Abstract
Recently it was demonstrated that PO activity is switched by calcium within the typical range of apoplastic free calcium concentrations (Plieth and Vollbehr, Plant Signal Behav 2012;7: 650–660). The heat stability of POs is also dependent on calcium. Here, a scenario is put forward which assigns calcium a switch-off function under heat: Peroxidases are switched off by heat stress-triggered apoplastic calcium depletion. It is assumed that this initiates apoplastic accumulation of reactive oxygen species (ROS) and eventually triggers a self-amplifying cascade of cellular events involving plasma membrane ion transport. Calcium depletion-initiated ROS accumulation (CaDIRA) may also trigger signal percolation and the formation of systemic responses to many different stress factors in plants. This hypothesis can explain some as yet unexplained observations.
Keywords: calcium signaling, innate tolerance, peroxidase, positive feed-back signaling, self-amplifying signal
Peroxidases Involved In Molecular Signaling
Class III Peroxidases (POs) of higher plants typically reside in the apoplast (http://peroxibase.toulouse.inra.fr/cellular_localisation.php). They are assumed to facilitate plants to withstand biotic stress situations and pathogen attack.1 But, the whole diversity of their functions is not yet clear.2 POs depend on peroxides and calcium. Both, hydrogen peroxide and calcium ions, are signals of plant abiotic stress response.3-5 Interestingly, POs are the most heat stable enzymes of plants.6-9 So, a typical questions at this point is: Are POs possibly nodes in signaling networks mediating heat stress response?
Previously we demonstrated that peroxidases (POs) are Ca2+ triggered molecular switches.10 We also showed that heat stability of peroxidases from higher plants is dependent on Ca2+ too. However, POs are switched from heat sensitive to heat tolerant in the nM-range of [Ca2+] whereas their activity is switched in the µM range. The latter is the typical range of apoplastic free calcium [Ca2+]apo.11 Since H2O2, is both, a signaling molecule and a PO substrate, it is assumed that POs play a role in plant innate heat tolerance, heat response, and possibly other stress response routes.
Heat Stress, And Acquired And Innate Heat Tolerance
Often plants are exposed to temperatures above their optimum for several hours a day. In their natural habitat, plants thrive because they employ many different mechanisms to cope with heat stress.12,13 The current general model is that during heat stress, signaling cascades involving calcium14-16 and H2O217,18 as signal molecules initiate the expression of heat shock proteins,12,19 and other effectors20 which then function as chaperones and in concert regenerate heat-damaged proteins and/or prevent further proteins misfolding.21,22 This is acquisition of thermotolerance and is based on cellular events resulting from the exposure to high but sublethal temperatures.13 Thermotolerance acquisition takes resources but enables the organism to cope better with subsequent periods of heat.
The innate (or basal) thermotolerance has to be distinguished from acquired thermotolerance.23 It is the ability to withstand high temperatures without heat preconditioning. The primary thermostability of unadapted cellular modules like proteins and membranes thus constitutes innate thermotolerance. Consequently, species from habitats of different maximum day temperature have different innate thermotolerance because evolution maintained molecular modules of appropriate thermostability.
Timing Is Decisive: Heat Shock-Induced Calcium Flux May Cause A Deactivation Of Peroxidases In The Apoplast And Trigger A Self-Amplifying Positive Feed-Back Loop
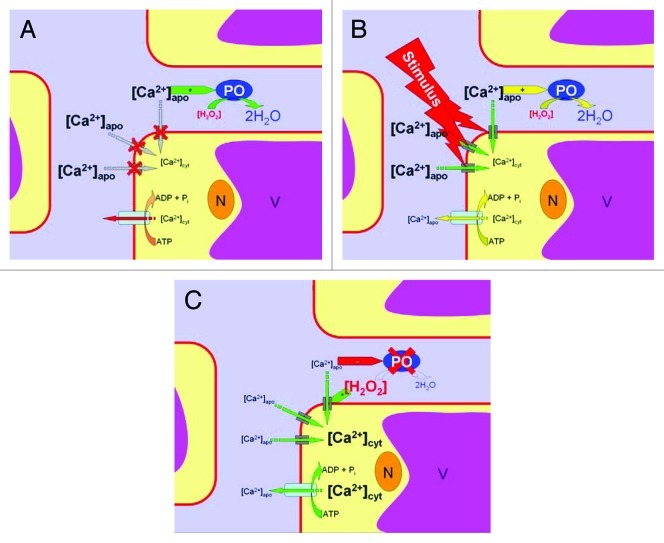
A movement of calcium ions between apoplast and cytoplasm during periods of heat is controversially reported. Some studies report an increase in cytoplasmic free calcium [Ca2+]cyt during heating14,16 whereas others do not.24 This is due to experimental timing as has been also shown for cold treatment.25,26 With rapid temperature changes a [Ca2+]cyt transient can be detected, however with moderate changes, [Ca2+]cyt transients are small or even below detection level. Thus, not the temperature alone but the rapidity of its change is the principal factor eliciting [Ca2+]cyt transients. Ca2+ involved in heat signaling is of extracellular (i.e., apoplastic) origin.12 Due to the high cytoplasmic calcium buffer capacity,27 a detectable increase of free [Ca2+]cyt in the cytoplasm reports a bulk Ca2+ ion flux from the apoplast into the cell. Thus, in a heat shock situation the cytoplasmic [Ca2+]cyt increases at the expense of apoplastic [Ca2+]apo. This causes massive Ca2+ depletion in the apoplast due to the much lower calcium buffer capacity in this compartment.28 In consequence, a shutdown of apoplastic PO activity occurs as has been demonstrated recently.10 This, in turn, causes a H2O2 accumulation in the apoplast because H2O2 consumption by POs is halted (Fig. 1). It is known that H2O2 activates cation transporters and favors further cellular calcium ion influx.29-32 This accelerates apoplastic calcium depletion. Thereby, PO inhibition and ROS accumulation is potentiated. ROS, accumulating in the apoplast, may then spread by diffusion and trigger other cells and serve as messengers for signal cascades. Hence, it is a calcium-depletion-initiated-ROS-accumulation (CaDIRA) in the apoplast (Fig. 1) providing a positive feed back amplification which probably makes an effect and initiates pathways of stress response in general and of thermotolerance acquisition in particular. In other words, calcium 'chairs' stimulus-response coupling and signal amplification in the apoplast through CaDIRA. Other scenarios have been proposed earlier. Among these are also models involving self-amplifying feed-back loops33 and depletion-refilling of Ca2+ stores.34
Figure 1. The calcium-depletion-initiated-ROS-accumulation (CaDIRA) scenario. Calcium 'chairs' stimulus-response coupling and signal amplification in the apoplast through a positive feed-back loop. Color coding: pale blue = apoplast, yellow = cytoplasm, magenta = vacuole, brown = nucleus, red = plasma membrane. (A) In a resting system cytoplasmic calcium [Ca2+]cyt is kept low by active transport. Apoplastic [Ca2+]apo in contrast is high. This promotes peroxidase (PO) activities which keep H2O2 concentration low in the apoplast. (B) Upon heat stress ('stimulus') cation channels are activated and Ca2+ ions flow downstream, producing a [Ca2+]cyt increase at the expense of extracellular Ca2+. The apoplastic Ca2+ pool starts to deplete. (C) At low [Ca2+]apo, PO activities are reduced. This, among other processes, causes H2O2 accumulation. H2O2 in turn promotes Ca2+ flux from the apoplast into the cell which causes persisting [Ca2+]apo depletion, reduced PO activity, increased H2O2, and finally a burst of extracellular ROS and intracellular [Ca2+]cyt.
The CaDIRA Scenario Can Answer Some Yet Open Questions
This self amplifying mechanism may further deplete calcium from the apoplast, so that finally nM levels of [Ca2+]apo are reached. This in turn switches POs from heat stable to heat sensitive.10 This way, under continuing heat, POs are irreversibly heat inactivated. Thus, CaDIRA (Fig. 1) can explain the finding that even moderate heat (42°C) is sufficient to reduce PO activity by almost 50%,35 when temperature was increased quickly (i.e., 'heat shock' was applied). A slower increase in temperature probably causes a reduced calcium depletion in the apoplast, since calcium pumps, employed for cytosolic calcium clearance and apoplast refilling, can keep up with heat-induced Ca2+ influx. Consequently, with moderate temperature changes, PO activity is reduced, but the heat stability of the enzymes is less affected since ROS fail to accumulate above precarious levels.
Other abiotic and biotic stimuli are known to elicit [Ca2+]cyt transients.36,37 Some [Ca2+]cyt transients originate from Ca2+ release from intracellular stores38 and others from the extracellular space. The latter can influence apoplastic enzymes and plasma membrane ion transport. This is known for chloride transporters for instance, when the plant is under salt stress. Chloride transport into the cells during salt stress is accelerated when Ca2+ is depleted outside the cells and conversely, Ca2+ addition outside the cell reduces chloride transport and alleviates salt stress.39 A direct interdependence between [Ca2+]cyt and [Ca2+]apo has been demonstrated.40 When salt is withdrawn from salt-adapted root cells then [Ca2+]apo decreases in parallel to a [Ca2+]cyt increase. This suggests that such an effect can also be seen with heat stress. Evidently, more detailed investigations are needed.
Often, physiological responses take place away from the primary site of stimulation. This requires cell-to-cell communication and a systemic spread of signals.41 Here, the CaDIRA scenario depicted above (Fig. 1) can put ideas across and provide explanations: With a local stimulation (e.g., attack of a phytophagous insect or local heat treatment) few cells around the site of stimulation undergo CaDIRA and switch to an 'excited state'. Since membrane depolarization and calcium flux are involved, apoplastic calcium depletion quickly occurs at the other end of the excited cell with almost no time lag and initiates CaDIRA at neighboring cells. This way, the signal could percolate through the plant tissue with velocities not limited to diffusion rates in the apoplast.
The notion of apoplastic calcium signaling is in line with other studies showing that [Ca2+]apo also controls other extracellular enzymes like cell wall phosphatases.42 In addition, apoplastic calmodulin plays an important role in signal transduction.43-45
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20007
References
- 1.Penel C, Dunand C. Signaling via Plant Peroxidases. In: Baluška F, Vivanco J, eds. Signaling in Plants: Springer Berlin Heidelberg, 2009:155-71. [Google Scholar]
- 2.Bakalovic N, Passardi F, Ioannidis V, Cosio C, Penel C, Falquet L, et al. PeroxiBase: a class III plant peroxidase database. Phytochemistry. 2006;67:534–9. doi: 10.1016/j.phytochem.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Knight H, Knight MR. Abiotic stress signalling pathways: specificity and cross-talk. Trends Plant Sci. 2001;6:262–7. doi: 10.1016/S1360-1385(01)01946-X. [DOI] [PubMed] [Google Scholar]
- 4.Neill S, Desikan R, Hancock J. Hydrogen peroxide signalling. Curr Opin Plant Biol. 2002;5:388–95. doi: 10.1016/S1369-5266(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 5.Laloi C, Apel K, Danon A. Reactive oxygen signalling: the latest news. Curr Opin Plant Biol. 2004;7:323–8. doi: 10.1016/j.pbi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Lee CY, Pennesi AP. Isolation and further characterization of a heat resistant peroxidase isoenzyme from Cauliflower. J Food Sci. 1984;49:1616–7. doi: 10.1111/j.1365-2621.1984.tb12859.x. [DOI] [Google Scholar]
- 7.Burnette FS. Peroxidase and its relationship to food flavor and quality: a review. J Food Sci. 1977;42:1–6. doi: 10.1111/j.1365-2621.1977.tb01204.x. [DOI] [Google Scholar]
- 8.Hendrickx M, Saraiva J, Lyssens J, Oliveira J, Tobback P. The influence of water activity on thermal stability of horseradish peroxidase. Int J Food Sci Technol. 1992;27:33–40. doi: 10.1111/j.1365-2621.1992.tb01175.x. [DOI] [Google Scholar]
- 9.Morales-Blancas EF, Chandia VE, Cisneros-Zevallos L. Thermal inactivation kinetics of peroxidase and lipoxygenase from broccoli, green asparagus and carrots. J Food Sci. 2002;67:146–54. doi: 10.1111/j.1365-2621.2002.tb11375.x. [DOI] [Google Scholar]
- 10.Plieth C, Vollbehr S. Calcium confers activity and innate heat tolerance on plant peroxidases. Plant Signal Behav. 2012;7:650–660. doi: 10.1111/psb.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felle HH, Hanstein S, Sattelmacher B, Horst WJ. Probing apoplastic ion relations in Vicia faba as influenced by nutrition and gas exchange. In: Sattelmacher B, Horst W, eds. The Apoplast of higher plants: Compartment of storage, transport and reactions. Dordrecht: Springer Netherlands, 2007:295-306. [Google Scholar]
- 12.Saidi Y, Finka A, Goloubinoff P. Heat perception and signalling in plants: a tortuous path to thermotolerance. New Phytol. 2011;190:556–65. doi: 10.1111/j.1469-8137.2010.03571.x. [DOI] [PubMed] [Google Scholar]
- 13.Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: An overview. Environ Exp Bot. 2007;61:199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- 14.Gong M, van der Luit AH, Knight MR, Trewavas AJ. Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol. 1998;116:429–37. doi: 10.1104/pp.116.1.429. [DOI] [Google Scholar]
- 15.Liu HT, Gao F, Cui SJ, Han JL, Sun DY, Zhou RG. Primary evidence for involvement of IP3 in heat-shock signal transduction in Arabidopsis. Cell Res. 2006;16:394–400. doi: 10.1038/sj.cr.7310051. [DOI] [PubMed] [Google Scholar]
- 16.Liu H-T, Li B, Shang Z-L, Li X-Z, Mu R-L, Sun D-Y, et al. Calmodulin is involved in heat shock signal transduction in wheat. Plant Physiol. 2003;132:1186–95. doi: 10.1104/pp.102.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dat JF, Lopez-Delgado H, Foyer CH, Scott IM. Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 1998;116:1351–7. doi: 10.1104/pp.116.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong M. Chen Bo, Li Z-G, Guo L-H. Heat-shock-induced cross adaptation to heat, chilling, drought and salt stress in maize seedlings and involvement of H2O2. J Plant Physiol. 2001;158:1125–30. doi: 10.1078/0176-1617-00327. [DOI] [Google Scholar]
- 19.Voellmy R, Boellmann F. Chaperone regulation of the heat shock protein response. In: Csermely P, Vígh L, eds. Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks: Springer New York, 2007:89-99. [DOI] [PubMed] [Google Scholar]
- 20.Sung D-Y, Kaplan F, Lee K-J, Guy CL. Acquired tolerance to temperature extremes. Trends Plant Sci. 2003;8:179–87. doi: 10.1016/S1360-1385(03)00047-5. [DOI] [PubMed] [Google Scholar]
- 21.Wang W, Vinocur B, Shoseyov O, Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9:244–52. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Waters ER, Lee GJ, Vierling E. Evolution, structure and function of the small heat shock proteins in plants. J Exp Bot. 1996;47:325–38. doi: 10.1093/jxb/47.3.325. [DOI] [Google Scholar]
- 23.Larkindale J, Hall JD, Knight MR, Vierling E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005;138:882–97. doi: 10.1104/pp.105.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larkindale J, Knight MR. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002;128:682–95. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plieth C, Hansen UP, Knight H, Knight MR. Temperature sensing by plants: the primary characteristics of signal perception and calcium response. Plant J. 1999;18:491–7. doi: 10.1046/j.1365-313X.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- 26.Nagel-Volkmann J, Plieth C, Becker D, Lüthen H, Dörffling K. Cold-induced cytosolic free calcium ion concentration changes in wheat. J Plant Physiol. 2009;166:1955–60. doi: 10.1016/j.jplph.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Plieth C, Sattelmacher B, Hansen UP. Cytoplasmic Ca2+-H+-exchange buffers in green algae. Protoplasma. 1997;198:107–24. doi: 10.1007/BF01282136. [DOI] [Google Scholar]
- 28.Oja V, Savchenko G, Jakob B, Heber U. pH and buffer capacities of apoplastic and cytoplasmic cell compartments in leaves. Planta. 1999;209:239–49. doi: 10.1007/s004250050628. [DOI] [PubMed] [Google Scholar]
- 29.Rentel MC, Knight MR. Oxidative stress-induced calcium signaling in Arabidopsis. Plant Physiol. 2004;135:1471–9. doi: 10.1104/pp.104.042663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price AH, Taylor A, Ripley SJ, Griffiths A, Trewavas AJ, Knight MR. Oxidative signals in tobacco increase cytosolic calcium. Plant Cell. 1994;6:1301–10. doi: 10.1105/tpc.6.9.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pei Z-M, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–4. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 32.Mori IC, Schroeder JI. Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol. 2004;135:702–8. doi: 10.1104/pp.104.042069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagi M, Fluhr R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006;141:336–40. doi: 10.1104/pp.106.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowler C, Fluhr R. The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci. 2000;5:241–6. doi: 10.1016/S1360-1385(00)01628-9. [DOI] [PubMed] [Google Scholar]
- 35.Saleh L, Plieth C. Fingerprinting antioxidative activities in plants. Plant Methods. 2009;5:2. doi: 10.1186/1746-4811-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plieth C. Calcium: just another regulator in the machinery of life? Ann Bot. 2005;96:1–8. doi: 10.1093/aob/mci144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plieth C. Plant calcium signaling and monitoring: pros and cons and recent experimental approaches. Protoplasma. 2001;218:1–23. doi: 10.1007/BF01288356. [DOI] [PubMed] [Google Scholar]
- 38.Plieth C, Sattelmacher B, Hansen UP, Thiel G. The action potential in Chara: Ca2+ release from internal stores visualized by Mn2+-induced quenching of fura-dextran. Plant J. 1998;13:167–75. doi: 10.1046/j.1365-313X.1998.00019.x. [DOI] [Google Scholar]
- 39.Lorenzen I, Aberle T, Plieth C. Salt stress-induced chloride flux: a study using transgenic Arabidopsis expressing a fluorescent anion probe. Plant J. 2004;38:539–44. doi: 10.1111/j.0960-7412.2004.02053.x. [DOI] [PubMed] [Google Scholar]
- 40.Gao D, Knight MR, Trewavas AJ, Sattelmacher B, Plieth C. Self-reporting Arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiol. 2004;134:898–908. doi: 10.1104/pp.103.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plieth C. Signal percolation through plants and the shape of the calcium signature. Plant Signal Behav. 2010;5:379–85. doi: 10.4161/psb.5.4.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeMarty M, Morvan C, Thellier M. Calcium and the Cell Wall. Plant Cell Environ. 1984;7:441–8. doi: 10.1111/j.1365-3040.1984.tb01434.x. [DOI] [Google Scholar]
- 43.Ma L, Sun DY. The effects of extracellular calmodulin on initiation of Hippeastrum rutilum pollen germination and tube growth. Planta. 1997;202:336–40. doi: 10.1007/s004250050135. [DOI] [Google Scholar]
- 44.Ma L, Xu X, Cui S, Sun D. The presence of a heterotrimeric G protein and its role in signal transduction of extracellular calmodulin in pollen germination and tube growth. Plant Cell. 1999;11:1351–64. doi: 10.1105/tpc.11.7.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang J, Wu S, Bai J, Sun DY. Extracellular calmodulin-binding proteins in plants: purification of a 21-kDa calmodulin-binding protein. Planta. 1996;198:510–6. doi: 10.1007/BF00262636. [DOI] [PubMed] [Google Scholar]