Abstract
We recently determined that CBSX proteins, which have only one pair of cystathionine β-synthase (CBS) domains, directly regulate the activation of thioredoxins and thereby control cellular H2O2 levels and modulate both plant development and growth. The Arabidopsis genome contains six CBSXs, and these are localized to different subcellular compartments‑ CBSX1 and CBSX2 in the chloroplast, CBSX3 in the mitochondria, CBSX4 in the cytosol, and CBSX5 and CBSX6 in the endoplasmic reticulum. The CBSXs have been identified in prokaryotes and plants, but not in animals. The considerable differences in length and amino acid sequence between CBSX members may result in variations in protein structure and in their specificity to interact with ligands and/or target proteins. Here, we discuss the possibility that the CBSXs are novel sensor relay proteins that use adenosine-containing molecules as a ligand.
Keywords: CBS domain, CBSX, adenosine-containing ligand, redox, sensor relay, thioredoxin
Due to their sessile nature plants have evolved various inducible defense mechanisms, such as intracellular signal sensor proteins and signaling transduction networks, to overcome many of the environmental challenges they may encounter. One of the more important intracellular signal sensing and transducing mechanisms is sensor relay. Sensor relay proteins have no specific function on their own and possess no intrinsic enzymatic activity; however, following binding to signal molecules, such as ligands or second messengers, they participate in regulating the activity of variable downstream partner proteins in a specific signal cascade. To date, only two types of proteins have been designated sensor relay proteins: calmodulins (CaM) and calcineurin B-like (CBL) proteins. CaMs and CBLs are representative sensor relay proteins; as such, following binding to the second messenger Ca2+, they transduce signals through bimolecular interactions.1 A total of seven CaMs and ten CBLs have been identified in the Arabidopsis genome. However, based on the amino acid sequence, there are actually only four different CaM proteins; consequently, the seven Arabidopsis CaM proteins are classified into four groups, with all CaM members in the same group having an identical amino acid sequence. The CaMs are able to interact with a wide variety of downstream proteins, including kinases, metabolic enzymes, transcription factors, channel proteins, among others.2-5 In comparison to CaMs, the CBLs have a relatively more restricted regulating capacity in that they interact with and regulate exclusively the activity of CBL-interacting protein kinases (CIPK). However, CBL3 has recently been reported to interact directly with and regulate the activity of methylthioadenosine nucleosidase, thereby indicating that CBLs may also have a variable regulating ability, similar to CaMs.6 Despite the wide diversity of regulatory activities shown by CaMs and CBLs, these two sensor relay proteins cannot account for all of the intracellular signal transduction mechanisms that function in plants. Moreover, judging from the multitude of signals initiated by various stimuli and the tremendous complexity of the targets with which the sensor relay proteins interact, it seems reasonable to assume that there may be other signal molecules and sensor relay proteins that interact in a manner similar to Ca2+ and the calcium sensor relay proteins.
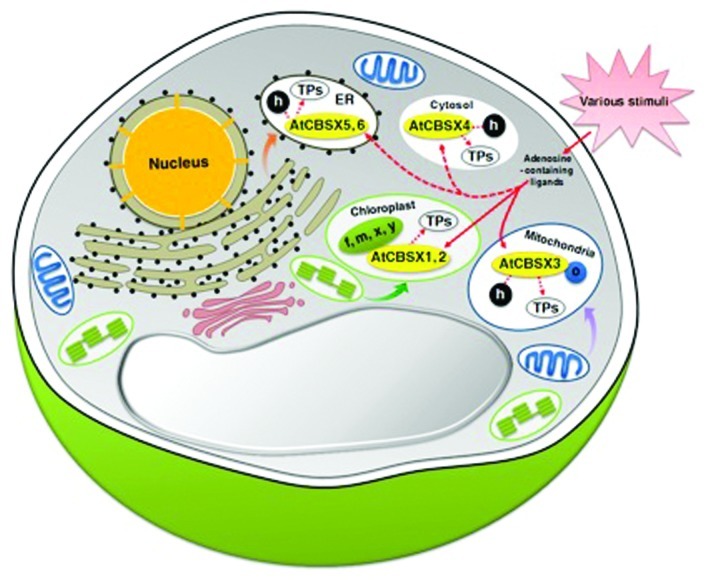
Possible candidate signal molecules/sensor relay proteins are the adenosine-containing metabolites and proteins, denoted CBSXs, which are characterized by containing only one pair of cystathionine β-synthase (CBS) domains—and no other protein domains. A total of six CBSXs have been identified in the Arabidopsis genome.7 CBSX1 and 2 activate thioredoxins (Trxs) in the chloroplast and in turn helps to regulate cellular redox levels.8 Those six Arabidopsis CBSX proteins are differentially localized in the subcellular compartments, with CBSX1 and CBSX2 found in the chloroplast, CBSX3 in the mitochondria, CBSX4 in the cytosol, and CBSX5 and CBSX6 in the endoplasmic reticulum. The six different types of Trxs are also differentially distributed throughout the cell, showing a similar subcellular localization as the CBSXs, with types f, m, x, and y in the chloroplast, o in the mitochondria, and h in the cytosol, mitochondria, endoplasmic reticulum, and extracellular compartment.9-13 Our group has reported that CBSX3, whose localization is predicted to be in the mitochondria, regulates mitochondrial Trx o. The activities of all of these CBSX members are augmented by binding with adenosine monophosphate (AMP).8 The reduced Trx has a function in that it is able to reduce the regulatory intramolecular disulfide bonds of target proteins, thereby altering the activity of these target proteins. A great many proteins have been identified as targets of Trxs.14 These results suggest that CBSXs sense changes in the adenosine-containing ligand (e.g., AMP) in most parts of the cellular compartments and transduce signals through their regulation of the activation of Trxs (Fig. 1). As such, this sensor relay protein system resembles the transduction of signals by CBLs through regulating the activities of CIPKs by sensing changes in cellular Ca2+ levels.
Figure 1. Schematic diagram of proposed function of all CBSX members in the cell. Extracellular stimuli alter the concentration of various adenosine-containing ligands, which in turn is sensed by CBSXs, and CBSXs are augmented by binding to a specific adenosine-containing ligand, resulting in a transduction of signals via a sensor relay mechanism. TPs, target proteins; f, Trx f; h, Trx h; m, Trx m; o, Trx o; x, Trx x; y, Trx y.
Bioinformatics studies of the CBSX proteins indicate that, with the exception of CBSX1 and 2, each CBSX that has been identified to date is distinct in terms of length, localization, and expression pattern (Table 1). In particular, the amino acid similarity of these proteins is apparently quite low among members. These features, namely, variable length and low amino acid homology, suggest that the actual structure of each CBSX member is different. These sequence and structural variations could affect their binding specificity and affinity with adenosine-containing ligands, as well as with their downstream partner proteins, leading to the notion that this variability of CBSXs in sensing ligands and in binding target proteins broadens the ability of the plant to recognize extracellular stimuli and subsequently to transduce the various signals much more appropriately. This notion is well supported by experimental results. First, CBSX1 has been shown to have a variable ability to interact with downstream partner proteins.8 Second, the CBS domains recognize their own specific adenosine-containing ligands, such as AMP, adenosine diphosphate (ADP), adenosine triphosphate (ATP), diadenosine polyphosphate, nicotinamide adenine dinucleotide (NADH), and S-adenosyl methionine (SAM).8,15-19 Third, the CBS domain-only protein in Methanocaldococcus jannaschii binds to double-stranded DNA.20 It may, therefore, safely be assumed that the CBSXs meet the requirements to be a sensor relay protein like CaM and CBL.
Table 1. CBSX proteins in Arabidopsis thaliana.
| Name | Locus | A.A. length | Subcellular localization | Stimuli response |
|---|---|---|---|---|
| CBSX1 |
At4g36910 |
236 |
Chloroplast |
Yes |
| CBSX2 |
At4g34120 |
238 |
Chloroplast |
No |
| CBSX3 |
At5g10860 |
206 |
Mitochondria |
No |
| CBSX4 |
At1g80090 |
402 |
Cytosol |
Yes |
| CBSX5 |
At4g27460 |
391 |
Endoplasmic reticulum |
Yes |
| CBSX6 | At1g65320 | 425 | Endoplasmic reticulum, Vacuole | Yes |
Furthermore, to date, CBS domain-containing proteins (CDCPs) have been found in all kingdoms of life form except viruses. For example, eight CBS domain-containing proteins have been identified in Escherichia coli, 12 in Saccharomyces cerevisiae, 34 in Arabidopsis thaliana, 59 in Oryza sativa, and 75 in Homo sapiens.7,21,22 Interestingly, however, as explained above, proteins comprising only a pair of CBS domains without any other protein domains have been identified in prokaryotes and plants—six in Arabidopsis and 12 in Oryza,7,22 but as yet no such proteins have been found in animal genomes. Moreover, four and eight proteins having two pairs of CBS domains (CBSCBS) without any other protein domains have been found in Arabidopsis and Oryza, respectively.7 As previously reported in our article, the CBSXs form a homodimer to achieve their proper function,8 which implies that these CBSCBS proteins may have similar functional properties with the CBSX proteins. As a consequence, the absence of CBSX and CBSCBS proteins in animals and their presence in prokaryotes and plants strongly imply that due to their sessile nature, plants may have evolved many more perception–response mechanisms than animals to perceive various extracellular stimuli.
Acknowledgments
This research was supported by a grant (2011‑0015848) from the National Research Foundation of Korea (NRF) from the Korea Research Foundation funded by the Ministry of Science and Technology, and grants (PJ008198 and PJ008160) from the Next-Generation BioGreen21 Program funded by the Rural Development Administration, Republic of Korea.
Glossary
Abbreviations:
- CaM
calmodulin
- CBL
calcineurin B-like
- CIPK
CBL-interacting protein kinase
- CBS
cystathionine β-synthase
- CBSX
protein having only one pair of CBS domains without any other protein domains
- CBSCBS
protein having two pairs of CBS domains
- CDCP
CBS domain-containing protein
- Trx
thioredoxin
- AMP
adenosine monophosphate
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- NADH
nicotinamide adenine dinucleotide
- SAM
S-adenosyl methionine
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19945
References
- 1.Jonak C, Okrész L, Bögre L, Hirt H. Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol. 2002;5:415–24. doi: 10.1016/S1369-5266(02)00285-6. [DOI] [PubMed] [Google Scholar]
- 2.Harper JF, Breton G, Harmon AC. Decoding Ca(2+) signals through plant protein kinases. Annu Rev Plant Biol. 2004;55:263–88. doi: 10.1146/annurev.arplant.55.031903.141627. [DOI] [PubMed] [Google Scholar]
- 3.Kim MC, Panstruga R, Elliott C, Müller J, Devoto A, Yoon HW, et al. Calmodulin interacts with MLO protein to regulate defence against mildew in barley. Nature. 2002;416:447–51. doi: 10.1038/416447a. [DOI] [PubMed] [Google Scholar]
- 4.Park CY, Lee JH, Yoo JH, Moon BC, Choi MS, Kang YH, et al. WRKY group IId transcription factors interact with calmodulin. FEBS Lett. 2005;579:1545–50. doi: 10.1016/j.febslet.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 5.Anandalakshmi R, Marathe R, Ge X, Herr JMJ, Jr., Mau C, Mallory A, et al. A calmodulin-related protein that suppresses posttranscriptional gene silencing in plants. Science. 2000;290:142–4. doi: 10.1126/science.290.5489.142. [DOI] [PubMed] [Google Scholar]
- 6.Oh SI, Park J, Yoon S, Kim Y, Park S, Ryu M, et al. The Arabidopsis calcium sensor calcineurin B-like 3 inhibits the 5′-methylthioadenosine nucleosidase in a calcium-dependent manner. Plant Physiol. 2008;148:1883–96. doi: 10.1104/pp.108.130419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kushwaha HR, Singh AK, Sopory SK, Singla-Pareek SL, Pareek A. Genome wide expression analysis of CBS domain containing proteins in Arabidopsis thaliana (L.) Heynh and Oryza sativa L. reveals their developmental and stress regulation. BMC Genomics. 2009;10:200–21. doi: 10.1186/1471-2164-10-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo KS, Ok SH, Jeong B-C, Jung KW, Cui MH, Hyoung S, et al. Single cystathionine β-synthase domain-containing proteins modulate development by regulating the thioredoxin system in Arabidopsis. Plant Cell. 2011;23:3577–94. doi: 10.1105/tpc.111.089847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson TC, Cao RQ, Kung JE, Buchanan BB. Thioredoxin and NADP-thioredoxin reductase from cultured carrot cells. Planta. 1987;171:321–31. doi: 10.1007/BF00398677. [DOI] [PubMed] [Google Scholar]
- 10.Marcus F, Chamberlain SH, Chu C, Masiarz FR, Shin S, Yee BC, et al. Plant thioredoxin h: an animal-like thioredoxin occurring in multiple cell compartments. Arch Biochem Biophys. 1991;287:195–8. doi: 10.1016/0003-9861(91)90406-9. [DOI] [PubMed] [Google Scholar]
- 11.Rivera-Madrid R, Mestres D, Marinho P, Jacquot JP, Decottignies P, Miginiac-Maslow M, et al. Evidence for five divergent thioredoxin h sequences in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1995;92:5620–4. doi: 10.1073/pnas.92.12.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelhaye E, Rouhier N, Gérard J, Jolivet Y, Gualberto J, Navrot N, et al. A specific form of thioredoxin h occurs in plant mitochondria and regulates the alternative oxidase. Proc Natl Acad Sci U S A. 2004;101:14545–50. doi: 10.1073/pnas.0405282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juárez-Díaz JA, McClure B, Vázquez-Santana S, Guevara-García A, León-Mejía P, Márquez-Guzmán J, et al. A novel thioredoxin h is secreted in Nicotiana alata and reduces S-RNase in vitro. J Biol Chem. 2006;281:3418–24. doi: 10.1074/jbc.M511687200. [DOI] [PubMed] [Google Scholar]
- 14.Montrichard F, Alkhalfioui F, Yano H, Vensel WH, Hurkman WJ, Buchanan BB. Thioredoxin targets in plants: the first 30 years. J Proteomics. 2009;72:452–74. doi: 10.1016/j.jprot.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Tuominen H, Salminen A, Oksanen E, Jämsen J, Heikkilä O, Lehtiö L, et al. Crystal structures of the CBS and DRTGG domains of the regulatory region of Clostridium perfringens pyrophosphatase complexed with the inhibitor, AMP, and activator, diadenosine tetraphosphate. J Mol Biol. 2010;398:410–3. doi: 10.1016/j.jmb.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Jin X, Townley R, Shapiro L. Structural insight into AMPK regulation: ADP comes into play. Structure. 2007;15:1285–95. doi: 10.1016/j.str.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Meyer S, Savaresi S, Forster IC, Dutzler R. Nucleotide recognition by the cytoplasmic domain of the human chloride transporter ClC-5. Nat Struct Mol Biol. 2007;14:60–7. doi: 10.1038/nsmb1188. [DOI] [PubMed] [Google Scholar]
- 18.Dong A, Xu X, Edwards AM, Chang C, Chruszcz M, Cuff M, et al. Midwest Center for Structural Genomics. Structural Genomics Consortium In situ proteolysis for protein crystallization and structure determination. Nat Methods. 2007;4:1019–21. doi: 10.1038/nmeth1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas M, Encinar JA, Arribas EA, Oyenarte I, García IG, Kortazar D, et al. Binding of S-methyl-5′-thioadenosine and S-adenosyl-L-methionine to protein MJ0100 triggers an open-to-closed conformational change in its CBS motif pair. J Mol Biol. 2010;396:800–20. doi: 10.1016/j.jmb.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Aguado-Llera D, Oyenarte I, Martínez-Cruz LA, Neira JL. The CBS domain protein MJ0729 of M. jannaschii binds DNA. FEBS Lett. 2010;584:4485–9. doi: 10.1016/j.febslet.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Ignoul S, Eggermont J. CBS domains: structure, function, and pathology in human proteins. Am J Physiol Cell Physiol. 2005;289:C1369–78. doi: 10.1152/ajpcell.00282.2005. [DOI] [PubMed] [Google Scholar]
- 22.Baykov AA, Tuominen HK, Lahti R. The CBS domain: a protein module with an emerging prominent role in regulation. ACS Chem Biol. 2011;6:1156–63. doi: 10.1021/cb200231c. [DOI] [PubMed] [Google Scholar]