Abstract
Different from unicellular organisms, metazoan cells require the presence of extracellular growth factors to utilize environmental nutrients. However, the underlying mechanism was unclear. We have delineated a pathway, in which glycogen synthase kinase 3 (GSK3) in cells deprived of growth factors phosphorylates and activates the acetyltransferase KAT5/TIP60, which in turn stimulates the protein kinase ULK1 to elicit autophagy. Cells with the Kat5/Tip60 gene replaced with Kat5S86A that cannot be phosphorylated by GSK3 are resistant to serum starvation-induced autophagy. Acetylation sites on ULK1 were mapped to K162 and K606, and the acetylation-defective mutant ULK1K162,606R displays reduced kinase activity and fails to rescue autophagy in Ulk1−/− mouse embryonic fibroblasts, indicating that acetylation is vital to the activation of ULK1. The GSK3-KAT5-ULK1 cascade seems to be specific for cells to sense growth factors, as KAT5 phosphorylation is not enhanced under glucose deprivation. Distinct from the glucose starvation-autophagy pathway that is conserved in all eukaryotic organisms, the growth factor deprivation response pathway is perhaps unique to metazoan organisms.
Keywords: GSK3, Tip60, Ulk1, acetylation, autophagy, growth factor, phosphorylation
One signature feature of metazoan cells is that the uptake of the extracellular nutrients is strictly controlled by growth factors. Without growth factor signaling, the surface expression of nutrient transporters is decreased, leading to exhaustion of energy and synthetic materials inside the cells. Autophagy, a self-eating catabolic process, is then activated to recycle unnecessary proteins and organelles for survival. A series of autophagy-related genes, known as ATG genes, have been identified and characterized, among which ATG1 (ULK1 and ULK2 in mammals) encodes a critical kinase for formation of autophagosomes. The energy sensor signaling pathway of MTOR is thought to act as a switch for autophagy initiation in response to nutrient shortage. By virtue of phosphorylation, MTOR inhibits ULK1 when the cell is under ample nutrient supply. The “inhibition pathway” by MTOR in autophagy control depicts that in cells with sufficient nutrients MTOR is activated and inhibits ULK1 through phosphorylation. Only when MTOR is inhibited can ULK1 be active and signal to elicit autophagy. In addition, recent studies have pointed out that the AMPK-ULK1/2 route is pivotal in mediating glucose deprivation, or a general nutrient shortage as in cells cultured in HBSS, to allow autophagy induction. However, signaling pathways specific for growth factor deprivation-induced autophagy, which may represent an evolutionarily advanced phenomenon, were largely unknown.
Our study started from characterizing the phosphorylation patterns of KAT5/TIP60, which led to the finding that GSK3 phosphorylates the Ser86 residue of KAT5 after a priming phosphorylation on Ser90, an event that is yet to be further defined. Although cyclin-dependent kinases can catalyze the priming phosphorylation, it remains unclear how Ser90 phosphorylation is regulated; in particular, phosphorylation of KAT5 Ser90 seems to be a constitutive event. As GSK3 is a common downstream kinase impinged upon by many signaling cascades including the PtdIns3K-AKT1 pathway, we explored whether KAT5 Ser86 phosphorylation was increased under serum deprivation by using a monoclonal antibody specifically against phospho-Ser86-KAT5. We showed that the phosphorylation on Ser86 of KAT5 was strongly elevated in cells deprived of serum, which was blocked by depleting cells with siRNA against Gsk3 or treating cells with GSK3 inhibitors. We further investigated the role of KAT5 and its phosphorylation in serum deprivation-induced autophagy. Knockdown of KAT5 by siRNA in HCT116 cells or knock-in of Kat5S86A in MEF cells hinders the induction of autophagy.
Next, we investigated the biochemical and biological consequence of KAT5 phosphorylation on Ser86. We found that KAT5 interacts with ULK1, which is enhanced in cells deprived of serum, concurrent with an increase in KAT5 Ser86 phosphorylation. KAT5 acetylates ULK1 on Lys162 and Lys606, and the KAT5S86A mutant shows reduced acetylation activity toward ULK1; inhibition of GSK3 by inhibitors also causes reduced acetylation on ULK1 in cells deprived of serum. An acetylation-phosphorylation coupled in vitro assay demonstrates an increased kinase activity of acetylated ULK1. Expression of the ULK1 mutant ULK1K162/606R that cannot be acetylated fails to rescue autophagy in Ulk1−/− MEFs. We have thus delineated a signaling pathway linking growth factor deprivation to autophagy induction (Fig. 1).
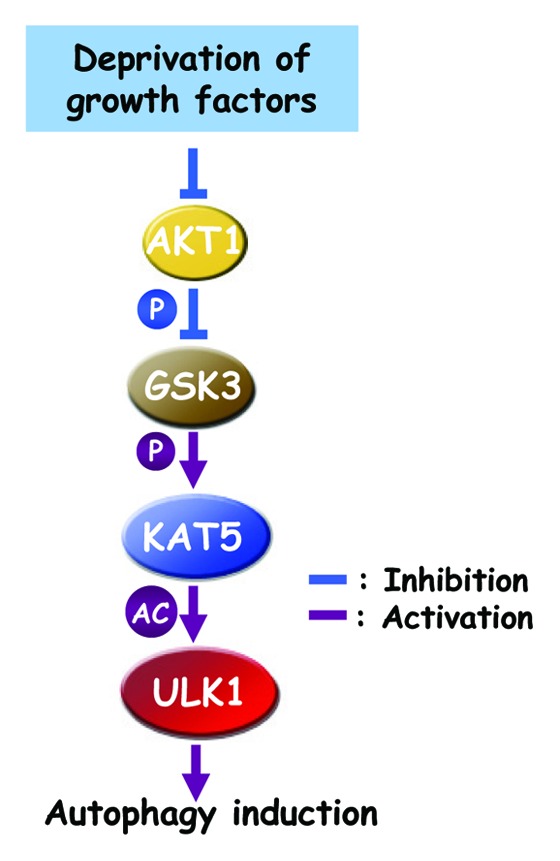
Figure 1. Simplified model of autophagy induction by growth factor deprivation.
We also demonstrated that glucose starvation and growth factor deprivation do not take the same signaling route to autophagy. In Ampkα−/− MEF cells, serum deprivation effectively induced KAT5 Ser86 phosphorylation and ULK1 acetylation that depend on the activity of GSK3. The product of ESA1 (yeast KAT5) is required in nitrogen depletion-induced autophagy in yeast, showing conservation of the involvement of this acetyltransferase in autophagy among different species. Whether amino acid deprivation would activate the GSK3-KAT5-ULK1 route and whether KAT5 would play a more general role in autophagy induced by a wider spectrum of signals await further investigation. More importantly, what actual pathophysiological conditions utilize the GSK3-KAT5-ULK1 pathway shall be of great interest in the future.
Acknowledgments
This work is supported by grants from the 973 Program (#2011CB910800), NSFC (#31130016, #30921005, and #31000621), Fundamental Research Funds for the Central Universities (#2010121094) and the 111 Project of MOE of China (#B06016).
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/20959


