Abstract
Accumulating evidence attests to a prosurvival role for autophagy under stress, by facilitating removal of damaged proteins and organelles and recycling basic building blocks, which can be utilized for energy generation and targeted macromolecular synthesis to shore up cellular defenses. These observations are difficult to reconcile with the dichotomous prosurvival and death-inducing roles ascribed to macroautophagy in cardiac ischemia and reperfusion injury, respectively. A careful reexamination of ‘flux’ through the macroautophagy pathway reveals that autophagosome clearance is markedly impaired with reperfusion (reoxygenation) in cardiomyocytes following an ischemic (hypoxic) insult, resulting from reactive oxygen species (ROS)-mediated decline in LAMP2 and increase in BECN1 abundance. This results in impaired autophagy that is ‘ineffective’ in protecting against cell death with ischemia-reperfusion injury. Restoration of autophagosome clearance and by inference, ‘adequate’ autophagy, attenuates reoxygenation-induced cell death.
Keywords: BECN1, LAMP2, autophagic flux, cell death, ischemia-reperfusion, reactive oxygen species
Myocardial infarction most commonly results from thrombotic occlusion of a coronary artery resulting in ischemia; and spontaneous or therapeutic reperfusion offers the best hope for myocardial salvage. However, reperfusion is accompanied by a burst of ROS generation and further injury, causing cardiomyocyte death and myocardial dysfunction. Despite significant research efforts, therapeutic options for reperfusion injury are limited to preventive pre-administration of pharmacological agents, which is impractical in the clinical setting. Understanding the role of autophagy in cardiomyocyte death in myocardial infarction, therefore, holds tremendous promise in development of strategies to promote myocardial salvage. Contemporaneous studies have employed autophagosome abundance as a readout for autophagy, and conclude that induction of autophagy via activation of adenosine monophosphate-activated kinase (AMPK) is beneficial during the ischemic phase, but further activation of autophagy, i.e. an increase in autophagosome prevalence, by BECN1 upregulation causes cell death during reperfusion, thus presenting a conundrum in the development of a therapeutic approach targeting autophagy in ischemia-reperfusion injury.
Since ‘autophagy’ is a process, and assessment of its efficiency should ideally require assessment of the rate of degradation of its substrates (such as damaged proteins and organelles) and/or the rate of generation of the end products (amino acids, sugars and lipids recycled back into the cytosol from the lysosomes), reliance on the levels of a single intermediate, such as autophagosomes, may not yield accurate conclusions. To overcome the limitation of the lack of a suitable single assay to track the efficiency of macroautophagy, we have employed complementary approaches to assess the autophagy process, namely autophagosome abundance in the presence or absence of an inhibitor of lysosomal acidification to assess ‘flux’; relative abundance of autophagosomes and autolysosomes; relative levels of SQSTM1/p62, an adaptor protein that gets consumed during autophagy; and the clearance of polyglutamine aggregates that are processed through macroautophagy. Our results indicate that autophagosome clearance may be partially impaired during ischemia and is severely impaired during reperfusion, resulting in accumulation of autophagosomes, and presumably undigested cargo, which manifests in further ROS generation, mitochondrial permeabilization and a necrotic mechanism of cell death (Fig. 1). Importantly, restoring autophagosome processing and reestablishing adequate flux through macroautophagy attenuates reoxygenation-induced cardiomyocyte death.
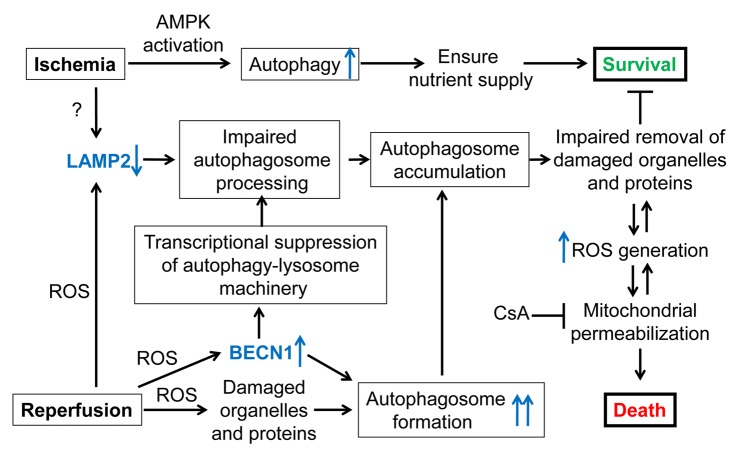
Figure 1. Schematic depicting the proposed outcomes of autophagy in myocardial ischemia-reperfusion injury. Autophagosome formation is induced by ischemia (hypoxia) via AMPK activation, and further stimulated by reperfusion (reoxygenation) secondary to ROS-induced cellular injury. Previous work suggests a critical prosurvival role for autophagy in ischemia, by ensuring nutrient supply. Our data demonstrate that both ischemia and reperfusion lead to a decline in LAMP2, which is important for autophagosome-lysosome fusion. Reperfusion-induced ROS also provokes increased BECN1 abundance, which stimulates autophagosome formation, but results in transcriptional inhibition of the autophagy-lysosome machinery. Together, these provoke marked impairment of autophagosome clearance with resultant autophagosome accumulation, and impaired removal of damaged cellular constituents. This sets up a vicious cycle of increased ROS generation and mitochondrial permeabilization, culminating in necrotic cell death, which can be attenuated by cyclosporin A (CsA), an inhibitor of mitochondrial permeability transition pore. ‘?’ indicates undefined mechanisms.
The mechanism for the impairment in autophagosome processing appears two-fold. Both ischemia and reperfusion induce a decline in levels of LAMP2, a lysosome membrane protein that plays an important role in autophagosome-lysosome fusion, which is driven by ROS generation with reperfusion. Accordingly, exogenous expression of either the LAMP2A or LAMP2B isoform results in restoration of autophagosome processing in cardiomyocytes subjected to hypoxia-reoxygenation. Additionally, reperfusion induces a ROS-driven increase in BECN1 abundance. However, shRNA mediated knockdown of Becn1 transcripts reveals surprising and dichotomous outcomes with reoxygenation, which are dependent on the degree of knockdown in hypoxia-reoxygenation-treated cardiomyocytes. Partial Becn1 knockdown results in restoration of relative predominance of autolysosomes, suggesting restored autophagosome processing; whereas a more complete knockdown prevents autophagosome formation. Importantly, partial Becn1 knockdown also prevents reoxygenation-induced cardiomyocte death, mirroring previous observations of reduced infarct size in Becn1 haplo-insufficient mice subjected to ischemia-reperfusion injury, in vivo; and a more complete Becn1 knockdown impairs autophagosome formation and increases cell death, confirming a critical prosurvival role for autophagy in this setting.
Furthermore, our data suggest that BECN1 levels transcriptionally regulate components of the autophagy-lysosome machinery such that Becn1 knockdown results in early (within 48 h) transcriptional upregulation of candidate genes as Map1lc3b, Sqstm1, Rab7 and Lamp1 with buildup of the respective protein levels, followed by transcriptional downregulation (except for the Sqstm1 transcript, which stimulates its own transcription) at 72 h. Interestingly, exogenous expression of BECN1 to supranormal levels transcriptionally downregulates these genes (except for Sqstm1); and taken together these observations point to a critical role for BECN1 protein abundance in regulating a transcriptional network that responds to activation of autophagy to prime the machinery for its successful and continued ‘adequate’ execution. Unraveling the details of this network is the focus of ongoing studies. Based on these data, we posit that reperfusion-induced ROS stabilizes BECN1 protein resulting in increased BECN1 abundance, which transcriptionally suppresses the autophagy-lysosome machinery and paradoxically causes an impairment in autophagosome processing, with resultant accumulation of autophagosomes (and undegraded cargo) and cell death. Partial knockdown of BECN1 in this setting relieves the transcriptional inhibition of the autophagy-lysosome machinery, enhancing autophagic flux, whereas adequate BECN1 protein is available for its obligate role in autophagosome formation. It is conceivable that other potential outcomes of increased BECN1 levels, such as interaction with KIAA0226/rubicon to inhibit autophagosome processing, pro-apoptogenic caspase-mediated cleavage, modulation of deubiquitinating enzymes and TP53/p53, and other hitherto undiscovered mechanisms contribute to its deleterious role in reperfusion-induced cell death, and will require further investigation.
In summary, our data demonstrate that autophagy is impaired at the late stages, i.e., after the formation of autophagosomes, in cardiac ischemia-reperfusion injury, which renders autophagy ‘inadequate’ in preventing cardiomyocyte death. The results also underscore the need to carefully evaluate the efficiency of the ‘process’ of autophagy with multiple readouts at various steps, spread out temporally, to prevent erroneous conclusions. Importantly, there remains an acute need for development of better assays to assess autophagic flux in vivo and in vitro, which will facilitate accurate conclusions regarding its role in health and disease.
Acknowledgements
We thank Joseph A. Hill of the University of Texas Southwestern Medical Center, Dallas; and Douglas L. Mann of Washington University, for their insightful comments and support. This manuscript was supported by an R01 grant from the NIH (HL107594) to A.D.
Glossary
Abbreviations:
- ROS
reactive oxygen species
- LAMP2
lysosome associated membrane protein 2
- AMPK
adenosine monophosphate-activated protein kinase
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/21036