Abstract
In bacteria, MscS-type mechanosensitive channels serve to protect cells from lysis as they swell during extreme osmotic stress. We recently showed that two MscS homologs from Arabidopsis thaliana serve a similar purpose in the epidermal plastids of the leaf, indicating that the plant cell cytoplasm can present a dynamic osmotic challenge to the plastid. MscS homologs are predicted to be targeted to both plastids and mitochondrial envelopes and have been found in the genomes of intracellular pathogens. Here we discuss the implications of these observations, and propose that MS channels provide an essential mechanism for osmotic adaptation to both intracellular and the extracellular environments.
Keywords: MscS-Like, intracellular pathogen, mechanosensitive channel, osmotic stress, plastid
Most cells must face the fundamental challenge of surviving a dynamic osmotic environment. The concentrations of ions and other molecules within the cell are tightly controlled in order to provide an optimal environment for biochemical processes and to properly regulate cell shape and turgor.1 Hyperosmotic shock (a sharp increase in the osmolarity of the extracellular environment) results in the immediate efflux of water out of the cell and subsequent reduction in cell volume. Osmoadaptation then requires the uptake and synthesis of ions and compatible solutes, favoring the influx of water back into the cell.2 Hypoosmotic shock (a sharp decrease in the osmolarity of the extracellular environment) has the opposite effect—the influx of water and swelling of the cell—and cells must jettison osmolytes rapidly in order to prevent lysis.3
Organellar Response to Osmotic Stress
It has long been suspected that osmoregulatory mechanisms are not restricted to the plasma membrane. For example, early studies on chloroplasts showed that they are capable of volume regulation when challenged with salt stress.4,5 More recently, it has been demonstrated that the nucleus can respond to osmotic stress by changing volume, potentially altering gene expression through changes in chromatin condensation or nucleocytoplasmic transport.6,7 To further investigate the molecular mechanisms underlying organellar osmotic stress, we have focused on plastids of the Arabidopsis thaliana leaf epidermis. Plastids are plant-specific organelles that arose from the ancient endosymbiosis of a photosynthetic bacterium.8 They are essential for plant life and exist in a variety of forms, each plastid type specialized for the functions of the tissue in which it resides.9 While the large, highly developed chloroplasts found in the leaf mesophyll are responsible for photosynthesis, the small heterotrophic plastids found in the pavement cells of the leaf epidermis (referred to here as “leaf epidermal plastids”) import energy from the cytoplasm to fuel several essential metabolic reactions, including the synthesis of fatty acids, amino acids, pigment molecules and lipid storage.10
Plastids Relieve Osmotic Stress in an Evolutionarily Conserved Manner
Not surprisingly—given their evolutionary history as endosymbionts—the molecular mechanisms by which plastids respond to osmotic stress resemble those used by bacteria. When challenged with hyperosmotic shock, bacteria selectively accumulate ions and compatible solutes such as glycine betaine,11 and experiments both in planta and in vitro suggest that the same is true of chloroplasts.12,13 When challenged with hypoosmotic shock, many bacterial species are thought to employ mechanosensitive (MS) channels as osmotic safety valves, releasing osmolytes in response to increased membrane tension.14 In Escherichia coli, at least three MS channels contribute to the ability of cells to survive hypoosmotic shock, including MscS (Mechanosensitive channel of Small conductance), MscL (Mechanosensitive channel of Large conductance), and YbdG, which is a component of MscM (Mechanosensitive channel of Mini conductance).15,16 We recently demonstrated that two homologs of MscS (MscS-Like (MSL)2 and MSL3) from Arabidopsis thaliana serve a similar role in the plastid envelope.17
The leaves of plants harboring mutations in MSL2 and MSL3 exhibit large, round epidermal plastids that lack the dynamic extensions of the envelope known as stromules.18 Unexpectedly, this phenotype does not require exposure of the plants to environmental osmotic stress, but is seen under normal growth conditions. We hypothesized that plastids from msl2 msl3 mutants are enlarged due to an inability to release osmolytes when the cytoplasm is hypoosmotic compared with the plastid stroma—just as the cells of E. coli mutants lacking MscS and MscL swell to the point of lysis under hypoosmotic shock.16 To test this hypothesis, we used genetic, environmental and growth media manipulations to increase the cytoplasmic osmolarity of intact plants or leaves. In each case, the large, round phenotype of the msl2 msl3 mutant plastids was suppressed. In addition, exposing excised leaves to extreme hypoosmotic shock resulted in the lysis of plastids from msl2 msl3 mutants but not from wild type plants. We concluded from these data that leaf epidermal plastids experience dynamic hypoosmotic stress under normal growth conditions, and that MSL2 and MSL3 play a critical role in protecting plastids from this stress.
MscS Family Members in Organelles and Intracellular Pathogens
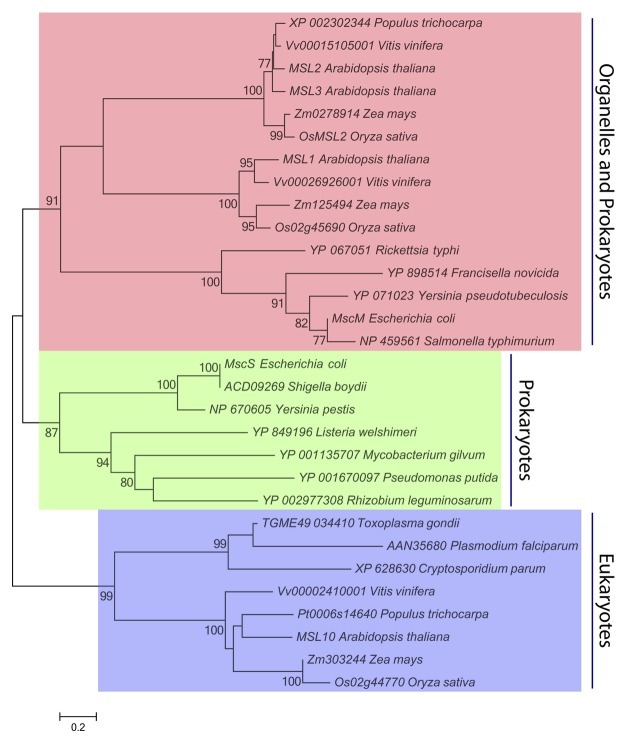
Phylogenetic analyses of the MscS family have suggested that the essential role of MscS-like channels might be extended to other organelles or to intracellular pathogens.19,20Figure 1 presents the inferred evolutionary relationship between 30 MscS homologs selected from both prokaryotic and eukaryotic species. The first of the three distinct clades in this tree is labeled “Organelles and Prokaryotes,” and comprises homologs from free-living and intracellular pathogenic bacterial species, homologs predicted to localize to plant mitochondria (e.g., Arabidopsis MSL1),24 and homologs predicted or known to localize to plant plastids (e.g., MSL2 and MSL3).18 The second clade, labeled “Prokaryotes,” comprises MscS homologs from free-living and pathogenic bacteria, including E. coli MscS and a homolog from the obligate intracellular pathogen Rickettsia typhi. The third clade, labeled “Eukaryotes,” comprises MscS homologs known or predicted to localize to the plasma membrane of plant cells (e.g., Arabidopsis MSL10)21 and homologs encoded in the genome of obligate eukaryotic intracellular pathogens (e.g., Plasmodium falciparum, the causative agent in malaria). Thus, if we consider the presence of MscS-like channels in the genome of an organism to indicate that it is likely to experience hypoosmotic stress during its life cycle, the phylogenetic tree shown in Figure 1 shows that neither endosymbiotic organelles nor intracellular pathogens are protected from osmotic stress by virtue of their location in the cytoplasm.
Figure 1. MscS Family Members in Organelles and Intracellular Pathogens. Thirty MscS homologs from 14 species of bacteria and 5 species of plants were identified by Aramemnon cluster analysis (http://aramemnon.botanik.uni-koeln.de/index.ep), previously published analyses,19,25 or inclusion in the Ortho MCL database (http://orthomcl.org/cgi-bin/OrthoMclWeb.cgi). The MscS-like region of each protein sequence was determined by InterProScan,26 and aligned using ClustalX27 with a multiple sequence alignment gap opening penalty of 3.0 and an extension penalty of 1.8. A total of 326 positions, including gaps, were present in the final data set. Evolutionary relationships were inferred by Neighbor-Joining28 and JTT distance matrix methods29 using MEGA5 software. The percentage of trees in which the associated taxa clustered together in the bootstrap test (n = 2000 replicates) are shown next to the branches.30 Only significant bootstrap values (greater than 75%) are shown, and branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. Scale bar, 0.2 amino acid substitutions per site.
Though MscS homologs are found in a wide array of prokaryotic and eukaryotic pathogens, a few obligate intracellular bacterial pathogens lack detectable MscS or MscL homologs, including Chlamydia, Clostridia, Mycoplasma and Ureaplasma.22These pathogens may have evolved alternate mechanisms for osmotic adjustment or may inhabit isoosmotic environments, thereby obviating the need for MS channels. Interestingly, MscL homologs (though not MscS homologs) are found in the genomes of Phytoplasmas, cell wall-less obligate intracellular pathogens that move between insect and plant hosts.23,24Perhaps, unlike the related Mycoplasmas and Ureaplasmas, Phytoplasmas require MS channels for osmotic stress relief as they move from the plant phloem (cells likely to contain high concentrations of photosynthate) to the insect gut, or from the phloem of source to that of sink tissue, or during diurnal cycles of photosynthate production.
In conclusion, the mechanosensitive channels MscS and MscL serve as osmotic safety valves in bacteria, presumably to deal with rapid osmotic changes in their extracellular environment. We recently reported that two MscS homologs from Arabidopsis serve the same function in the envelope of plastids. A phylogenetic analysis of MscS homologs presented here suggests that the cytoplasm may present osmotic stresses to mitochondria and to intracellular pathogens as well. Thus, the evolutionary relationship between bacterial and eukaryotic MscS homologs provides a unique opportunity to study the osmoregulatory mechanisms used by cells, organelles, and intracellular pathogens to survive dynamic environmental challenges. Furthermore, an investigation into the contribution of MscS homologs to the replication and virulence of both prokaryotic and eukaryotic intracellular pathogens may lead to the identification of a new class of therapeutic targets.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The research from our laboratory described in this manuscript was funded by NSF Award 0816627 and NIH Award GM084211.
Glossary
Abbreviations:
- MS
mechanosensitive
- MscS
Mechanosensitive channel of Small conductance
- MSL
MscS-Like
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19991
References
- 1.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev. 2007;87:1441–74. doi: 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- 2.Alfieri RR, Petronini PG. Hyperosmotic stress response: comparison with other cellular stresses. Pflugers Arch. 2007;454:173–85. doi: 10.1007/s00424-006-0195-x. [DOI] [PubMed] [Google Scholar]
- 3.Hohmann S, Krantz M, Nordlander B. Yeast osmoregulation. Methods Enzymol. 2007;428:29–45. doi: 10.1016/S0076-6879(07)28002-4. [DOI] [PubMed] [Google Scholar]
- 4.Robinson SP. Osmotic adjustment by intact isolated chloroplasts in response to osmotic stress and its effect on photosynthesis and chloroplast volume. Plant Physiol. 1985;79:996–1002. doi: 10.1104/pp.79.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCain DC. Combined effects of light and water stress on chloroplast volume regulation. Biophys J. 1995;69:1105–10. doi: 10.1016/S0006-3495(95)79984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finan JD, Guilak F. The effects of osmotic stress on the structure and function of the cell nucleus. J Cell Biochem. 2010;109:460–7. doi: 10.1002/jcb.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finan JD, Leddy HA, Guilak F. Osmotic stress alters chromatin condensation and nucleocytoplasmic transport. Biochem Biophys Res Commun. 2011;408:230–5. doi: 10.1016/j.bbrc.2011.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archibald JM. The puzzle of plastid evolution. Curr Biol. 2009;19:R81–8. doi: 10.1016/j.cub.2008.11.067. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Juez E, Pyke KA. Plastids unleashed: their development and their integration in plant development. Int J Dev Biol. 2005;49:557–77. doi: 10.1387/ijdb.051997el. [DOI] [PubMed] [Google Scholar]
- 10.Weber AP, Linka N. Connecting the plastid: transporters of the plastid envelope and their role in linking plastidial with cytosolic metabolism. Annu Rev Plant Biol. 2011;62:53–77. doi: 10.1146/annurev-arplant-042110-103903. [DOI] [PubMed] [Google Scholar]
- 11.Wood JM, Bremer E, Csonka LN, Kraemer R, Poolman B, van der Heide T, et al. Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:437–60. doi: 10.1016/S1095-6433(01)00442-1. [DOI] [PubMed] [Google Scholar]
- 12.Robinson S, Jones G. Accumulation of Glycinebetaine in Chloroplasts Provides Osmotic Adjustment During Salt Stress. Funct Plant Biol. 1986;13:659–68. [Google Scholar]
- 13.Chen TH, Murata N. Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 2008;13:499–505. doi: 10.1016/j.tplants.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Haswell ES, Phillips R, Rees DC. Mechanosensitive channels: what can they do and how do they do it? Structure. 2011;19:1356–69. doi: 10.1016/j.str.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumann U, Edwards MD, Rasmussen T, Bartlett W, van West P, Booth IR. YbdG in Escherichia coli is a threshold-setting mechanosensitive channel with MscM activity. Proc Natl Acad Sci U S A. 2010;107:12664–9. doi: 10.1073/pnas.1001405107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levina N, Tötemeyer S, Stokes NR, Louis P, Jones MA, Booth IR. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–7. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veley KM, Marshburn S, Clure CE, Haswell ES. Mechanosensitive channels protect plastids from hypoosmotic stress during normal plant growth. Curr Biol. 2012;22:408–13. doi: 10.1016/j.cub.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haswell ES, Meyerowitz EM. MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr Biol. 2006;16:1–11. doi: 10.1016/j.cub.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 19.Balleza D, Gómez-Lagunas F. Conserved motifs in mechanosensitive channels MscL and MscS. Eur Biophys J. 2009;38:1013–27. doi: 10.1007/s00249-009-0460-y. [DOI] [PubMed] [Google Scholar]
- 20.Pivetti CD, Yen MR, Miller S, Busch W, Tseng YH, Booth IR, et al. Two families of mechanosensitive channel proteins. Microbiol Mol Biol Rev. 2003;67:66–85. doi: 10.1128/MMBR.67.1.66-85.2003. [table of contents.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr Biol. 2008;18:730–4. doi: 10.1016/j.cub.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 22.Pivetti CD, Yen MR, Miller S, Busch W, Tseng YH, Booth IR, et al. Two families of mechanosensitive channel proteins. Microbiol Mol Biol Rev. 2003;67:66–85. doi: 10.1128/MMBR.67.1.66-85.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oshima K, Ishii Y, Kakizawa S, Sugawara K, Neriya Y, Himeno M, et al. Dramatic transcriptional changes in an intracellular parasite enable host switching between plant and insect. PLoS One. 2011;6:e23242. doi: 10.1371/journal.pone.0023242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christensen NM, Axelsen KB, Nicolaisen M, Schulz A. Phytoplasmas and their interactions with hosts. Trends Plant Sci. 2005;10:526–35. doi: 10.1016/j.tplants.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Haswell ES. MscS-Like Proteins in Plants. Current Topics in Membranes: Academic Press, 2007:329-59. [Google Scholar]
- 26.Zdobnov EM, Apweiler R. InterProScan--an integration platform for the signature-recognition methods in InterPro. Bioinformatics. 2001;17:847–8. doi: 10.1093/bioinformatics/17.9.847. [DOI] [PubMed] [Google Scholar]
- 27.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 28.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–82. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 30.Felsenstein J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution. 1985;39:783–91. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]