Abstract
Chromosome segregation errors are highly frequent in mammalian female meiosis, and their incidence gradually increases with maternal age. The fate of aneuploid eggs is obviously dependent on the stringency of mechanisms for detecting unattached or repairing incorrectly attached kinetochores. In case of their failure, the newly formed embryo will inherit the impaired set of chromosomes, which will have severe consequences for its further development. Whether spindle assembly checkpoint (SAC) in oocytes is capable of arresting cell cycle progression in response to unaligned kinetochores was discussed for a long time. It is known that abolishing SAC increases frequency of chromosome segregation errors and causes precocious entry into anaphase; SAC, therefore, seems to be essential for normal chromosome segregation in meiosis I. However, it was also reported that for anaphase-promoting complex (APC) activation, which is a prerequisite for entering anaphase; alignment of only a critical mass of kinetochores on equatorial plane is sufficient. This indicates that the function of SAC and of cooperating chromosome attachment correction mechanisms in oocytes is different from somatic cells. To analyze this phenomenon, we used live cell confocal microscopy to monitor chromosome movements, spindle formation, APC activation and polar body extrusion (PBE) simultaneously in individual oocytes at various time points during first meiotic division. Our results, using oocytes from aged animals and interspecific crosses, demonstrate that multiple unaligned kinetochores and severe congression defects are tolerated at the metaphase to anaphase transition, although such cells retain sensitivity to nocodazole. This indicates that checkpoint mechanisms, operating in oocytes at this point, are essential for accurate timing of APC activation in meiosis I, but they are insufficient in detection or correction of unaligned chromosomes, preparing thus conditions for propagation of the aneuploidy to the embryo.
Keywords: anaphase, aneuploidy, cell cycle, chromosome segregation, meiosis, oocytes
Introduction
Chromosome segregation errors during mammalian female meiosis frequently create gametes with aberrant number of chromosomes. After fertilization of such an egg, the incomplete chromosomal set is transferred to the newly formed embryo, which has devastating consequences for its further development, including embryonic lethality and severe developmental disorders.1 The incidence of aneuploidy is usually higher in egg than in sperm, and a factor contributing highly to human and mouse oocyte aneuploidy is advanced maternal age.2 It is, however, important to mention that not all mammalian species are suffering from the maternal age-related aneuploidy; for example, in porcine oocytes, this phenomenon was not established, which might reflect species specific differences in chromosome segregation mechanisms.3 One of the factors contributing to such high occurrence of aneuploidy in aged egg is a weakening of centromeric cohesion,4,5 arising due to the inability of the oocyte to replace cohesins during prolonged prophase arrest.6,7 Reduced connection between sister chromatids is subsequently causing their precocious segregation in meiosis I. The propagation of meiosis I segregation defects to the embryo would, however, require also a failure of SAC controlling chromosome segregation in meiosis II. Although the function of SAC in oocytes was studied for quite some time,8-10 it is still unresolved whether it is similar to somatic cells. So far we have clear evidence that SAC is required for precise timing of APC activity in meiosis I since depletion of its component Bub1 causes premature chromosome segregation, accompanied by extensive aneuploidy.11 Also in mitosis, SAC components ensure proper timing of anaphase;12 therefore, this function seems to be preserved. However, in contrast to mitosis, where the anaphase onset is blocked until last kinetochore is aligned on the metaphase plate,13-15 it seems that oocytes are unable to respond to unaligned kinetochores by prolonging SAC activity.16,17 To analyze this phenomenon more quantitatively, we used oocytes with extraordinarily high rate of aneuploidy, in which chromosome segregation errors are expected to be more frequent, and we monitored chromosome movements, spindle assembly, APC activity and polar body extrusion simultaneously in individual cells using confocal live imaging. Our data demonstrate that oocytes are not able to respond to multiple unaligned kinetochores and massive congression defects by delaying anaphase.
Results
The duration of meiosis I is not affected by presence of univalent chromosomes
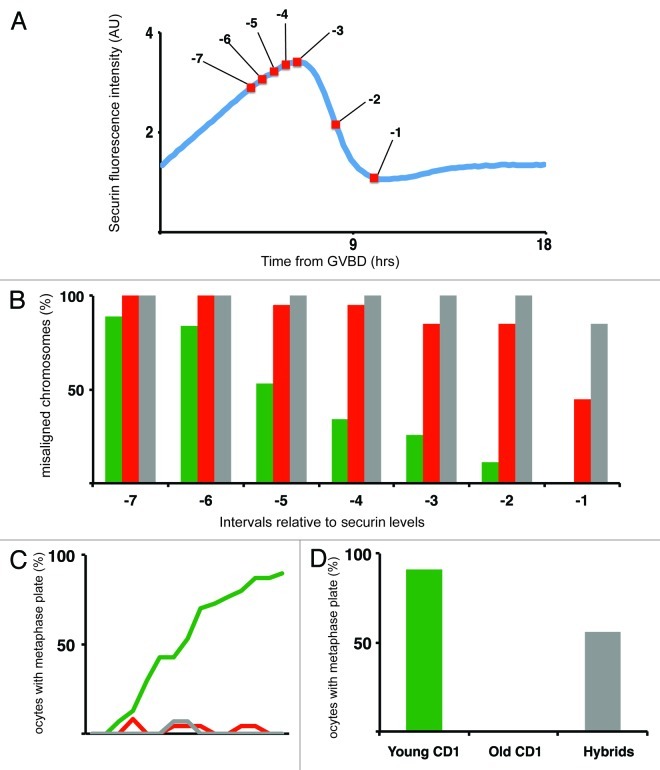
For our study, we used oocytes isolated from naturally aged CD1 outbred mice at age 19–25 mo (“old CD1”) and oocytes isolated from Mus musculus × Mus spretus F1 hybrids (“hybrids”). These groups were selected based on previously reported high level of aneuploidy,18,19 which would also increase probability of detecting chromosome segregation defects in vivo. Both groups were compared with the oocytes from CD1 animals 2–5 mo old (“young CD1”). To assess aneuploidy levels in all three groups, the oocytes were matured in vitro for 18 h, and subsequently the frequency of aneuploidy was measured by kinetochore-counting assay in monastrol-treated oocytes19 (Fig. 1A). Our results showed that 4.1% of young CD1 (n = 73), 42.5% of old CD1 (n = 47) and 72.7% of hybrid oocytes (n = 33) had numerical chromosomal aberrations. Values obtained for both CD1 groups were similar to previously published figures,19 and small variations were likely caused by age or strain differences. The aneuploidy observed in hybrid oocytes was, however, significantly higher compared with the previously published report.18 Possible explanation for such high aneuploidy levels is the origin of mice. Whereas Mus spretus animals used for our interbreeding were derived from natural population, mice used in previous study were inbred. To address the question whether configuration of chromosomes in meiosis I is similar in all three groups, chromosome spreads were prepared from oocytes in meiosis I, matured for 7.5 h after IBMX removal. At this time, oocytes were still in meiosis I, verified by the absence of polar bodies. Chromosome spreads revealed that young CD1 (n = 46) and hybrids (n = 18) contained no univalents, whereas 19.2% of the old CD1 oocytes (n = 26) contained closely positioned univalents without visible chiasmata (data not shown). Single chromatids were not detected in any of the groups. Based on multiple evidence, including nocodazole treatment and measurement of meiosis I length, it was previously shown that SAC is intact in old oocytes.19 Sixteen hour exposure of oocytes to a low level (0.04 μg/ml) of nocodazole, which was added to the media 4.5 h after germinal vesicle break down (GVBD), showed that 75% of young CD1 (n = 68), 71% of Old CD1 (n = 17) and 86% of hybrid oocytes (n = 21) remained arrested in meiosis I, indicating that in all three groups, the SAC was intact (data not shown). To analyze whether the presence of univalents in old CD1 group is affecting metaphase plate formation and timing of anaphase I, we used live-cell imaging. Oocytes were microinjected with mRNAs encoding histone H2B, β-tubulin 2a and securin fused to different fluorescent proteins and then matured on microscope. Images were taken every 10 to 15 min for period of 18 h. Such conditions allowed to simultaneously monitor various processes, including GVBD, chromosome movements, assembly of the meiotic spindle, polar body extrusion (PBE) and activity of APC in individual cells, representative movie frames are shown in Figure 2. Our results showed that the length of meiosis I, measured from GVBD to DNA masses segregation, was not significantly different in young CD1 oocytes (8.75 h ± 0.94, n = 50) and hybrid oocytes (9.20 h ± 1.88, n = 21). It was slightly shorter in old CD1 (8.22 ± 0.78, n = 46) compared with young CD1 (p = 0.037) and hybrids (p = 0.035) (Fig. 1B), although the difference was minor. This indicates that the presence of univalent chromosomes in oocytes is not causing meiosis I extension by prolonging SAC activity, which might be consistent with the idea that univalent chromosomes are escaping SAC detection by bipolar attachment.20-22 Securin levels measured during time-lapse experiments showed that APC was activated in all three groups with similar timing relative to GVBD (Fig. 1C).
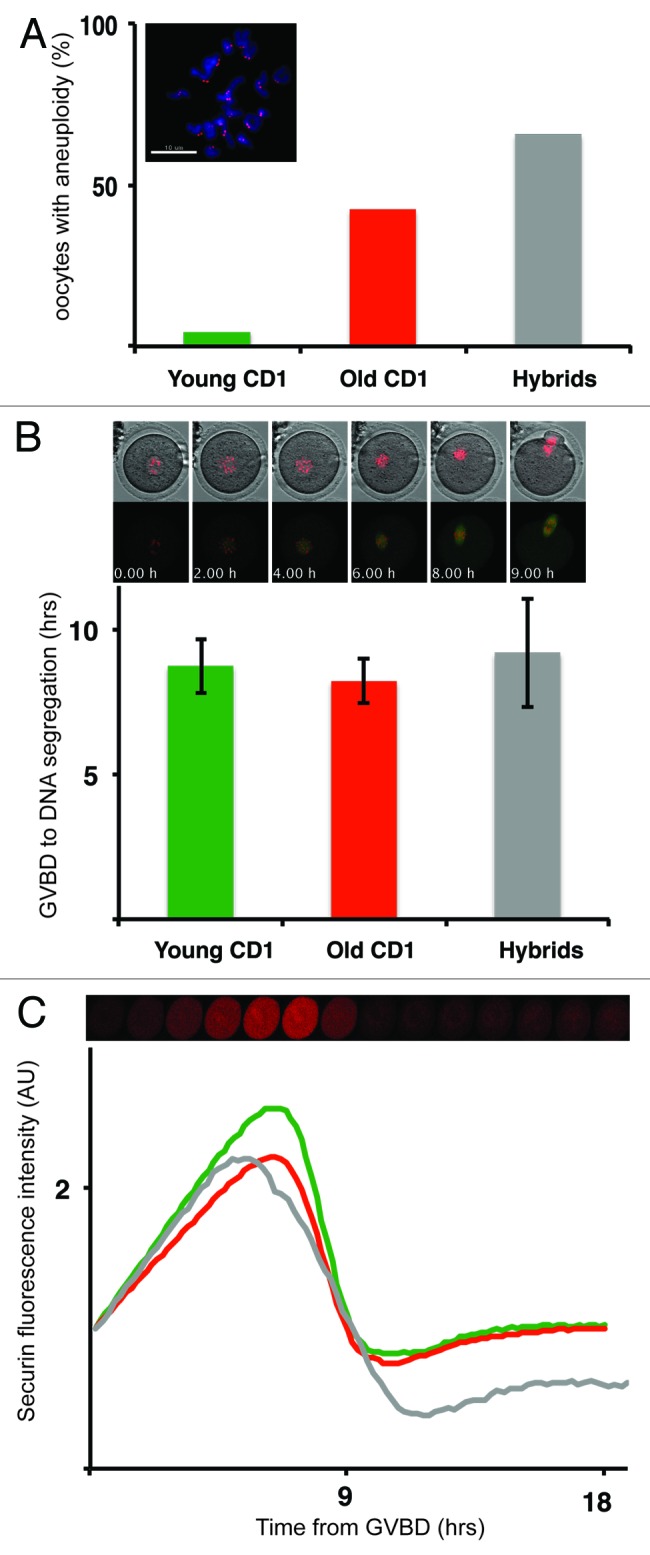
Figure 1. The duration of meiosis I is not affected by high frequency of aneuploidy. (A) Aneuploidy frequency in Young CD1 (green, n = 73), Old CD1 (red, n = 47) and Hybrid (gray, n = 33) was scored after monastrol treatment in meiosis II. Image represents typical staining of chromosomes with DAPI (blue), and kinetochores (red). (B) The length of meiosis I - oocytes were microinjected with fluorescently labeled histone H2B (red) and β-tubulin 2A (green) and analyzed by live imaging. Movie frames showing intervals from GVBD until DNA separation, time is indicated for each frame. Lower chart – the average length (hrs) of meiosis I in Young CD1 (green, n = 50), Old CD1 (red, n = 46) and Hybrid (green, n = 21) oocytes, error bars showing SD. (C) Dynamics of securin degradation during meiosis I. Average securin expression levels in Young CD1 (blue, n = 18), Old CD1 (red, n = 21) and Hybrid oocytes (n = 8) are shown, expression levels were normalized to the first frame.
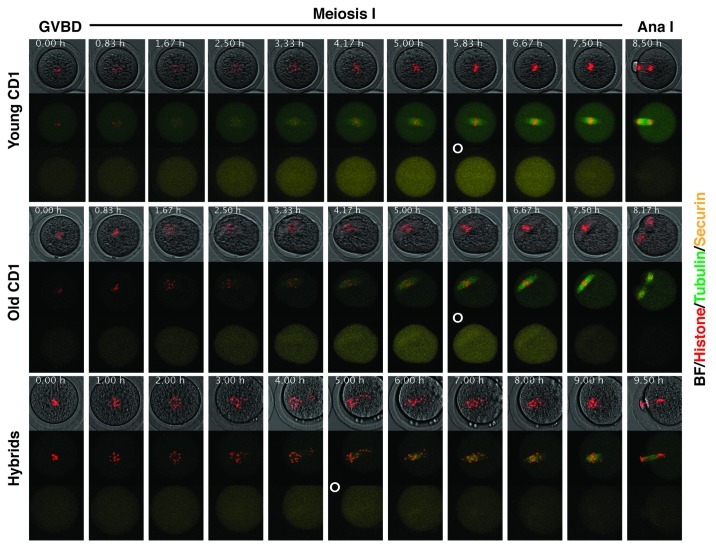
Figure 2. Time-lapse analysis of chromosome alignment and securin degradation. Oocytes were microinjected with fluorescently labeled histone H2B (red), β-tubulin 2A (green) and securin (yellow). Movie frames from representative Young CD1, Old CD1 and Hybrid oocyte are shown, the frame with maximal level of securin is marked with white circle.
Metaphase plate formation is compromised in oocytes with high aneuploidy
Analysis of the time-lapse movies also revealed that significant fraction of old CD1 oocytes and even more hybrid oocytes, contained unaligned chromosomes at the time of APC activation, assessed by measuring securin degradation (Fig. 2). In mitosis, alignment of all kinetochores on equatorial plane of the spindle is required for satisfying SAC, which, in turn, leads to activation of APC and subsequent destruction of multiple substrates, including securin and cyclin B.15,23 Securin stability is therefore an excellent marker of initiation of metaphase to anaphase transition also in oocytes.11 Quantification of securin levels and simultaneous detection of fluorescently labeled chromosomes in individual cells allowed to correlate dynamics of the metaphase plate formation with APC activation, which should coincide with SAC switching off (Fig. 3). We were interested in whether the metaphase plate formation, when all chromosomes congress on equatorial plane without a single chromosome visibly separated, is achieved in oocytes before initiation of securin destruction. For each cell, two time intervals were identified; the frame with the highest level of securin and then the frame with all chromosomes aligned on metaphase plate (Fig. 3A). Our results showed that 89% of young CD1 (n = 19) are able to form a metaphase plate before initiation of securin destruction; however, only 15% of old CD1 (n = 20) and 0% of hybrids (n = 13) achieved this. Following the same cells further as they were heading toward anaphase, showed that 55% of old CD1 and 92% of hybrid oocytes were unable to form metaphase plate at any time interval before anaphase in contrast to young CD1 group, in which 100% formed the metaphase plate before reaching anaphase (Fig. 3B).
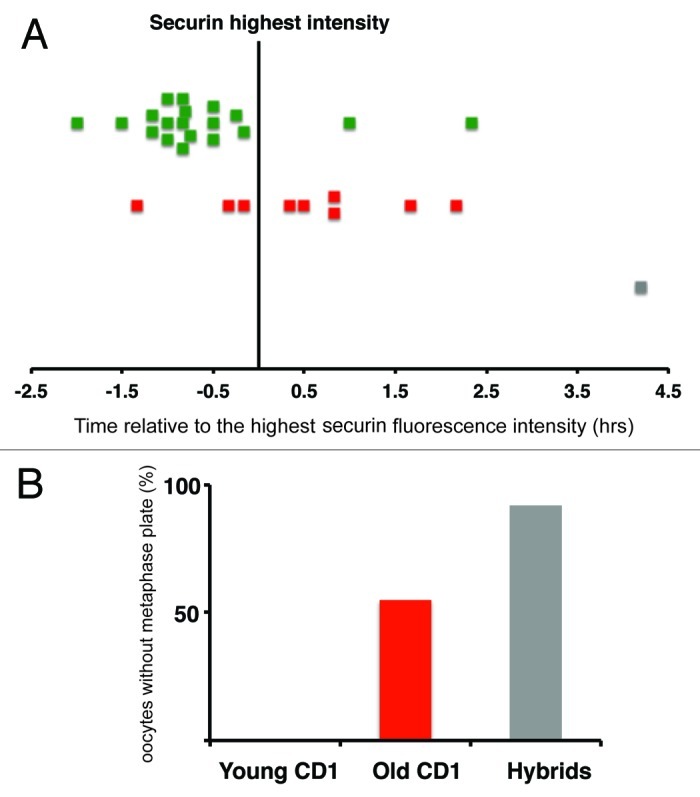
Figure 3. Metaphase plate formation vs. APC activation. (A) The highest level of securin is represented by vertical line. Squares are showing time of aligning of all chromosomes on equatorial plane in individual oocytes. Young CD1 (green, n = 19), Old CD1 (red, n = 20) and Hybrid (gray, n = 13). (B) Chart showing frequency of oocytes never forming metaphase plate before anaphase onset - Young CD1 (green, n = 19) 0%, Old CD1 (red, n = 20) 55% and Hybrid (gray, n = 13) 92%.
SAC inactivation in oocytes is independent on chromosome alignment
To study more quantitatively the correlation between chromosome alignment and APC activity, we used again oocytes injected with β-tubulin 2a, histone H2B and securin, all labeled with fluorescent proteins. Misaligned chromosomes, visibly separated from the metaphase plate, were scored in seven selected intervals: at 120 (-7), 90 (-6), 60 (-5) and 30 min (-4) before peak of securin, at highest securin level (-3), at half of the securin level after initiation of its destruction (-2) and finally at the last frame before anaphase (-1) (Fig. 4A). These intervals were selected to cover part of meiosis I with full SAC activity, APC activation during metaphase to anaphase transition and also time when cells were entering anaphase. Nineteen young CD1, 20 old CD1 and 13 hybrid oocytes in which the chromosomes were visible in each interval were used for this study. Our results (Fig. 4B) showed that all three groups contained unaligned chromosomes during two hours prior to the highest securin level, and therefore our observation is consistent with recently published data showing that SAC is turned off in the presence of unaligned chromosomes.16,17 It is, however, important to mention that while young CD1 eventually organized their chromosomes on the metaphase plate before reaching anaphase, 50% of old CD1 and 85% of hybrid oocytes were entering anaphase with chromosomes separated from the metaphase plate. Further, we tested formation of metaphase II plate in all three groups of oocytes. In this analysis, the misaligned chromosomes were scored in 15 consecutive intervals throughout first 150 min after anaphase I (Fig. 4C) and also at 18 h after IBMX removal (Fig. 4D). Our results showed that 90% of young CD1 (n = 30) reached the stage, with all chromosomes aligned on metaphase plate within 150 min after anaphase I. In contrast to this, almost all old CD1 and hybrid oocytes, perhaps due to the aneuploidy resulting from separase activation in anaphase I, were unable to form metaphase plate within selected interval. This was confirmed by analysis of the chromosome alignment at 18 h (Fig. 4C and D), which indicated that the alignment defect in those cells might be permanent.
Figure 4. Correlation between chromosome alignment and securin levels. (A) Typical securin expression curve showing intervals for scoring chromosome alignment: -7 = 120, -6 = 90, -5 = 60, -4 = 30 min to highest securin, -3 = highest securin, -2 = half of the securin level, -1 = one frame before anaphase. (B) Scoring of unaligned chromosomes in meiosis I in intervals described in A – Young CD1 (green, n = 19), Old CD1 (red, n = 20) Hybrid (gray, n = 13). (C) Scoring oocytes with all chromosomes aligned on metaphase II plate during 150 min following anaphase I. Young CD1 (green, n = 30), Old CD1 (red, n = 24) Hybrid (gray, n = 15). (D) % of oocytes with metaphase plate at 18 h - Young CD1 (green, n = 22) 91%, Old CD1 (red, n = 20) 0% and Hybrid (gray, n = 13) 56%.
Severe alignment and congression defects are not preventing SAC inactivation in oocytes
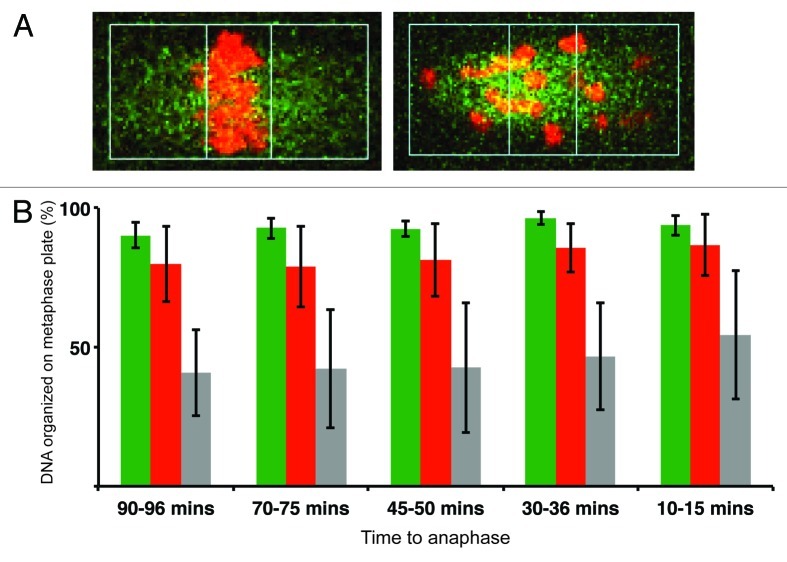
Scoring cells with unaligned chromosomes does not fully reveal the severity of alignment defects in individual cells. To analyze this more quantitatively, we adapted method previously used to score congression defects.11 In this experiment, we measured amount of DNA located inside a rectangle, which represented 25% of the spindle length (Fig. 5A). For this analysis we used only oocytes, in which the spindle axis was parallel with the focus plane. Cells were analyzed in five frames: at 90–96, 70–75, 45–50, 30–36 and 10–15 min prior to anaphase (Fig. 5B). Except for the last interval just before anaphase, young CD1 (n = 10) oocytes had significantly more (p ≤ 0.05) DNA inside the rectangle compared with old CD1 oocytes (n = 6). In contrast to both CD1 groups, hybrid oocytes (n = 5) showed extensive chromosome alignment and congression defects, and except for the last frame before anaphase, less than 50% of the DNA were localized at the equatorial plane. This demonstrates that the oocytes were unable to detect or respond to unaligned chromosomes resulting in chromosomes scattered all over the spindle, when oocytes were entering anaphase.
Figure 5. Severe congression defects in meiosis I in Old CD1 and Hybrid oocytes. (A) Oocytes were microinjected with fluorescently labeled histone H2B (red) and β-tubulin 2A (green). DNA was measured within a rectangle representing 25% of the spindle length. Typical images of oocytes with chromosomes aligned on equatorial plane (left panel) and unaligned (right panel). (B) Scoring of congression defects in meiosis I in Young CD1 (green, n = 10), Old CD1 (red, n = 6) Hybrid (gray, n = 5) oocytes, time of selected intervals relative to anaphase is indicated.
Discussion
The data presented here are showing that mammalian oocytes are unable to respond to multiple unaligned kinetochores and to severe congression defects by prolonging SAC activity. Crucially, the anaphase in such oocytes is timely executed, despite the defects in chromosome alignment. Recent reports showed that a critical mass of chromosomes congressed on metaphase plate before anaphase onset is required.16,17 At the time of APC activation, oocytes isolated from F1 hybrids of Mus musculus and Mus spretus have more than 50% of DNA positioned outside of the central quarter of the spindle, demonstrating that the required critical mass is either very low, or the alignment of the chromosomes is not prerequisite for APC activation. Importantly, in old and hybrid oocytes, which are both showing extensive spindle defects with no impact on timing of anaphase onset, their response to nocodazole depolymerizing the spindle microtubules is preserved, demonstrating that SAC is intact. Our data contribute to the recent discussion about the function of SAC in mammalian oocytes and its role in etiology of maternal age-related aneuploidy (reviewed in refs. 9, 16, 24 and 25). In mitosis, SAC was shown to be extremely efficient, delaying anaphase until all kinetochores are aligned on metaphase plate, and even one single unattached kinetochore is able to block the cell cycle progression.13 Although it seems that the attachment of chromosomes to spindle microtubules is sufficient for switching SAC off and the kinetochore tension is sensed by attachment correcting mechanisms, it is likely that both mechanisms are linked by several key players participating in both processes.15,26,27 From previous studies, we have strong evidence that SAC in mouse oocytes is essential for faithful chromosome segregation and its abolition, by depleting Bub1, has catastrophic consequences, including precocious APC activation and chromosome missegregation and resulting in extensive aneuploidy.11 But we also know that unaligned chromosomes, both univalents and bivalents, in meiosis I are well tolerated (see refs. 16, 17, 28 and 29 and Fig. 4B ). These data, together with our results, strongly suggest that attachment of the chromosomes to the spindle microtubules is sufficient for entering anaphase in mammalian oocytes. This is consistent with the hypothesis that SAC is fully functional in mammalian oocytes, even in the situation when oocytes are isolated from aged animals or interspecific hybrids. We can speculate that in those cells, the high frequency of aneuploidy is due to the absence or failure of mechanisms correcting chromosome attachment errors, which are in somatic cells closely cooperating with SAC activity.
Material and Methods
Mice strains
CD1 mice were purchased from Velaz, Czech Republic at the age of 6 weeks and then aged naturally. Mus spretus were obtained from Jaroslav Pialek, Institute of Vertebrate Biology, AS CR. F1 hybrids resulted from mating Mus musculus C57BL/6 females (purchased from Anlab) and Mus spretus males. All animal work was conducted according to Act No 246/1992 Coll., on the protection of animals against cruelty under supervision of Central Commission for Animal Welfare, approval ID 018/2010.
Oocyte culture and microinjection
GV-stage oocytes were harvested from ovaries in M2 medium (Sigma-Aldrich) containing 200 mM 3-isobutyl-1-methylxanthine (IBMX, Sigma-Aldrich). After releasing from surrounding cumulus cells, oocytes were cultured in drops of M16 medium (Millipore) supplemented with IBMX and covered with mineral oil (Sigma-Aldrich) at 37°C in the presence of 5% CO2. Microinjection was performed in M2 with IBMX. mRNAs for microinjection were prepared by in vitro transcription of plasmids containing ORFs of mouse histone H2B, β-tubulin 2a and securin. Oocytes were microinjected with I-10 microinjector (Narishige) with mixture of up to three different mRNAs into the cytoplasm. Microinjection was performed on Leica DM IL inverted microscope equipped with N PLAN 5 × /0.12 and HI PLAN I 20 × /0.3 objectives. Oocytes were matured in M16 medium without IBMX at 37°C in the presence of 5% CO2. In some experiments, oocytes were cultured in the presence of 0.04 μg/ml nocodazole (Sigma-Aldrich) in the culture media.
Kinetochore counting assay
This method was adapted from reference 19. After 18 h of maturation, MII-stage oocytes were incubated in M16 containing 100 μM monastrol (Sigma-Aldrich) at 37°C and 5% CO2. Zona pelucida and polar body were removed in Tyrode’s solution (Sigma-Aldrich), fixation was performed using 2% paraformaldehyde (Sigma-Aldrich) for 20 min and cells were stained with human anti-centromere antibody (HCT-0100, 1:500, Immunovision) and Alexa Fluor 488 goat anti human secondary antibody (A11013, 1:500, Invitrogen, Life Technologies). Cells were mounted into Vectashield with Dapi (H-1200, Vector Laboratories) and finally scanned by Leica SP5 confocal microscope equipped with HCX PL APO 40 × /1.3 OIL CS objective. For detection of DAPI and Alexa Fluor 488 we used excitation wavelengths 405 nm and 488 nm and AOBS and AOTF filter system. By choosing appropriate Z resolution the optimal coverage of the whole cell volume was achieved.
Chromosome spreads
Chromosome spreads from MI-stage oocytes were prepared as described previously.11,19 Oocytes were matured for 7.5 h. Only oocytes that achieved GVBD within 1.5 h after IBMX removal were used and matured subsequently for additional 6 h. Spreads were stained with antibody against centromere (HCT-0100, 1:500, Immunovision). Primary antibodies were detected by Alexa Fluor 594 goat anti human (A11014, 1:500, Invitrogen, Life technologies). Slides were mounted into Vectashield containing DAPI (H-1200, Vector Laboratories) and scanned with Leica SP5 confocal microscope with AOBS equipped with HCX PL APO 40 × /1.3 OIL CS objective. For detection of DAPI and Alexa Fluor 594 excitation wavelengths 405 nm and 561 nm were used. Some of the spreads were scanned using Leica AF6000 inverted fluorescence microscope equipped with HC PL APO 20x/0.7 IMM CORR CS and HCX PL APO 100 × /1.4–0.7 OIL objectives.
Live imaging
1–2 h after microinjection oocytes were transferred to Leica SP5 confocal microscope equipped with EMBL stage incubator and HCX PL APO 20 × /0.7 IMM CORR λBL and HCX PL APO 40 × /1.3 OIL CS objectives. For excitation 405, 488, 514 and 561 nm wavelengths were used. Fluorescently tagged proteins were detected with internal PMTs or HyDs. 9–13 Z-stacks were captured every 10–15 min for 18 h. Labeled chromosomes were tracked with Leica High Content Screening Automation software.
Analysis of data
Mean and standard deviation values were calculated using MS Excel, statistical significance of the differences between groups were tested using paired and unpaired Student’s t-test (GraphPad Prism software for Macintosh). Time-lapse experiments were analyzed using ImageJ (http://rsbweb.nih.gov/ij), Imaris (www.bitplane.com) or Huygens (www.svi.nl/tiki-index.php?page=HomePage) software.
Acknowledgments
We are grateful to Jaroslav Pialek, Institute of Vertebrate Biology, A.S. C.R. for Mus spretus breading pairs, Prof. Jiri Rubes and to all members of Martin Anger’s laboratory for helpful discussion and Dr. Frank Sieckmann (Leica Microsystems CMS GmbH) for optimizing High content Screening Software. M.A. was supported by Czech Science Foundation grant 523/09/0743, EMBO installation grant 1817 and Purkyne Fellowship. J.S., A.D. and L.N. were supported by above grants and also by Czech Science Foundation 204/09/H084. M.K. was supported by Czech Science Foundation grant P502–10–0944. Work in M.A. laboratory is supported by the project “CEITEC – Central European Institute of Technology” (CZ.1.05/1.1.00/02.0068) from European Regional Development Funds.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21398
References
- 1.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–91. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 2.Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Hornak M, Jeseta M, Musilova P, Pavlok A, Kubelka M, Motlik J, et al. Frequency of aneuploidy related to age in porcine oocytes. PLoS One. 2011;6:e18892. doi: 10.1371/journal.pone.0018892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol. 2010;20:1522–8. doi: 10.1016/j.cub.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol. 2010;20:1511–21. doi: 10.1016/j.cub.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Tachibana-Konwalski K, Godwin J, van der Weyden L, Champion L, Kudo NR, Adams DJ, et al. Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev. 2010;24:2505–16. doi: 10.1101/gad.605910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Revenkova E, Herrmann K, Adelfalk C, Jessberger R. Oocyte cohesin expression restricted to predictyate stages provides full fertility and prevents aneuploidy. Curr Biol. 2010;20:1529–33. doi: 10.1016/j.cub.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holt JE, Jones KT. Control of homologous chromosome division in the mammalian oocyte. Mol Hum Reprod. 2009;15:139–47. doi: 10.1093/molehr/gap007. [DOI] [PubMed] [Google Scholar]
- 9.Homer H. New insights into the genetic regulation of homologue disjunction in mammalian oocytes. Cytogenet Genome Res. 2011;133:209–22. doi: 10.1159/000324118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogt E, Kirsch-Volders M, Parry J, Eichenlaub-Ritter U. Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error. Mutat Res. 2008;651:14–29. doi: 10.1016/j.mrgentox.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 11.McGuinness BE, Anger M, Kouznetsova A, Gil-Bernabé AM, Helmhart W, Kudo NR, et al. Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr Biol. 2009;19:369–80. doi: 10.1016/j.cub.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 12.Meraldi P, Draviam VM, Sorger PK. Timing and checkpoints in the regulation of mitotic progression. Dev Cell. 2004;7:45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Rieder CL, Schultz A, Cole R, Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol. 1994;127:1301–10. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clute P, Pines J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat Cell Biol. 1999;1:82–7. doi: 10.1038/10049. [DOI] [PubMed] [Google Scholar]
- 15.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–93. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 16.Lane SI, Yun Y, Jones KT. Timing of anaphase-promoting complex activation in mouse oocytes is predicted by microtubule-kinetochore attachment but not by bivalent alignment or tension. Development. 2012;139:1947–55. doi: 10.1242/dev.077040. [DOI] [PubMed] [Google Scholar]
- 17.Nagaoka SI, Hodges CA, Albertini DF, Hunt PA. Oocyte-specific differences in cell-cycle control create an innate susceptibility to meiotic errors. Curr Biol. 2011;21:651–7. doi: 10.1016/j.cub.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koehler KE, Schrump SE, Cherry JP, Hassold TJ, Hunt PA. Near-human aneuploidy levels in female mice with homeologous chromosomes. Curr Biol. 2006;16:R579–80. doi: 10.1016/j.cub.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Duncan FE, Chiang T, Schultz RM, Lampson MA. Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol Reprod. 2009;81:768–76. doi: 10.1095/biolreprod.109.077909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouznetsova A, Lister L, Nordenskjöld M, Herbert M, Höög C. Bi-orientation of achiasmatic chromosomes in meiosis I oocytes contributes to aneuploidy in mice. Nat Genet. 2007;39:966–8. doi: 10.1038/ng2065. [DOI] [PubMed] [Google Scholar]
- 21.Dudas A, Ahmad S, Gregan J. Sgo1 is required for co-segregation of sister chromatids during achiasmate meiosis I. Cell Cycle. 2011;10:951–5. doi: 10.4161/cc.10.6.15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakuno T, Tanaka K, Hauf S, Watanabe Y. Repositioning of aurora B promoted by chiasmata ensures sister chromatid mono-orientation in meiosis I. Dev Cell. 2011;21:534–45. doi: 10.1016/j.devcel.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Peters JM. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell. 2002;9:931–43. doi: 10.1016/S1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- 24.Jones KT, Lane SI. Chromosomal, metabolic, environmental, and hormonal origins of aneuploidy in mammalian oocytes. Exp Cell Res. 2012;318:1394–9. doi: 10.1016/j.yexcr.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Wang ZB, Schatten H, Sun QY. Why is chromosome segregation error in oocytes increased with maternal aging? Physiology (Bethesda) 2011;26:314–25. doi: 10.1152/physiol.00020.2011. [DOI] [PubMed] [Google Scholar]
- 26.Khodjakov A, Pines J. Centromere tension: a divisive issue. Nat Cell Biol. 2010;12:919–23. doi: 10.1038/ncb1010-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musacchio A. Spindle assembly checkpoint: the third decade. Philos Trans R Soc Lond B Biol Sci. 2011;366:3595–604. doi: 10.1098/rstb.2011.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt P, LeMaire R, Embury P, Sheean L, Mroz K. Analysis of chromosome behavior in intact mammalian oocytes: monitoring the segregation of a univalent chromosome during female meiosis. Hum Mol Genet. 1995;4:2007–12. doi: 10.1093/hmg/4.11.2007. [DOI] [PubMed] [Google Scholar]
- 29.LeMaire-Adkins R, Radke K, Hunt PA. Lack of checkpoint control at the metaphase/anaphase transition: a mechanism of meiotic nondisjunction in mammalian females. J Cell Biol. 1997;139:1611–9. doi: 10.1083/jcb.139.7.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]