. 1950;15:114–24. doi: 10.1111/j.1365-2621.1950.tb16457.x. [DOI] [PubMed] [Google Scholar]
- 51.Mühling KH, Plieth C, Hansen UP, Sattelmacher B. Apoplastic pH of intact leaves of Vicia faba as influenced by light. J Exp Bot. 1995;46:377–82. doi: 10.1093/jxb/46.4.377. [DOI] [Google Scholar]
- 52.da Silva E, Lourenco EJ, Neves VA. Soluble and bound peroxidases from papaya fruit. Phytochemistry. 1990;29:1051–6. doi: 10.1016/0031-9422(90)85401-Z. [DOI] [Google Scholar]
- 53.Khan AA, Robinson DS. The thermostability of purified mango isoperoxidases. Food Chem. 1993;47:53–9. doi: 10.1016/0308-8146(93)90302-V. [DOI] [Google Scholar]
- 54.Lin TY, Timasheff SN. On the role of surface tension in the stabilization of globular proteins. Protein Sci. 1996;5:372–81. doi: 10.1002/pro.5560050222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Larkindale J, Knight MR. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002;128:682–95. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plieth C. Calcium: just another regulator in the machinery of life? Ann Bot. 2005;96:1–8. doi: 10.1093/aob/mci144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plieth C. Plant calcium signaling and monitoring: pros and cons and recent experimental approaches. Protoplasma. 2001;218:1–23. doi: 10.1007/BF01288356. [DOI] [PubMed] [Google Scholar]
- 58.Laohavisit A, Davies JM. Annexins. New Phytol. 2011;189:40–53. doi: 10.1111/j.1469-8137.2010.03533.x. [DOI] [PubMed] [Google Scholar]
- 59.Jami SK, Clark GB, Turlapati SA, Handley C, Roux SJ, Kirti PB. Ectopic expression of an annexin from Brassica juncea confers tolerance to abiotic and biotic stress treatments in transgenic tobacco. Plant Physiol Biochem. 2008;46:1019–30. doi: 10.1016/j.plaphy.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 60.Gorecka KM, Konopka-Postupolska D, Hennig J, Buchet R, Pikula S. Peroxidase activity of annexin 1 from Arabidopsis thaliana. Biochem Biophys Res Commun. 2005;336:868–75. doi: 10.1016/j.bbrc.2005.08.181. [DOI] [PubMed] [Google Scholar]
- 61.Gaspar T, Penel C, Thorpe T, Greppin H. Peroxidases - a survey of their biochemical and physiological roles in higher plants (1970-1980). Geneve: Universite de Geneve - Centre de Botanique, 1982. [Google Scholar]
- 62.Lewis LA, McCourt RM. Green algae and the origin of land plants. Am J Bot. 2004;91:1535–56. doi: 10.3732/ajb.91.10.1535. [DOI] [PubMed] [Google Scholar]
- 63.Sørensen I, Rose JK, Doyle JJ, Domozych DS, Willats WG. The Charophycean green algae as model systems to study plant cell walls and other evolutionary adaptations that gave rise to land plants. Plant Signal Behav. 2012;7:1–3. doi: 10.4161/psb.7.1.18574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Graham L. Green algae to land plants: An evolutionary transition. J Plant Res. 1996;109:241–51. doi: 10.1007/BF02344471. [DOI] [Google Scholar]
- 65.Thongsook T, Whitaker JR, Smith GM, Barrett DM. Reactivation of broccoli peroxidases: structural changes of partially denatured isoenzymes. J Agric Food Chem. 2007;55:1009–18. doi: 10.1021/jf062242+. [DOI] [PubMed] [Google Scholar]
- 66.Plieth C. Aequorin as a reporter gene. In: Salinas J; Sanchez-Serrano JJ (Eds) “Arabidopsis Protocols” 2nd Edition, Humana Press 2006; 323:307-27. [DOI] [PubMed] [Google Scholar]
- 67.Navas Díaz A, García Sánchez F, González Garcia JA. Phenol derivatives as enhancers and inhibitors of luminol-H2O2-horseradish peroxidase chemiluminescence. J Biolumin Chemilumin. 1998;13:75–84. doi: 10.1002/(SICI)1099-1271(199803/04)13:2<75::AID-BIO469>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 68.Patton C, Thompson S, Epel D. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium. 2004;35:427–31. doi: 10.1016/j.ceca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 69.de Montellano PRO. Catalytic Mechanisms of Heme Peroxidases. In: Torres E, Ayala M, eds. Biocatalysis Based on Heme Peroxidases. Berlin Heidelberg: Springer-Verlag 2010:79-107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.