Abstract
Quantitative and qualitative defects in CD1-restricted natural killer T cells have been reported in several autoimmune-prone strains of mice, including the nonobese diabetic (NOD) mouse. These defects are believed to be associated with the emergence of spontaneous autoimmunity. Here we demonstrate that both CD1d-null NOD and CD1d-null NOD/BDC2.5 T cell receptor transgenic mice have an accelerated onset and increased incidence of diabetes when compared with CD1d+/− and CD1d+/+ littermates. The acceleration of disease did not seem to result from changes in the T helper (Th)1/Th2 balance because lymphocytes purified from lymphoid organs and pancreatic islets of wild-type and CD1d-null mice secreted equivalent amounts of IFN-γ and IL-4 after stimulation. In contrast, the pancreata of CD1d-null mice harbored significantly higher numbers of activated memory T cells expressing the chemokine receptor CCR4. Notably, the presence of these T cells was associated with immunohistochemical evidence of increased destructive insulitis. Thus, CD1d-restricted T cells are critically important for regulation of the spontaneous disease process in NOD mice.
Type 1 diabetes mellitus (IDDM) is an autoimmune disorder characterized by lymphocyte-mediated destruction of insulin-producing β cells in the pancreatic Islets of Langerhans (1, 2). At present, the primary immune defects that initiate the autoimmune destruction of islets and that lead to IDDM remain elusive. Studies with the nonobese diabetic (NOD) mouse model of IDDM have revealed that multiple factors, in particular the presence or absence of regulatory cells, can affect the initiation of disease (1, 2). In mice, rats, and humans, a specific population of regulatory T cells, the natural killer T cell (NKT) subset, have been suggested recently to play an important role in the progression of autoimmune diabetes (3, 4). The regulatory functions of this population of T cells have been associated primarily with the CD1d-restricted subset (1, 3–5). CD1d is a nonpolymorphic class Ib protein expressed on the cell surface of antigen presenting cells, whose function is to present lipid antigens in its antigen-binding groove to CD1d-restricted T cells (3, 4). A role for CD1d-restricted T cells in the progression to IDDM was suggested by the findings of quantitative and qualitative defects in these cells in both humans with type 1 diabetes (5, 6) and rodent models of IDDM—the diabetes-prone NOD mice and the BioBreeding (BB) rat (4, 7–9). Furthermore, diabetes in NOD mice can be inhibited partially through passive transfer of NKT cells enriched for CD1d-restricted T cells (10) or through the introduction of the Vα14Jα281 transgene, which increased the number of invariant CD1d-restricted cells (11).
Neither the passive transfer of cells enriched for CD1d-restricted T cells nor the introduction of the Vα14Jα281 transgene were able to protect NOD mice fully from diabetes (10, 11). In the passive-transfer models, the infusion of thymic “NKT” cells was significantly more efficacious than those lines derived from the spleen, which conferred little or no protection on transfer (7). Moreover, in the Vα14Jα281 transgene model, only 3 of 6 founder lines (those with the most robust and largest numbers of NKT cells) were protected partially from diabetes (11). To demonstrate the association of spontaneous autoimmunity in NOD mice with CD1d-restricted T cell depletion, we have crossed CD1d-deficient mice to NOD and NOD/BDC2.5 T cell receptor transgenic (tg) mice (12). The BDC2.5 T cell antigen receptor (TCR) was cloned from an MHC class II-restricted T cell clone specific to an islet-derived antigen. Both CD1−/− NOD and CD1−/− NOD/BDC2.5 mice have an accelerated onset and increased incidence of diabetes when compared with their heterozygote and wild-type littermates. Therefore, CD1d-regulated events are critical to the maintenance of peripheral tolerance in NOD mice.
Methods
Mice.
Females from the NOD/shi strain and NOD/severe combined immunodeficient (SCID) mice were maintained at the pathogen-free condition at The Scripps Research Institute (La Jolla, CA). CD1d-deficient mice (CD1−/−, with a disruption of both CD1.1 and CD1.2 genes; ref. 13) on a C57BL/6 × 129 sv background were crossed to NOD/shi background, and offspring from the 13th generation were intercrossed to obtain mice homozygous for the CD1d-null mutation (NOD CD1−/−). NOD/BDC2.5 TCR mice (12) were backcrossed onto the NOD background for 20 generations. The NOD CD1−/− mice were crossed to NOD/BDC2.5 mice and intercrossed to generate CD1−/− NOD/BDC2.5 and CD1+/+ NOD/BDC2.5 mice. Mice were considered diabetic after two consecutive measurements of nonfasting blood glucose level over 300 mg/dl.
Cell Transfer.
Ten-week-old donor splenocyte suspensions were prepared (7) at a concentration of 108 cells per milliliter in PBS and injected i.v. into the 8-week-old NOD/SCID females in a total volume of 200 μl.
Antigen and Antibody.
The islet autoantigen glutamic acid decarboxylase (GAD) was purified as described (14). Con A was purchased from Sigma. For flow-cytometry analysis, purified or conjugated antibodies to mouse CD1, CD4, CD8, TCR αβ, Vβ4, DX5 (pan NK marker), CD25, CD44, CD62L, and chemokine receptors (CCR) 3, 4, and 5 were purchased from PharMingen.
Histology.
Sections of pancreas were examined histologically and stained with mAb to CD4, CD8, Vβ4, Mac-1, and CD25, respectively (15).
Lymphocyte Isolation.
The pancreatic and hepatic lymphocytes were isolated according to the methods described (7, 15).
Cell Proliferation and Cytokine ELISA.
Lymphocyte proliferation assay was performed (7) and results were expressed as stimulation index (SI). IFN-γ, IL-12 (p40), and IL-4 production in supernatant after 48 h of culture was measured by using optEIA kits (PharMingen) according to the manufacturer's guidelines. The sensitivity was 30 pg/ml for IFN-γ and IL-12, and 8 pg/ml for IL-4.
Results
Exacerbation of Spontaneous Diabetes in CD1d-Deficient NOD Mice.
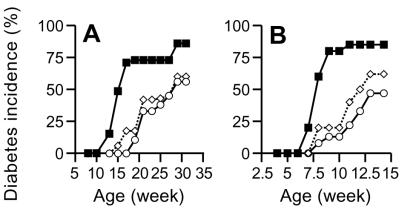
To investigate the contribution of CD1d-restricted T cells to the control of autoimmune diabetes in the NOD mouse, a CD1d-deficient genotype (CD1.1−/− and CD1.2−/−) was crossed onto the NOD genetic background for 13 generations. As determined by fluorescence-activated cell sorter (FACS) analysis, the level of CD1d expression in the NOD CD1+/− mice was reduced to half of that in the NOD CD1+/+ mice and did not exceed the background level in the NOD CD1−/− mice (data not shown). Introgression of the CD1d-null phenotype resulted in the marked loss of NKT cells (TCR + DX5 + cells), and presumably CD1d-restricted T cells, in the spleens (0.1%), pancreatic lymph nodes (0.2%), and pancreata (0.5%) of CD1−/− NOD mice (Table 1). To determine whether CD1d-restricted T cells participated in the development of spontaneous disease, cohorts of CD1−/−, CD1+/−, and CD1+/+ NOD mice were followed for disease incidence. Female CD1−/− NOD mice developed diabetes with a relatively early onset compared with their CD1-expressing littermate controls. A portion (5–8%) of CD1−/− NOD mice became diabetic as early as 12 week of age and 50% were diabetic at 15 weeks. However, none of the CD1+/− NOD or CD1+/+ NOD mice became diabetic before 15 weeks of age, at which time the incidence was 0–5% in the CD1+/− and CD1+/+ littermates (Fig. 1A). By 32 weeks, the diabetes incidence in CD1−/− NOD mice (82%) was significantly increased over that seen in CD1+/+ NOD mice (56%; P < 0.05, Kaplan–Meier, statistics).
Table 1.
Flow cytometric analysis of lymphocyte infiltration and cell activation status in control and CD1d-deficient NOD mice
| Mice | n | TCRαβ + DX5, % | CD4+
|
CD8+
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD25, % | CD44, % | CD62L, % | CCR4, % | CD25, % | CD44, % | CD62L, % | CCR4, % | |||
| Spleens | ||||||||||
| CD1d+/+ | 5 | 4.1 ± 0.2 | 7.3 ± 1.5 | 20.2 ± 4.9 | 66.3 ± 4.5 | 38.2 ± 6.6 | 5.2 ± 1.5 | 17.5 ± 2.5 | 68.7 ± 2.5 | 13.7 ± 3.4 |
| CD1d+/− | 8 | 2.6 ± 0.2 | 6.8 ± 2.7 | 17.3 ± 1.2 | 78.5 ± 2.7 | 35.0 ± 7.2 | 7.1 ± 1.7 | 16.6 ± 2.0 | 65.9 ± 1.0 | 15.4 ± 5.0 |
| CD1d−/− | 8 | 0.1 ± 0.0* | 8.2 ± 1.3 | 25.4 ± 3.0 | 74.2 ± 2.2 | 42.3 ± 3.3 | 6.2 ± 1.1 | 28.0 ± 2.7 | 72.2 ± 2.3 | 15.0 ± 7.1 |
| Pancreatic lymph nodes | ||||||||||
| CD1d+/+ | 5 | 3.6 ± 0.1 | 4.3 ± 0.2 | 31.5 ± 5.1 | 57.4 ± 6.5 | 47.0 ± 2.8 | 4.1 ± 0.5 | 21.0 ± 2.3 | 53.22 ± 5.05 | 14.5 ± 6.0 |
| CD1d+/− | 8 | 2.0 ± 0.1 | 4.2 ± 0.4 | 27.3 ± 3.8 | 66.5 ± 8.7 | 51.3 ± 8.0 | 6.3 ± 1.0 | 26.6 ± 1.8 | 60.00 ± 6.31 | 18.3 ± 6.1 |
| CD1d−/− | 8 | 0.2 ± 0.0* | 5.2 ± 0.3 | 30.2 ± 2.9 | 68.2 ± 3.4 | 58.2 ± 4.2 | 7.2 ± 0.2 | 32.5 ± 4.7 | 77.05 ± 5.60 | 20.2 ± 4.4 |
| Pancreata | ||||||||||
| CD1d+/+ | 5 | 5.5 ± 0.4 | 3.8 ± 0.6 | 23.7 ± 2.0 | 50.3 ± 2.4 | 45.0 ± 8.2 | 5.2 ± 1.0 | 18.2 ± 1.4 | 53.2 ± 5.0 | 18.4 ± 6.0 |
| CD1d+/− | 8 | 2.2 ± 0.2 | 4.1 ± 0.4 | 29.6 ± 2.8 | 62.2 ± 2.1 | 52.5 ± 6.3 | 7.0 ± 3.2 | 29.3 ± 2.8 | 40.0 ± 3.1 | 25.2 ± 4.5 |
| CD1d−/− | 8 | 0.5 ± 0.8* | 8.7 ± 0.6* | 38.7 ± 1.2* | 55.5 ± 6.1 | 77.2 ± 4.4* | 14.2 ± 2* | 32.5 ± 5* | 59.2 ± 6.2 | 31.0 ± 4* |
Mononuclear cells were isolated from spleens, pancreatic lymph nodes, and pancreata and labeled with mAbs as indicated. Data (mean ± SD) shown in the table are derived from 8-week-old female mice. *, P < 0.05, ANOVA.
Figure 1.
(A) Diabetes development in female CD1d-deficient NOD mice. CD1−/− (■, n = 22), CD1+/−, (▵, n = 17), and CD1+/+, (○, n = 30). (B) Diabetes development after adoptive transfer of splenocytes. Splenocytes from each individual donor (n = 6) were transferred into two recipient female NOD/SCID mice (n = 12).
To determine further in vivo the diabetogenicity of NOD CD1−/− T cells, splenocytes from prediabetic (10-week-old) mice were transferred into immunodeficient NOD/SCID recipients that could not otherwise develop diabetes. Importantly, there no differences were detected in the fraction of the ratios of CD4+ to CD8+ T cells or transferred T cells when compared with donor splenocytes from NOD CD1−/− to CD1+/− or CD1+/+ NOD mice. The incidence of diabetes in NOD/SCID mice receiving splenocytes from CD1−/− mice was accelerated significantly also and increased over that seen in NOD/SCID recipients of cells from CD1+/+ NOD (85% vs. 47%, P < 0.05, Kaplan–Meier statistics, Fig. 1B). Thus, a deficiency of CD1d-restricted T cells leads to exacerbation of both spontaneous and adoptively transferred diabetes in NOD mice.
NKT Cell Deficiency Did Not Alter the T Helper (Th)1/Th2 Balance.
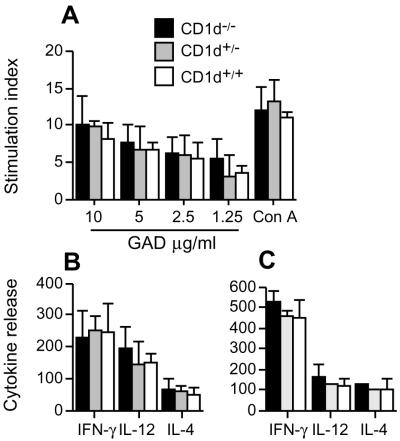
To investigate whether autoreactive T cell priming was altered by a CD1d-restricted T cell deficiency, the capacity of T cells in the spleen to proliferate and secrete cytokines in response to in vitro challenge with a dominant autoantigen (GAD) in IDDM was determined. No changes in the capacity of splenocytes from CD1−/−, CD1+/−, and CD1+/+ mice to proliferate and secrete IL-4, IFN-γ, and IL-12 in response to GAD were detected (Fig. 2A and B). To ensure that there were no islet-specific effects of the CD1-null phenotype on the Th1/Th2 balance, the responses of lymphocytes isolated from the islets of NOD mice were examined also. Lymphocytes infiltrating the islets were isolated from 10-week-old CD1−/−, CD1+/−, and CD1+/+ mice and challenged in vitro with GAD. As was seen with the splenocytes, the cells infiltrating the islets produced equivalent amounts of IL-4, IFN-γ, and IL-12 in response to in vitro challenge with GAD (Fig. 2C). Thus, differentiation of Th1/Th2 cells in the spleen and islets was unaffected by the introgression of a CD1-null phenotype.
Figure 2.
NKT cell deficiency did not alter T cell proliferative capacity and T helper (Th)1/Th2 balance in NOD mice. (A) Splenocytes were tested for proliferation in response to GAD and Con A. (Background = 938 cpm; n = 4.) (B) Cytokine release from splenocytes was determined 48 h after stimulation with 10 μg/ml of GAD (n = 4, spontaneous release, IFN-γ 120 ± 36 pg/ml; IL-12, 89 ± 17 pg/ml; IL-4, undetectable). (C) Cytokine release from pancreatic-infiltrating cell was determined 48 h after stimulation with 10 μg/ml of GAD (spontaneous release, IFN-γ 188 ± 45 pg/ml; IL-12, 112 ± 26 pg/ml; IL-4, undetectable). All data represent three independent experiments with similar results.
Pancreata in CD1−/− Mice Harbor Higher Proportions of the Activated Memory T Cells.
To investigate the participation of CD1d-restricted T cells in the diabetogenicity of autoreactive T cells, T cell-activation status in the spleen and pancreatic lymph nodes, as well as in pancreata of the CD1−/− NOD mice, was compared. Lymphocytes from these organs were isolated and stained with antibodies to cell surface molecules including CD4, CD8, CD25 (IL-2 receptor), CD44 and CD62 L (l-selectin). The total number of infiltrating cells was 6.3 (±0.4) × 105, 9.8 (±1.6) × 105, and 17.4 (±2.3) × 105/pancreas in CD1−/−, CD1+/−, and CD1+/+ mice, respectively (CD1−/− vs. CD1+/+, P < 0.05). No significant differences in CD4 or CD8 populations expressing these activation markers were observed in the spleen and pancreatic lymph nodes in CD1−/−, CD1+/−, and CD1+/+ mice. Interestingly, the percentage of activated memory CD4+ and CD8+ T cells, as indicated by expression of CD25 and CD44, was found to be increased in the pancreata of CD1−/− mice relative to CD1+/− and CD1+/+ mice (Table 1). Thus, in the absence of CD1d, an enhanced frequency of activated memory T cells in the target organ, the pancreatic islets, was observed.
CD1−/− NOD Mice Develop Early and Progressive Insulitis.
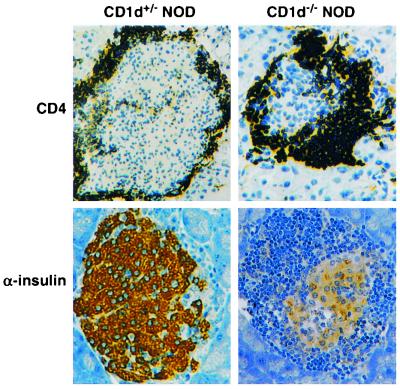
To determine whether the greater incidence of diabetes was associated with the degree of lymphocyte infiltration in the islets in the CD1−/− NOD mice, a study of the insulitis in the young female CD1−/− NOD mice was undertaken. CD1−/− NOD mice at 10 weeks of age demonstrated a markedly high insulitis index (nearly 100%). In contrast, only 67% of the islets from female CD1+/+ NOD mice showed insulitis (Table 2). To assess the kinetics of infiltration, the insulitis in 6-week-old mice was analyzed. At this time point, 56% of islets demonstrated periinsulitis and 44% insulitis in CD1−/− NOD mice that was accompanied by diminished staining for insulin, whereas only 38% of islets showed periinsulitis and 29% insulitis in CD1+/+ NOD mice (Table 2, Fig. 3). Thus, the infiltration of islets by T cells progressed more rapidly in the CD1−/− NOD mice.
Table 2.
Summary of histological analysis of pancreatic sections from 10-week-old female CD1−/−, CD1+/−, and CD1+/+ NOD mice
| Mice | Total islets | No insulitis | Periinsulitis | Insulitis |
|---|---|---|---|---|
| CD1d−/− | 169 | 2% | 53% | 45% |
| CD1d+/− | 138 | 27% | 43% | 30% |
| CD1d+/+ | 188 | 33%* | 38% | 29%* |
| NOD/shi | 169 | 28% | 41% | 31% |
, P < 0.05. Comparison was made between the CD1d+/+ and CD1d−/− group of mice by ANOVA.
Figure 3.
Analysis of islet integrity and function in 6-week-old CD1d-deficient NOD mice by immunohistochemical staining, using antibodies to CD4 (Left) and α-insulin (Right). Note the periinsulitis in the CD1+/− mice surrounding the intact insulin-stained islet (Left Lower). In contrast, however, at this stage, insulitis and reduced insulin staining are observed in the CD1−/− mice (Right Lower).
Expression of CD1d Determines the Diabetogenicity of NOD/BDC2.5tg T Cells.
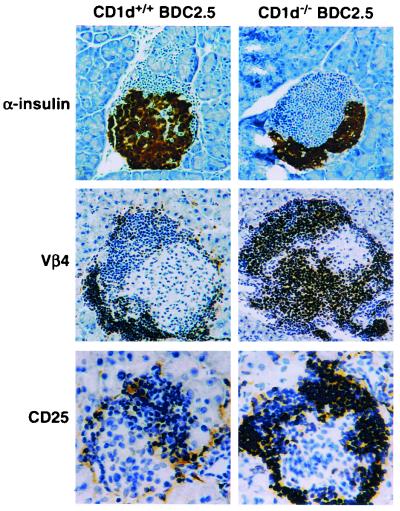
NOD/BDC2.5 mice bear rearranged genes for the TCR-α(Vα1) and TCR-β (Vβ4) chains (13). In our colony, NOD/BDC2.5 mice do not develop spontaneous diabetes but maintain a large number of resting islet-specific memory T cells (16, 17). The diminished incidence of diabetes in the NOD/BDC2.5 background is consistent with the 10–20% rate seen in published studies (13, 16). To test whether CD1d-restricted events were important for the regulation of diabetes incidence in the NOD/BDC2.5 transgene model, the CD1−/− phenotype was bred onto the NOD/BDC2.5 background. The numbers of DX5 + TCRαβ cells in the spleen of CD1+/− NOD/BDC2.5, CD1+/+ NOD/BDC2.5 and CD1−/− NOD/BDC2.5 were 1.37, 1.85, and 0.21%, and in the liver they were 6.38, 6.62, 0.52%, respectively. Expression of DX5, a pan-NK marker, was used as a surrogate marker for NKT cells because NOD do not express the NK1.1 allele. Although there is not complete overlap, DX5 is coexpressed with NK1.1 (CD161) on NKT cells in strains that express the NK1.1 allele (4). Consistent with previous results, none of the control female NOD/BDC2.5 mice developed diabetes over the period of observation (0 of 20 mice, followed for over 40 weeks). Interestingly, the CD1−/− NOD/BDC2.5 mice began to develop diabetes at 23 weeks of age, with a 43% incidence of diabetes by 35 weeks of age (7 of 16; Fig. 4). At that time, there was a significant increase in the total number of lymphocytes infiltrating the islets of CD1−/− NOD/BDC2.5 mice [18.0 (+1.2) × 105/pancreas compared with 8.2 (+0.3) × 105 lymphocytes for NOD/BDC2.5 controls; P < 0.05; ANOVA]. The increased islet infiltration by BDC2.5+ T cells was associated also with a loss of insulin-containing β cells (Fig. 5 Top) and a significant increase in the severity of insulitis. Like the CD1−/− NOD mice, over 95% of the islets in 10-week-old CD1−/− NOD/BDC2.5 mice had insulitis, compared with only 52% in the NOD/BDC2.5 control mice (data not shown). Immunohistochemical staining of these islets revealed that the majority of infiltrating cells were Vβ4+ (tg TCR) and/or CD4+ cells (Fig. 5 Middle). Furthermore, infiltrating lymphocytes in the islets of CD1−/− NOD/BDC2.5 mice stained more intensely for the activation marker CD25 when compared with the staining seen in NOD/BDC2.5 control mice (Fig. 5 Bottom). To confirm whether CD1d-restricted T cell deficiency affects the activation of memory Vβ4+ tg T cells in the peripheral lymphoid organs and in the target organ, a fluorescence-activated cell sorter analysis of lymphocytes isolated from the spleen, pancreatic lymph nodes, and pancreas was performed. Compared with control NOD/BDC2.5 mice, Vβ4+ cells isolated from islets of CD1−/− NOD/BDC2.5 mice expressed significantly higher levels of CD25 and CD44 (Table 3).
Figure 4.
(A) Diabetes development in CD1d-deficient NOD/BDC2.5 mice. CD1−/− BDC2.5 (■, n = 16); CD1+/+ BDC2.5 (○, n = 40). (B) Diabetes development after adoptive transfer of splenocytes. Splenocytes from each donor (n = 4) were transferred into two recipient NOD/SCID mice (n = 8).
Figure 5.
Analysis of islet integrity and function. Sections from 6-week-old CD1d-deficient NOD/BDC2.5 mice by immunohistochemical staining, using antibodies to α-insulin (Top), vβ4 (Middle), and CD25 (Bottom).
Table 3.
Flow cytometric analysis of Vβ4+ tg cell infiltration and activation status in then CD1d−/− and CD1d+/+ BDC2.5 mice
| Mice | n | Spleen
|
Pancreatic
lymph nodes
|
Pancreata
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD25, % | CD44, % | CD62L, % | CD25, % | CD44, % | CD62L, % | CD25, % | CD44, % | CD62L, % | CCR4, % | ||
| CD1d+/+ BDC2.5 | 4 | 6.2 ± 0.3 | 19.8 ± 1.3 | 79.4 ± 4.5 | 5.28 ± 1.21 | 22.3 ± 3.2 | 66.7 ± 4.2 | 6.33 ± 1.1 | 25.4 ± 3.3 | 58.8 ± 4.3 | 68.8 ± 8.0 |
| CD1d−/− BDC2.5 | 4 | 6.8 ± 0.4 | 22.5 ± 2.0 | 77.5 ± 3.3 | 8.08 ± 2.04 | 38.1 ± 2.6 | 45.8 ± 2.2 | 13.4 ± 1.2* | 42.0 ± 3.1* | 44.5 ± 2.7 | 96.2 ± 4.3* |
Mononuclear cells were isolated from spleens, pancreatic lymph nodes, and pancreata and labeled with mAbs as indicated. Data (mean ± SD) shown in the table are double-positive population for Vδ4 and each of the indicated Abs, derived from 8-week-old female mice. *, P < 0.05, ANOVA.
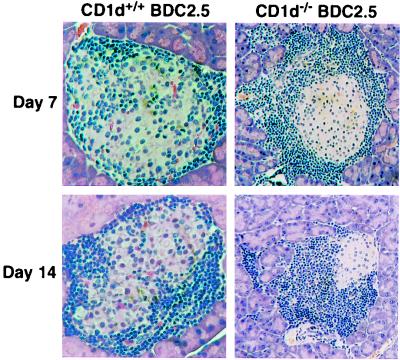
Finally, if CD1d-restricted T cells were able to regulate the diabetogenic potential of Vβ4+ tg T cells, transfer of these tg T cells from CD1−/− NOD/BDC2.5 mice into naïve NOD/SCID recipients should result in the development of diabetes. Notably, 15 weeks after transfer of splenocytes into NOD/SCID mice, 45% of the recipients developed diabetes, whereas the transfer of splenocytes from CD1+/+ NOD/BDC2.5 littermates did not result in the development of diabetes (P < 0.01, Kaplan–Meier statistics; Fig. 4B). Importantly, there was no difference in the activation status of splenocytes from CD1−/− NOD/BDC2.5 and NOD/BDC2.5 donors (Table 3). To confirm that diabetes development was associated also with accelerated insulitis in this model, the kinetics of infiltration of the islets was determined. The pancreata of recipients of splenocytes from CD1−/− NOD/BDC2.5 mice and from NOD/BDC2.5 mice were evaluated by immunohistochemistry on days 7 and 14 after transfer. Insulitis began to appear in splenocyte recipients from both types of donors by day 7, but on days 7 and day 14, insulitis was much more severe and aggressive in those receiving CD1−/− NOD/BDC2.5 splenocytes (Fig. 6).
Figure 6.
Splenocytes from CD1+/+ and CD1−/− NOD/BDC2.5 mice were transferred into NOD/SCID recipients. Pancreata from individual recipients were removed at days 7 and 14 after transfer, and paraffin sections were stained with hematoxylin/eosin and examined for infiltration.
CCR Expression in Pancreatic Infiltration.
To investigate potential lymphocyte recruitment signals differentiating CD1−/− NOD and CD1+/+ NOD mice, an analysis of T cell CCR and endothelial adhesion molecule expression was undertaken (18). A fluorescence-activated cell sorter analysis of lymphocytes purified from the peripheral lymphoid organs and pancreata was done to determine CCR status for chemokine receptors CCR3, CCR4, and CCR5, whose increased cell-surface expression or intraislet concentration of ligand were associated with increased diabetogenicity (ref. 19; S.-H.K. and N.S., unpublished data). Consistent with the nontransgenic mice, the expression of both CD25 and CD44 was up-regulated on CD4+ T cells in the islets (Table 3). Interestingly, higher levels of CCR4 on T cells infiltrating the islets of CD1−/− NOD/BDC2.5 mice were found compared with CD1+/+ NOD/BDC2.5 controls (Table 3, as well as in CD1−/− NOD mice shown in Table 1). In addition, the pancreatic endothelium from CD1−/− NOD/BDC2.5 mice expressed higher levels of lymphocyte function-associated antigen (LFA)-1 and intercellular adhesion molecule (ICAM)-1 when compared with control mice (data not shown). No significant differences in expression of CCR3 and CCR5 in those mice were found. Thus, the introduction of a CD1d-null phenotype into NOD and NOD/BDC2.5 mice resulted in the acceleration of insulitis with activated CCR4+ wild-type and tg T cells.
Discussion
Recent evidence indicates that in NOD mice, autoimmune destruction of pancreatic islet cells is caused in part by a deficiency or dysfunction of regulatory T cells (1, 2). As with other autoimmune diseases (19), a number of pathogens and/or the expression of inflammatory mediators such as IFN-γ (20) and TNFα (21) are thought to delete or inhibit these regulatory cells. These regulatory cell populations have been found in rodents and seem to be present in humans as well (22). Recently, the CD4+CD25+ T cell subset of ill-defined specificity was demonstrated to contain regulatory cells capable of controlling autoimmunity to islet autoantigens in mice (23) and in rats (24). A separate population of regulatory T cells specific for lipid antigens, CD1d-restricted T cells that include the TCR α-chain rearrangements Vα14Jα281 in the mouse or Vα24JαQ in humans, are thought to regulate autoimmunity also (3, 4). In the NOD mouse, the transfer of lymphocyte populations enriched for CD1d-restricted T cells or transgenic over-expression of the Vα14Jα281 TCR α-chain from invariant CD1d-restricted T cells have been demonstrated to regulate the progression of diabetes (4, 10, 11). Here we report that in the NOD mouse, the loss of CD1d results in profound islet infiltration by activated T cells and the exacerbation of autoimmune diabetes. Several mechanisms by which this particular cell population could function have been suggested. The presentation of lipid antigens to T cells by the CD1d molecule is a critical first step eliciting adaptive T and B cell responses (3). Once activated, CD1d-restricted T cells then are thought to initiate and regulate events such as tumor surveillance, parasite recognition, and autoimmune responses (3, 4). In addition to regulatory functions, CD1d-restriected T cells can act as NK-like effector cells also (4). An understanding of the mechanism by which CD1d-restricted T cells regulate autoimmune diabetes is complicated by the undefined nature of the event or events that initiate the autoimmune destruction of the islets and the lack of a clearly identified target cell for CD1d-restricted T cells (1, 3, 4).
The restricted population of autoreactive T cells in NOD/BDC2.5 tg mice provides a unique model to investigate the mechanisms by which regulatory lymphocytes control an autoreactive T cell population in vivo. NOD/BDC2.5/SCID, but not NOD/BDC2.5, mice developed diabetes with early onset (in our colony, 3–4 weeks) and 100% incidence (ref. 17; S. Pakala and N.S., unpublished observations). The mechanism underlying this observation is believed to be the presence of regulatory T cells that are lost in the SCID background. The introduction of a CD1d-null phenotype onto the NOD/BDC2.5 background resulted in the rapid influx of activated Vβ4+ T cells and the subsequent development of diabetes in ≈50% of females. The conversion of NOD/BDC2.5 mice that were diabetes-free to the disease-susceptible phenotype of CD1−/− NOD/BDC2.5 mice demonstrates that the diabetogenic potential of autoreactive BDC2.5 T cells was controlled significantly by CD1d-dependent mechanisms. In addition, the differences in disease incidence observed when comparing NOD/BDC2.5/SCID or CD1−/− NOD/BDC2.5 to NOD/BDC2.5 mice suggests that regulatory mechanisms that are independent of CD1d are also important. One caveat to this interpretation arises from the strategy used to generate the NOD/CD1−/− phenotype. Because the NOD/CD1−/− phenotype was generated from a 129/B6 CD1−/− founder, there is the possibility that results reflect the introduction of an adjacent gene cotransferred during the backcross of the mutated 129 locus onto the NOD genetic background. The backcross of the mutated locus into NOD mice resulted in the introgression of a 2-centimorgan region of DNA on chromosome 3 that was centromeric to Idd10 (data not shown).
It has been argued that burst secretion of IFN-γ and IL-4 by CD1d-restricted T cells is critical to the initiation of adaptive immune response and may facilitate the differentiation of autoreactive T helper (Th)1 or Th2 cells (19, 25). Previous studies reported that the protection of diabetes by transfer of NKT cells (10) or expression of the Vα14Jα281 transgene (11) depended on IL-4 and IL-10 production. However, in the CD1−/− NOD mouse model, CD1d-restricted events had little effect on T cell cytokine profiles and were important for inhibiting the accumulation of activated CCR4+ T cells in the islets. Furthermore, the regional changes noted in lymphocyte CCRs and expression of adhesion molecules on endothelial cells indicates that CD1d-restricted T cells are regulating lymphocyte activation and trafficking locally. This hypothesis is supported by the findings of elevated CCR4, CD25, and CD44 proteins on T cells infiltrating the islets, but will require molecular quantitation of CD1d-restricted T cell distribution and activation.
The elaboration of chemokines and expression patterns of their receptors is known to be critical for cellular homing into and out of sites of inflammation (18). Notably, CCR4 expression identified a markedly diabetogenic T cell population, and the transfer of these cells precipitated severe diabetes in naïve NOD recipients (S.-H.K. and N.S., unpublished data). Therefore, CD1d-restricted T cells partially determine the diabetogenicity of potentially autoreactive T cells by using a mechanism associated with the regulation of CCR expression on autoreactive T cells.
Stimulation of CD1d-restricted T cells in vivo results in the rapid activation of NK, T, and B cells (26, 27). This relationship between innate defense mechanisms and adaptive cellular phenomena was used to argue that CD1-restricted T cells function at the interface between innate and adaptive immunity (1–3, 19). In rodents and humans, CD1d-restricted T cells seem to be dysfunctional in autoimmune-prone individuals and as a consequence, NOD mice would be predicted to have an impaired transition to appropriate adaptive responses (6, 7). Despite this prediction, the exacerbation of diabetes by germ-line deletion of the CD1 locus suggests that CD1d-dependent events are important, albeit less effective in NOD mice, for the control of autoimmunity. In contrast, in recent studies CD1d-restricted T cell deficiencies did not significantly alter the course of autoimmune diseases induced by autoantigens and bacterial adjuvants, i.e., experimental autoimmune myasthenia gravis (28) and experimental allergic encephalomyelitis in C57BL6 mice (L. Van Kaer, personal communication). The potent adjuvants used in these studies directly induce a prompt series of cellular activation events in NK and dendritic cells, and the subsequent adaptive responses of B and T cells. The induction of costimulatory molecules and the abrupt release of inflammatory mediators as a consequence of such activation is likely to bypass the role for CD1d-restricted T cells in these settings.
Thus, CD1d-restricted T cells regulate the diabetogenicity of both heterogeneous (NOD) and homogeneous (BDC2.5) autoreactive T cell populations, and the regulatory events controlled by CD1d-restricted T cells are associated with the control of autoreactive T cell activation and maintenance of self-tolerance.
Vigorous efforts are being made to identify diabetes susceptibility genes outside of the MHC in both mouse and man (IDDM or Idd loci, respectively) (e.g., refs. 29–31). Although many large genetic regions that putatively encode one or more such genes have been identified, no definitive identification has been reported, with the exception of the insulin gene and possibly IL-2. CD1d, encoded on mouse chromosome 3 or human chromosome 1, may from the present study be a candidate, but much further work would be needed to implicate it in either the murine or the human disease.
Acknowledgments
We thank L. Van Kaer and H. G. Ljunggren for discussions and advice; L. Tucker, M. Cleary, L. Mocnick, E. Rodriguez, and B. Eckelman for maintaining the mouse colony and technical assistance; and P. Minick for editorial assistance. This is manuscript 13888-IMM from The Scripps Research Institute. This study was supported by the Juvenile Diabetes Foundation International (F.D.S.), the National Multiple Sclerosis Society (M.F.), and the National Institutes of Health (J.L.S., S.B.W., and N.S.).
Abbreviations
- GAD
glutamic acid decarboxylase
- IDDM
insulin-dependent diabetes mellitus
- NOD
nonobese diabetic
- NKT
natural killer T
- tg
transgenic
- TCR
T cell antigen receptor
- SCID
severe combined immunodeficient
- CCR
chemokine receptor
- Th
T helper
References
- 1.Delovitch T L, Singh B. Immunity. 1997;7:727–738. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson M A, Leiter H E. Nat Med. 1999;5:601–604. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 3.Park S H, Bendelac A. Nature (London) 2000;406:788–792. doi: 10.1038/35021233. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey D I, Hammond K J L, Poulton L D, Smyth M J, Baxter A G. Immunol Today. 2000;21:573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 5.Wilson S B, Kent S C, Patton K T, Orban T, Jackson R A, Exley M, Porcelli S, Schatz D A, Atkinson M A, Balk S P, et al. Nature (London) 1998;391:177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 6.Wilson S B, Kent S C, Horton H F, Hill A A, Bollyky P L, Hafler D A, Strominger J L, Byrne M C. Proc Natl Acad Sci USA. 2000;97:7411–7416. doi: 10.1073/pnas.120161297. . (First Published June 6, 2000; 10.1073/pnas.120161297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falcone M, Yeung B, Tucker L, Rodriguez E, Sarvetnick N. J Exp Med. 1999;190:963–972. doi: 10.1084/jem.190.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda K, Dennert G. J Exp Med. 1993;177:155–164. doi: 10.1084/jem.177.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mieza M A, Itoh T, Cui J Q, Makino Y, Kawano T, Tsuchida K, Koike T, Shirai T, Yagita H, Matsuzawa A, et al. J Immunol. 1996;156:4035–4040. [PubMed] [Google Scholar]
- 10.Hammond K J L, Poulton L D, Palmisano L, Silverira P, Godfrey D I, Baxter A G. J Exp Med. 1998;187:1047–1056. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehuen A, Lantz O, Beaudoin L, Laloux V, Carnaud C, Bendelac A, Bach J-F, Monteiro R C. J Exp Med. 1998;188:1831–1839. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz J D, Wang B, Haskins K, Benoist C, Mathis D. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 13.Sonoda K H, Exley M, Snapper S, Balk S P, Stein-Streilein J. J Exp Med. 1999;190:1215–1225. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman D L, Clare-Salzer M, Tian J, Forsthuber T, Ting G, Robinson G, Atkinson M A, Sercarz E E, Tobin A J, Lehmann P V. Nature (London) 1993;366:69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trembleau S, Penna G, Gregori S, Chapman H D, Serreze D V, Magram J, Adorini L. J Immunol. 1999;163:2960–2968. [PubMed] [Google Scholar]
- 16.Mueller R, Bradley L M, Krahl T, Sarvetnick N. Immunity. 1997;7:411–418. doi: 10.1016/s1074-7613(00)80362-3. [DOI] [PubMed] [Google Scholar]
- 17.Suri A, Katz J D. Immunol Rev. 1999;169:55–65. doi: 10.1111/j.1600-065x.1999.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 18.Youn B S, Mantel C, Broxmeyer H E. Immunol Rev. 2000;177:150–174. doi: 10.1034/j.1600-065x.2000.17701.x. [DOI] [PubMed] [Google Scholar]
- 19.Shi F-D, Ljunggren H G, Sarvetnick N. Trends Immunol. 2001;22:97–101. doi: 10.1016/s1471-4906(00)01821-4. [DOI] [PubMed] [Google Scholar]
- 20.Sarvetnick N, Liggitt D, Pitts S L, Hansen S E, Stewart T A. Cell. 1988;52:773–782. doi: 10.1016/0092-8674(88)90414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green E A, Flavell R A. Immunity. 2000;12:459–469. doi: 10.1016/s1074-7613(00)80198-3. [DOI] [PubMed] [Google Scholar]
- 22.Shevack E M. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 23.Salomon B, Lenschow, Rhee D J, Ashourian L N, Singh B, Sharpe A, Bluestone J A. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 24.Mason D, Stephens L A. J Immunol. 2000;165:3105–3110. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 25.Mendiratta S K, Martin W D, Hong S, Boesteanu A, Joyce S, Van Kaer L. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 26.Carnaud C, Lee D, Donnars O, Park S-H, Beavis A, Koezuka Y, Bendelac A. J Immunol. 1999;163:4647–4645. [PubMed] [Google Scholar]
- 27.Eberl G, MacDonald H R. Eur J Immunol. 2000;30:985–992. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Shi F-D, Wang H, Li H, Hong S, Taniguchi M, Link H, Van Kaer L, Ljunggren H G. Nat Immunol. 2000;1:245–251. doi: 10.1038/79792. [DOI] [PubMed] [Google Scholar]
- 29.Wicker L S, Todd J A, Peterson L B. Annu Rev Immunol. 1995;13:179–200. doi: 10.1146/annurev.iy.13.040195.001143. [DOI] [PubMed] [Google Scholar]
- 30.Gonzales A, Katz J D, Mattei M-G, Kikutani H, Benoist C, Mathis D. Immunity. 1997;7:873–883. doi: 10.1016/s1074-7613(00)80405-7. [DOI] [PubMed] [Google Scholar]
- 31.Encinas J A, Kuchroo V K. Curr Opin Immunol. 2000;12:691–697. doi: 10.1016/s0952-7915(00)00164-3. [DOI] [PubMed] [Google Scholar]