Abstract
Comment on: Tanaka Y, et al. Proc Natl Acad Sci USA 2012; 109:4515-20.
Keywords: Runx1, development, hematopoiesis, hematopoietic stem cells, yolk sac
Hematopoiesis starts with generation of “primitive” erythroblasts and macrophage progenitors in the visceral yolk sac. The earliest strictly defined adult-type or definitive HSCs can be detected in the AGM region at around E11.1 Accumulation of stem cells activity in the explant cultures of the AGM region strengthened a growing perception that HSCs originate intraembryonically and mammalian hematopoiesis as a whole has multiple origins.1 Several lines of evidence suggest that dHSCs derive from a transitory subset of endothelial cells called hemogenic endothelium. HSC precursors were directly traced to conceptus cells that express VE-cadherin,2 a specific marker of endothelial lineage. Selective deletion of Runx1 in VE-cadherin+ cells resulted in the ablation of all hematopoietic progenitors including HSCs and development of the characteristic Runx1-null phenotype.3 Emergence of HIACs during midgestation has also been regarded as evidence in support of dHSC generation by dorsal aorta endothelium. A strong lymphoid potential of the pre-circulation embryo proper suggests that dHSC precursors segregate in situ from the P-Sp mesoderm4 that precedes the appearance of the aorta hemogenic endothelium.
This theory, however, has some important caveats. First, endothelial cells of midgestation dorsal aorta do not proliferate,5 leaving HIACs to be generated either through vascular remodeling or blood cell clustering.6 Robust dHSC expansion in reaggregation AGM explant cultures7 indicates that dHSC maturation in the AGM region is largely structure-independent and dHSCs are unlikely to arise through the remodeling mechanism. Second, the failure of E9.5 P-Sp/AGM explant cultures to produce dHSCs8 is not consistent with the proposed key role of the P-Sp/AGM region in stem cell origin. Third, the earliest dHSCs are also found in other vascular territories at similar frequencies and at the same time as in the AGM region.1 Taken together, these observations imply that there exists an early anatomical source of dHCS precursors, which enter those territories after E9.5.
This source might be located in the visceral yolk sac, as was proposed in the early studies.9 In our recent work we systematically analyzed the anatomical origin of hematopoietic system and, specifically, the HSC lineage.10 To put our investigation in the context of published data, we first re-examined the lymphoid potential of the early conceptus. The results demonstrated how much the outcome of cell potential measurements is dependent on the assay conditions. Replacing S17 stromal cell line for M-CSF-deficient OP9 cells allowed for detection of a robust lymphoid potential of the pre-circulation yolk sac precursors. These precursors highly coexpress Runx1 and VE-cadherin, which may explain the results of VE-cadherin-dependent Runx1 ablation experiments.3 At this stage, cells with this phenotype could not be found in the embryo proper and it would be interesting to trace the progeny of the Runx1+VE-cadherin+ precursors in the model of Zovein and colleagues.2 It has to be noted, however, that the artificial in vitro conditions may influence initial commitment of early conceptus cells and entice them to enter alternative developmental pathways.
In our non-invasive in vivo rescue system, Runx1, a key hematopoietic transcriptional factor, is reactivated in Runx1-null conceptuses at specific stages of gestation. Restoration of Runx1 activity at E7.5 efficiently rescued the development of fetal liver dHSCs. All hematopoietic cells in the rescued embryos descended exclusively from the early Runx1+ precursor cells that sustained reactivation of Runx1 locus. This is a strongest indication of the cell-autonomous function of Runx1 in the hematopoietic development. To locate the Runx1+ rescuable precursors of dHSCs in way that avoids LacZ staining artifacts and excludes cells with incompletely transcribed Runx1 locus, we performed in situ hybridization of the early conceptuses with the downstream probe (Runx1 exon 5–exon 8). These precursors were pinned down to the nascent extraembryonic mesoderm at E6.5 and blood island anlage at E7.5, which revitalizes the idea of common origin for “primitive” and “definitive” hematopoiesis. It therefore becomes evident that dHSCs precursors emerge in the early extraembryonic mesoderm and maturate on their way to fetal liver.
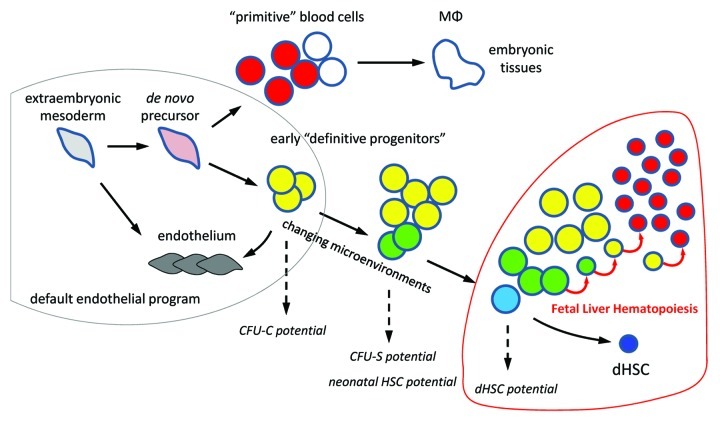
Post-E7.5 hematopoietic rescue attempts, both in vivo and in vitro, were essentially unsuccessful despite presence of many intraembryonic cells that efficiently respond to gene reactivation. It seems that between E7.5 and E8.0 there is a drastic drop in the rescuable precursors capable to revert the Runx1-null phenotype. In the absence of functional Runx1 transcription factor, many Runx1-expressing yolk sac precursors convert to endothelial cells and fuse to yolk sac vasculature. The conversion becomes evident by E9.0–E9.5, but the endothelial recommitment might be irreversible already at E8.0, which explains the failure of rescue attempts beyond this stage. The endothelialization of certain circulating blood cells may also happen in the normal embryo.6 In sum, these findings suggest a default endothelial program for the extraembryonic mesoderm (Fig. 1) and establish Runx1 as a trigger for a long journey toward dHSCs.
Figure 1. Epigenetic progenitor selection. Extraembryonic mesoderm cells progressively acquire the epigenetic makeup of hematopoietic progenitors. Few of these progenitors are selected as dHSCs. The process is manifested by acquisition of hematopoietic potential in standard assays. MФ, tissue macrophages; CFU-C, colony-forming unit in culture; CFU-S, colony-forming unit spleen.
Glossary
Abbreviations:
- dHSCs
definitive hematopoietic stem cells
- AGM region
aorta-gonad-mesonephros region
- E11
embryonic day 11
- HIACs
hematopoietic intra-aortic cell clusters
- P-Sp
para-aortic splanchnopleura
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21388
References
- 1.Medvinsky A, et al. Development. 2011;138:1017–31. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 2.Zovein AC, et al. Cell Stem Cell. 2008;3:625–36. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen MJ, et al. Nature. 2009;457:887–91. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cumano A, et al. Cell. 1996;86:907–16. doi: 10.1016/S0092-8674(00)80166-X. [DOI] [PubMed] [Google Scholar]
- 5.North T, et al. Development. 1999;126:2563–75. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- 6.Samokhvalov IM, et al. Nature. 2007;446:1056–61. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 7.Taoudi S, et al. Cell Stem Cell. 2008;3:99–108. doi: 10.1016/j.stem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Cai Z, et al. Immunity. 2000;13:423–31. doi: 10.1016/S1074-7613(00)00042-X. [DOI] [PubMed] [Google Scholar]
- 9.Moore MAS, et al. Br J Haematol. 1970;18:279–96. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka Y, et al. Proc Natl Acad Sci USA. 2012;109:4515–20. doi: 10.1073/pnas.1115828109. [DOI] [PMC free article] [PubMed] [Google Scholar]