Summary
Campylobacter rectus is associated with fetal exposure and low-birth weight in humans. C. rectus also invades placental tissues and induces fetal intrauterine growth-restriction (IUGR) in mice, along with Toll-like receptors (TLR4) overexpression, suggesting that TLR4 may mediate placental immunity and IUGR in mice. To test this hypothesis we examined the effect of in vitroTLR4 neutralization in trophoblastic proinflammatory activity and studied the IUGR phenotype in a congenic TLR4-mutant mouse strain after in vivo C. rectus infection. Human trophoblasts were pretreated with TLR4 neutralizing antibodies and infected with C. rectus; pro-inflammatory cytokine production was assessed by cytokine multiplexing assays. Neutralizing TLR4 antibodies significantly impaired the production of pro-inflammatory cytokines in trophoblastic cells after infection in a dose-dependent manner. We used a subcutaneous chamber model to provide a C. rectus challenge in BALB/cAnPt (TLR4Lps-d) and wild-type(WT) females. Females were mated with WT or TLR4Lps-dmales once/week; pregnant mice were infected at (E)7.5 and sacrificed at (E)16.5 to establish IUGR phenotypes. Maternal C. rectus infection significantly decreased fetal weight/length in infected WT when compared to sham WT controls(P<0.05, ANOVA). However, infected TLR4Lps-d−/− mice did not show statistically significant differences in fetal weight and length when compared to WT controls(P>0.05). Furthermore, heterozygous TLR4Lps-d −/+fetuses showed IUGR phenotype rescue. We concluded that TLR4 is an important mediator of trophoblastic proinflammatory responses and TLR4-deficient fetuses do not develop IUGR phenotypes after C. rectus infection, suggesting that placental cytokine activation is likely to be mediated by TLR4 during low birth weight/preterm delivery pathogenesis.
Keywords: periodontitis, preterm delivery, animal models, mice, fetal growth retardation, Campylobacter rectus, Toll-like receptors
Introduction
Periodontal diseases (gingivitis and periodontitis) are among the most common infectious diseases affecting up to 50% of Americans (Albandar, 2002). As a chronic infection in nature, periodontitis exposes the host to microbial challenge for extended periods of time leading to a persistent oral inflammatory response that ultimately causes alveolar bone resorption and tooth loss. Concomitantly, the susceptible host is exposed to repeated bacteremias and systemic inflammatory mediators that have been shown to contribute to the pathogenesis of some systemic diseases including atherosclerosis and cardiovascular diseases (Beck and Offenbacher, 2005). Periodontitis has also been associated with an increased risk for preterm delivery (PTD) and preeclampsia in different human populations, suggesting that maternal periodontitis and the associated bacteria may represent an important systemic stressor for both the mother and fetus (Ruma et al., 2008). Moreover, maternal oral pathogens may reach the developing fetus through hematogenous dissemination (Han et al., 2009). In particular, Campylobacter rectus is an exclusively oral Gram negative anaerobe that has experimentally shown the competence to selectively translocate to the fetoplacental unit and operate as a fetal infectious agent eliciting prematurity and growth restriction in animals (Bobetsis et al., 2007;Offenbacher et al., 2005). Furthermore, our studies have found that maternal C. rectus infection in the presence of low serum antibody is associated with high fetal exposure and preterm delivery, as demonstrated by high fetal IgM antibody responses (Madianos et al., 2001). Fetal exposure to oral organisms is associated with higher levels of several pro-inflammatory mediators in cord blood, including IL-1β, IL-6 and TNFα, especially among preterm births. However, the underlying biological mechanisms leading to fetal-placental inflammation and preterm delivery after C. rectus exposure still remain to be elucidated.
PTD is defined by the World Health Organization as birth at less than 37 completed gestational weeks(1970). PTD is still the major cause of neonatal mortality and morbidity in the world, associated with low birth weight (<2500 grams) and fetal intrauterine growth-restriction(IUGR) (MacDorman et al., 2005). Preterm delivery can be initiated by multiple mechanisms including infection, local inflammation, uteroplacental ischemia, hemorrhage, stress and other immunologically mediated processes (Romero et al., 2006). Although precise triggering mechanisms have not been established, the development of a proinflammatory condition is a common pathway that centralizes all multiple PTD risk factors(Romero et al., 1994). Particularly, uterine infections account for 25–40% of preterm births and they are strongly linked to local proinflammatory cytokines, metalloproteinases and prostaglandins. The increased expression of these inflammatory mediators may lead to membrane weakening, early membrane rupture and uterine contraction initiation (Shoji et al., 2007). Such proinflammatory responses are likely to be initiated and/or mediated by the host innate immune system via activation of Toll-like receptors (TLRs) which have a primary role in pathogen recognition and innate immunity initiation(Brikos and O’Neill, 2008). TLRs receptors bind to several microbial components or end-products known as pathogen-associated molecular patterns (PAMPs). After binding and recognition, TLRs are able to trigger an array of signaling pathways that ultimately activate downstream molecules such as nuclear factor kB (NF-kB) and interferon regulatory factor 3 (IRF-3)(Uematsu and Akira, 2006), which in turn mediate the expression of several proinflammatory cytokines as demonstrated in several tissues, including the maternal-fetal interface(Koga and Mor, 2008). TLR regulation has been suggested to play a critical role in mediating the innate immune response during pregnancy, which in turn has significant implications for the success or failure of pregnancies in both early and late gestation (Patni et al., 2007). To date, about 13 mammalian Toll-like receptors homologues have been identified and designated, and their expression has been described in the human placenta, being dominantly expressed by trophoblasts (Abrahams et al., 2004; Holmlund et al., 2002; Kumazaki et al., 2004). Notably, trophoblasts have also been proposed to be involved in coordinating the immune response during both embryonic implantation, placental development and immunosurveillance (Mor, 2008).
Our overall goal is to better understand the maternal and fetal biological mechanisms leading to preterm delivery in response to C. rectus infection. Specifically, we have hypothesized that C. rectus induces a placental innate inflammatory response mediated by Toll-like receptors (TLRs). This hypothesis is based on previous studies using C. rectus as a model of systemic infection in pregnant mice, in which we have demonstrated: 1) the systemic dissemination of C. rectus from distant sites of infection (dorsal subcutaneous chamber and oral cavity) to the placenta(Arce et al., 2010); 2) Increased local placental inflammatory response confined to the decidua along with placental structural alterations (wider junctional zone)(Offenbacher et al., 2005); 3) Fetal intrauterine growth restriction induction (lighter and shorter fetal pups)(Yeo et al., 2005); 4) Altered gene expression along with down-regulation of several imprinted genes (i.e. Insulin growth-factor 2) via changes in DNA methylation patterns (hypermethylation) (Bobetsis et al., 2007; Bobetsis et al., 2010); 5)increased trophoblastic TLR-4 expression (2-fold) after C. rectus oral infection(Arce et al., 2009) and 6) in vitro trophoblastic production of TNFα and IL-6 in a dose-dependent response to C. rectus infection (Arce et al., 2010). Based upon these experimental observations, we believe that the local placental inflammatory response may play a significant role in mediating IUGR. However, it is still unclear whether TLRs activation mediate the placental inflammatory responses and IUGR in response to C. rectus exposure in vivo. The purpose of this investigation was to determine the role of TLR-4 in mediating in vitro (human trophoblasts) cytokine synthesis following C. rectus challenge and to determine whether TLR4 deficient mice would lose the IUGR phenotype in response to C. rectus exposure.
Methods
Mammalian cell lines
The human trophoblast cell line BeWo (ATCC CCL-98) was used for cytokine assays (Pattillo and Gey, 1968). BeWo cells are the first human trophoblastic endocrine cell type to be maintained in continuous culture, initiated from a malignant gestational choriocarcinoma of the fetal placenta. Briefly, BeWo cells were grown in Ham’s F12K medium with 2 mM L-glutamine adjusted to contain 10% fetal bovine serum (FBS) according to ATCC propagation instructions. Cells were grown in T-25 flasks (Corning, Life Sciences, MA) or onto cover slips placed in 6-well plates for the experiments. All cells were grown at 37°C in 10% CO2.
Bacterial cultures
C. rectus 314 aliquots were maintained in Wilkins Chalgren anaerobic broth medium (WC broth; DSMZ, Braunschweig, Germany) containing 10% skim milk at −80°C. C. rectus aliquots were reconstituted on PRAS ETSA plates (Enriched Tryptic Soy Agar from Anaerobe Systems, Morgan Hill CA). Bacteria were anaerobically grown under 5% CO2, 10% H2-85% N2 atmosphere at 37°C for 4–6 days. Bacterial suspensions were prepared from primary cultures at their log phase of growth and resuspended in tissue culture medium without antibiotics (in vitro experiments) or PBS (in vivo experiments) to an optical density of 1.00 (600 nm) determined by spectrophotometry (Cecil Instruments, Cambridge, UK) corresponding to 1×109 bacteria/ml.
In vitro trophoblast infection assays
BeWo cells were grown onto 6-well plates until 80–90% confluency. BeWo cell monolayers were also washed 3 times with cell culture medium without antibiotics prior to inoculation with bacteria. Bacterial cells were added to obtain a multiplicity of infection (MOIs) of 500 bacteria/BeWo cell, after which plates were centrifuged at 250 ×g for 5 min, incubated for 12h at 37°C in 10% CO2 and washed with PBS. This time point and MOI were chosen based on previous experiments demonstrating a dose-dependent pro-inflammatory activity (Arce et al., 2010). All experiments were done in triplicates and in two independent times.
TLR 4 neutralization
Additional infection experiments were performed to evaluate the effect of using a TLR4 neutralizing antibody in the proinflammatory phenotype. Briefly, BeWo cells were treated with 1 or 2ug of anti-human TLR4 antibody (AF1478 goat IgG, R&D systems, Minneapolis MN) for 2 hours before infection. Then BeWo cell monolayers were washed 3 times with cell culture medium without antibiotics and were followed by the infection protocol as explained before. The concentration for human TLR4 bioactivity neutralization for this antibody was chosen based on the lowest dose recommended by the manufacturer (1.5 μg/mL). Additional experiments were also performed to include ultrapure E. coli LPS (0111:B4 strain, Invivogen, San Diego, CA) using 1 μg/well as a positive control for the production of proinflammatory cytokines.
Cytokine multiplexing assays
infected cells and non-infected controls were washed 3 times with PBS to remove non-adhered cells. The quantification of IL-6 and TNFα in cell supernatants was performed by means of xMAP multiplexing cytokine assays. Briefly, cell supernatants were collected after timed infection, centrifuged at 1500 × g for 5 min and then frozen until analysis. Multianalyte kits for human and mouse IL-6 and TNFα were used following the manufacturer’s instructions (Fluorokine MAP Kits, R&D systems, Minneapolis, MN). Experiments were performed in duplicates and two independent assays
Congenic TLR4-deficient mouse model of systemic C. rectus infection
All procedures were in accordance with the animal welfare guidelines and approved by the University of North Carolina-Chapel Hill Institutional Animal Care and Use Committee. The mouse infection model used was similar to that described before (Yeo et al., 2005). BALB/cAnPt TLR4-deficient (TLR4Lps-d) mice and congenic BALB/cByJ Wild-type (WT) controls were obtained from the Jackson Laboratory (C.C3-Tlr4Lps-d/J stock number 002930 and BALB/cByJ wild type stock number 001026, The Jackson Laboratory, Bar Harbor, MA). All mice were housed under controlled and standardized conditions with 12-hour light-dark cycles. Regular mouse diet and water were provided ad libitum. Females were enrolled in the experiments at approximately 6 weeks of age and immediately had a steel chamber implanted subcutaneously as previously described (Yeo et al., 2005). After one month of healing, females were mated overnight with males of the same or different background (6 females per group). The next morning, females were removed from the male cages and examined for vaginal plugs. If a plug was found, that day was recorded as embryonic day E0.5. At E7.5, pregnant mice received an intra-chamber injection of 100 μl of 109 CFU/mL live C. rectus in PBS. Mice were then sacrificed at E16.5 and fetuses (n=47 from homozygous TLR4lps-d−/− infected dams and n=49 from heterozygous TLR4lps-d+/− infected dams) and their respective placental tissues were collected for further analyses (fetal length/weight). For comparison purposes, we included fetal weight and length data from experiments using sham-infected WT controls (n=143) and C. rectus-infected WT mice (n=37) that were subjected to the same experimental protocol.
Statistical analysis
A minimal sample size of 6 mice per group was calculated [power (1−β) of >0.90% with alpha-error threshold of (α) = 0.05] based on our previous results on fetal growth restriction after C. rectus systemic infection at E16.5 (Arce et al., 2010). Continuous variables were expressed as means and standard errors. mRNA fold differences between the infected cells (test) and non-infected controls were compared using the unpaired T-test. Protein concentration differences were compared using the one-way Anova test. Mean placental/fetal weight and fetal length values for all groups were compared using the Anova (Kruskal-wallis) test with Dunn’s post-hoc comparisons. The frequency of resorptions and litter sizes in all groups were compared by using the Chi-square test. The threshold for statistical significance was set at a P-value less than 0.05. No corrections were made for multiple comparisons. All analyses were performed using GraphPad software (San Diego California USA).
Results
Neutralizing anti-human TLR 4 antibodies impaired the production of pro-inflammatory cytokines in BeWo cells after C. rectus infection in a dose-dependent manner
We evaluated the production of the pro-inflammatory cytokines IL-6 and TNFα by multiplexing assays in response to C. rectus treatment and the effects of anti- TLR4 neutralizing antibodies on cytokine synthesis. As depicted in Figure 1, there was a dose-dependent suppression of the cytokine production by the addition of anti-TLR4AB in BeWo cells. For example, for total IL-6 production in response to MOI-500 there was a statistically significant decrease to 46.4% when cells were pre-treated with 1ug TLR4AB (34.0±9.02 pg/mL), and a further decrease to 17.1% was evident when using 2ug of TLR4AB (12.5±4.18, P<0.05, Anova). A similar trend was also observed for TNFα, in which total production was decreased to 48.1% when using 1ug TLR4AB (4.9±1.80) and to 33.6% when using 2ug TLRAB (3.4±0.32, P<0.05, Anova). We also evaluated the pro-inflammatory responses to a classical TLR4 agonist (E. coli LPS) as a positive control for the experiment, finding a significant decrease for both IL-6 (21.5%) and TNFα (31%).
Figure 1. TLR4 neutralization in trophoblastic cells affects cytokine production after in vitro C. rectus infection.
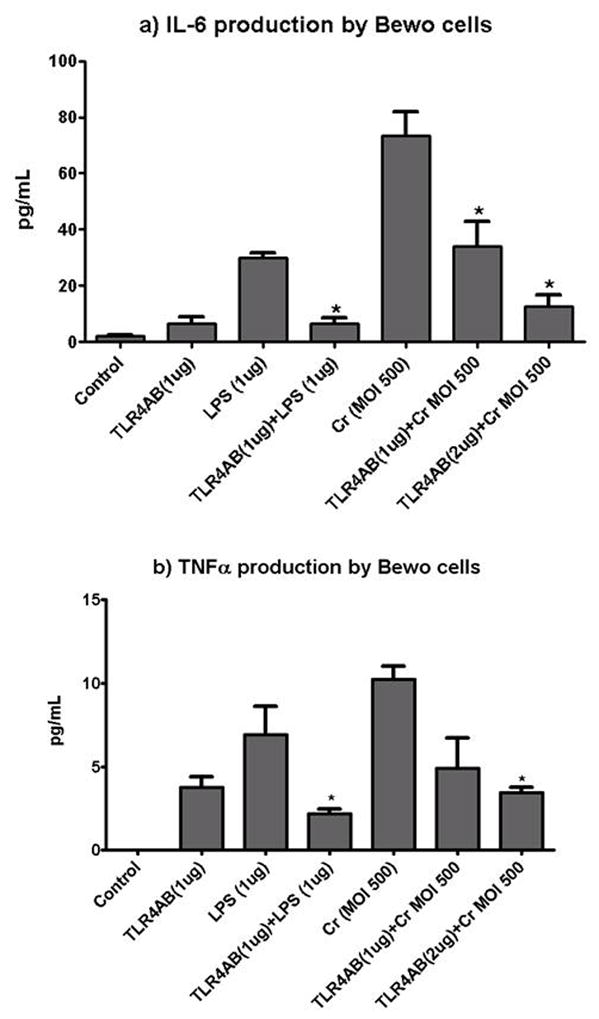
BeWo cells were pre-treated with 1ug or 2ug of a TLR4AB showing a dose-response decrease in IL-6 and TNFα production. Cr=C. rectus. * P<0.05
TLR4-deficient fetuses are less susceptible to IUGR after maternal C. rectus systemic infection
We sought to evaluate the in vivo effect of a deficient TLR4 receptor congenic murine strain on IUGR after systemic C. rectus exposure. In terms of litter size and frequency of fetal resorptions, there were no statistical differences among all experimental groups (P>0.05, Chi-square). This suggests there were no effects of TLR4 deficiency on implantation. However, differences in fetal weight are shown in Figure 2. WT fetuses from sham-infected dams had an average weight of 0.47±0.005 grams. When infected with C. rectus, WT dams delivered IUGR fetuses that had an average weight of 0.42±0.009. However, when TLR4lps-ddams were mated with males of the same genetic background and then infected with C. rectus, the TLR4lps-d−/− homozygous fetuses appeared not to be affected by the infection as their average weight of 0.46±0.010 did not statistically differ from the WT control dams (P>0.05, Kruskal-wallis). Furthermore, when TLR4lps-ddams were mated with a male from a different genetic background (WT) and after C. rectus infection, the IUGR phenotype of TLR4lps-d+/− heterozygous fetuses had an average fetal weight of 0.43±0.008 was statistically different from the WT controls (P<0.05, Kruskal-wallis) and intermediate between WT and homozyogousTLR4lps-d−/−. A very similar trend was also observed for the fetal length (expressed in cms) as illustrated in Figure 3. For example, WT controls had an average size of 1.440±0.008 and when WT dams were infected by C. rectus fetuses were found to be significantly smaller (1.229±0.01, P<0.05, Kruskal-wallis). However, infected homozygous TLR4lps-d−/− fetuses were larger than infected WT (1.396±0.019) and were not statistically different from WT controls (P>0.05). On the other hand, infected heterozygous TLR4lps-d+/− mice showed smaller fetuses (1.251±0.012) that were significantly different from fetuses from the WT controls.
Figure 2. Fetal weight is not significantly affected in TLR4-deficient fetuses from C. rectus-infected dams.
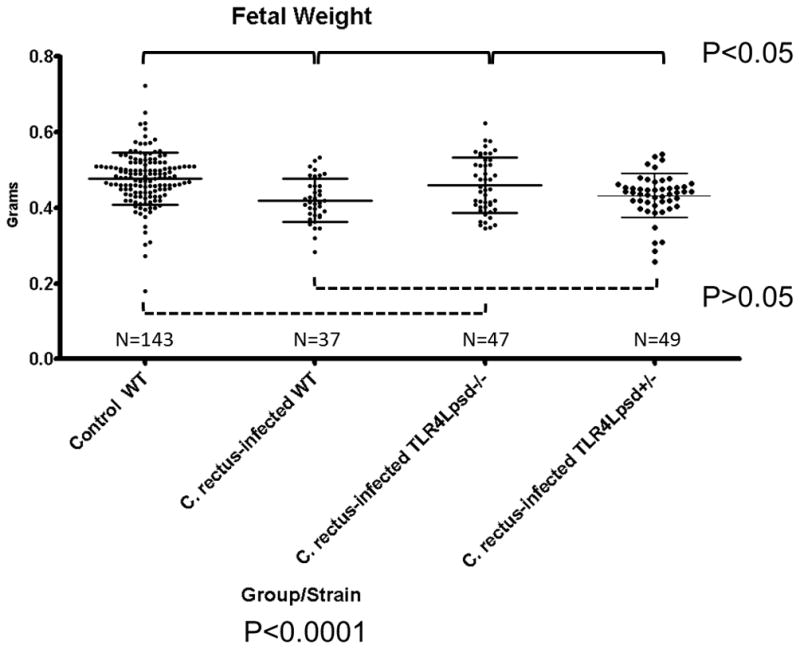
Box-plots depicting average ± standard errors. The overall Kruskal-Wallis test was highly statistically significant (P<0.0001). Dunn’s post-hoc multiple comparison tests found statistically significant differences (P<0.05) for Control WT vs. C. rectus-infected WT, Control WT vs. C. rectus-infected TLR4Lpsd+/− and C. rectus-infected WT vs. C. rectus-infected TLR4Lpsd−/−. There were not significant differences (P>0.05) for Control WT vs. C. rectus-infected TLR4Lpsd−/−, C. rectus-infected WT vs. C. rectus-infected TLR4Lpsd+/− and C. rectus-infected TLR4Lpsd−/− vs. C. rectus-infected TLR4Lpsd+/−. Solid lines indicate statistical significance (P<0.05). Dotted lines indicate no statistical significance (P>0.05).
Figure 3. Fetal length is unaffected in TLR4-deficient fetuses from C. rectus-infected dams.
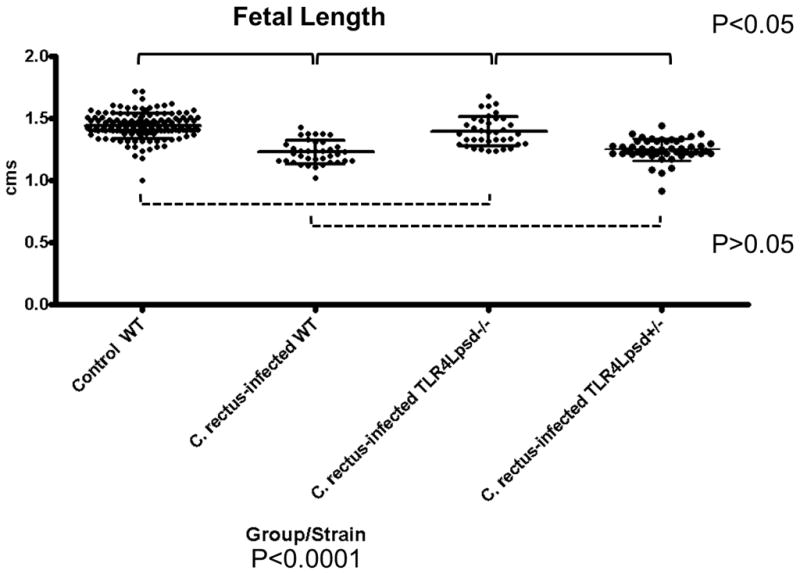
Box-plots depicting average ± standard errors. The overall Kruskal-Wallis test was highly statistically significant (P<0.0001). Dunn’s post-hoc multiple comparison tests found statistically significant differences (P<0.05) for Control WT vs. C. rectus-infected WT, Control WT vs. C. rectus-infected TLR4Lpsd+/−, C. rectus-infected WT vs. C. rectus-infected TLR4Lpsd−/− and for C. rectus-infected TLR4Lpsd−/− vs. C. rectus-infected TLR4Lpsd+/−. There were no significant differences for Control WT vs. C. rectus-infected TLR4Lpsd−/− and C. rectus-infected WT vs. C. rectus-infected TLR4Lpsd+/−. Solid lines indicate statistical significance (P<0.05). Dotted lines indicate no statistical significance(P>0.05).
Discussion
Preterm delivery is the major cause of neonatal morbidity/mortality in the world, affecting 12.5% of live births the United States (Abrahams, 2008). Even though the etiology of pregnancy complications remains somewhat elusive, there is strong evidence supporting the association between infections and PTD, mostly mediated by systemic inflammation (Goldenberg et al., 2000; Romero et al., 2007). These observations in humans have been confirmed by a number of animal models demonstrating a PTD phenotype in response to experimental infection with heat-killed, live bacteria or isolated bacterial components that are able to trigger an inflammatory cascade at the fetoplacental level (Elovitz, 2006; Han et al., 2004).
Toll-like receptors (TLRs) have gained a lot of attention in PTD research in the last decade. TLRs are a family of transmembrane proteins that play a key role in activating the innate immune system in different organs including the fetal maternal interphase (Uematsu and Akira, 2006). In particular, TLR receptors type 4 (TLR4) has been found to be particularly important because TLR4 senses the major Gram negative component: lipopolysaccharide (LPS). LPS delivered systemically, intrauterine or intra-amniotically triggers PTD in vivo(Elovitz and Mrinalini, 2004; Liu et al., 2007). Furthermore, mice deficient for TLR4 show protection against bacterial and LPS-induced PTD (Wang and Hirsch, 2003; Elovitz et al., 2003). Experimental TLR4 antagonism suppresses pro-inflammatory responses to bacteria including oral microorganisms in vivo(Liu et al., 2007;ms Waldorf et al., 2008). At the cellular level, Trophoblasts are thought to be primary sentinel cells that are involved in microbial clearance preventing microbial translocation of infectious agents from mother to fetus (Levy, 2007). Upon recognition of microbes through TLRs, trophoblasts coordinate NK cells and neutrophils recruitment to the maternal-fetal interface, a key feature in PTD and pre-eclampsia pathogenesis (Abrahams and Mor, 2005). Trophoblastic cells upregulate the secretion of pro-inflammatory chemokines (IL-8 and MCP-1) and cytokines (IL1β, IL6 and TNFα) following the ligation of TLR-4 by bacterial LPS, exerting trophoblastic cells to differentially modulate the maternal immune system both during normal pregnancy and in the presence of an intrauterine infection (Mor, 2008).
A similar proinflammatory phenotype has also been observed in our experiments using the oral periodontal pathogen Campylobacter rectus. C. rectus is an orally Gram negative anaerobe and motile bacterium that plays a pathogenic role in human periodontitis (Rams et al., 1993; Yokoyama et al., 2008). C. rectus shows a wide array of virulence factors including LPS (Ogura et al., 1995; Ogura et al., 1996; Takiguchi et al., 1996). C. rectus is part of the Campylobacteraceae family which has been associated with other diseases showing important pro-inflammatory mechanistic similarities such as Campylobacter jejuni in acute gastroenteritis (Allos, 2001) and Campylobacter fetus in sheep and cattle abortion (Guerrant et al., 1978; Macuch and Tanner, 2000; Fujihara et al., 2006). In animal models, C. jejuni and C. fetus infections result in impaired fetal development and intrauterine growth restriction (O’Sullivan et al., 1988a; O’Sullivan et al., 1988b). Noteworthy, our in vivo and in vitro C. rectus infection experiments in pregnant mice and placental cells have consistently shown an upregulation of maternal inflammatory serum markers following challenge(Yeo et al., 2005), as well as placental translocation. In culture C. rectus is capable of trophoblastic cell invasion. These observations suggest a pathway that involves translocation of C. rectus to placental tissue, activation of trophoblastic TLR4 consequent placental pro-inflammatory response that ultimately result in IUGR (Arce et al., 2010).
We previously reported a dose-dependent response for human IL-6 and TNFα production at the protein levels in human BeWo trophoblasts in response to C. rectus in vitro infection (Arce et al., 2010). In this report we provide new data that demonstrate that the antagonism of TLR4 by means of a neutralizing TLR4 antibody (TLR4AB) impairs the activation of inflammatory responses (Fig. 1). When BeWo cells were pre-treated with 2 different doses (1 or 2 ug) of TLR4AB for 2 hours before live bacterial exposure, a statistically significant dose-dependent inhibition in the level of IL-6 was observed (Fig 1a, P<0.05, Anova for both doses when compared to Cr MOI500). A similar trend was observed for TNFα production (Fig. 1b, P<0.05, Anova for 2ug dose when compared to Cr MOI500). The ligation of TLR4 by the antagonist also induced some degree of cytokine activation that was not statistically significant when compared to the blank control (P>0.05, Anova), but may indicate a slight partial agonist activity. Collectively, these results strongly suggest a TLR4 dependent activity of BeWo cells in response to C. rectus infection. TLR4 experimental antagonism has also demonstrated significant effects on a PTD phenotype in different animal models. For example, TLR4 receptors have been shown to mediate the murine placental inflammatory response and fetal death to Fusobacterium nucleatum, another oral periodontal bacterial species associated with PTD (Liu et al., 2007). Moreover, the selective antagonism of TLR4 inhibits inflammation and preterm uterine contractility in a nonhuman intra-amniotic LPS model in Rhesus monkeys (ms Waldorf et al., 2008).
Lastly, we aimed to evaluate our current murine model of systemic bacterial exposure of C. rectus in pregnant mice on a congenic TLR4-mutant mouse strain on a Balb/C background. As reported before, a remote subcutaneous maternal C. rectus infection increases fetal growth restriction in Balb/C mice (Yeo et al., 2005; Offenbacher et al., 2005). As shown in Figures 2 and 3, when WT dams were infected with C. rectus, there was a statistically significant decrease in fetal weight (12.5%) and length reduction (14.7%)(P<0.05, Kruskal-wallis). However, in the absence of functional TLR4 in homozygous fetuses, no IUGR was detected as TLR4Lps-d−/− fetuses were not statistically different from sham-infected WT controls for both weight and length(P>0.05, Dunn’s). Furthermore, when fetuses carried only 1 allele with a functional TLR4 in the heterozygous TLR4Lps-d+/− group the IUGR phenotype was again observed as these fetuses were significantly different from WT controls (P<0.05, Dunn’s) and comparable to C. rectus-infected WT dams (P>0.05, Dunn’s). To our interpretation, the observed phenotype in our experiments completely supported our hypothesis that TLR4 receptors are mediating IUGR after systemic C. rectus infection in mice. This is also in agreement with Liu et al (Liu et al., 2007), who reported that the fetal death rate was significantly reduced in TLR4-deficient mice on a TLR4 knock/out strain following F. nucleatum infection.
In conclusion, TLR4 neutralization leads to a significant reduction in trophoblastic pro-inflammatory activity can be observed in vitro. Moreover, in the absence of a functional TLR4 in mice seems to lead to decreased susceptibility to C. rectus infection (IUGR) in vivo. Collectively, these results suggest that the placental proinflammatory responses are mediated by TLR4 during low birth weight/preterm delivery pathogenesis. We also speculate that TLR4-antagonistic therapies may be used to specifically block infection-associated inflammation during pregnancy as proposed by others (Abrahams, 2008; Liu et al., 2007). TLR4 as a therapeutic target may provide new future studies that could have promise to bring new anti-inflammatory agents for the treatment or prevention of bacterial-induced, preterm delivery.
Acknowledgments
This study was funded by the National Institutes of Health grants RO1-DE-12453, P-60-DE-13079, and U01 DE1457.
References
- The prevention of perinatal mortality and morbidity. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1970;457:1–60. [PubMed] [Google Scholar]
- Abrahams VM. Antagonizing toll-like receptors to prevent preterm labor. Reprod Sci. 2008;15:108–109. doi: 10.1177/1933719108314574. [DOI] [PubMed] [Google Scholar]
- Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- Abrahams VM, Mor G. Toll-like receptors and their role in the trophoblast. Placenta. 2005;26:540–547. doi: 10.1016/j.placenta.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Albandar JM. Global risk factors and risk indicators for periodontal diseases. Periodontol 2000. 2002;29:177–206. doi: 10.1034/j.1600-0757.2002.290109.x. [DOI] [PubMed] [Google Scholar]
- Allos BM. Campylobacter jejuni Infections: update on emerging issues and trends. Clin Infect Dis. 2001;32:1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- Arce RM, Barros SP, Wacker B, Peters B, Moss K, Offenbacher S. Increased TLR4 expression in murine placentas after oral infection with periodontal pathogens. Placenta. 2009;30:156–162. doi: 10.1016/j.placenta.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce RM, Diaz PI, Barros SP, Galloway P, Bobetsis Y, Threadgill D, Offenbacher S. Characterization of the invasive and inflammatory traits of oral Campylobacter rectus in a murine model of fetoplacental growth restriction and in trophoblast cultures. J Reprod Immunol. 2010;84:145–153. doi: 10.1016/j.jri.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76:2089–2100. doi: 10.1902/jop.2005.76.11-S.2089. [DOI] [PubMed] [Google Scholar]
- Bobetsis YA, Barros SP, Lin DM, Arce RM, Offenbacher S. Altered gene expression in murine placentas in an infection-induced intrauterine growth restriction model: a microarray analysis. J Reprod Immunol. 2010;85:140–148. doi: 10.1016/j.jri.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobetsis YA, Barros SP, Lin DM, Weidman JR, Dolinoy DC, Jirtle RL, et al. Bacterial infection promotes DNA hypermethylation. J Dent Res. 2007;86:169–174. doi: 10.1177/154405910708600212. [DOI] [PubMed] [Google Scholar]
- Brikos C, O’Neill LA. Signalling of toll-like receptors. Handb Exp Pharmacol. 2008:21–50. doi: 10.1007/978-3-540-72167-3_2. [DOI] [PubMed] [Google Scholar]
- Elovitz MA. Anti-inflammatory interventions in pregnancy: now and the future. Semin Fetal Neonatal Med. 2006;11:327–332. doi: 10.1016/j.siny.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab. 2004;15:479–487. doi: 10.1016/j.tem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara N, Takakura S, Saito T, Iinuma Y, Ichiyama S. A case of perinatal sepsis by Campylobacter fetus subsp. fetus infection successfully treated with carbapenem--case report and literature review. J Infect. 2006;53:e199–e202. doi: 10.1016/j.jinf.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- Guerrant RL, Lahita RG, Winn WC, Jr, Roberts RB. Campylobacteriosis in man: pathogenic mechanisms and review of 91 bloodstream infections. Am J Med. 1978;65:584–592. doi: 10.1016/0002-9343(78)90845-8. [DOI] [PubMed] [Google Scholar]
- Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004;72:2272–2279. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47:38–47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmlund U, Cebers G, Dahlfors AR, Sandstedt B, Bremme K, Ekstrom ES, Scheynius A. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology. 2002;107:145–151. doi: 10.1046/j.1365-2567.2002.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Mor G. Expression and function of toll-like receptors at the maternal-fetal interface. Reprod Sci. 2008;15:231–242. doi: 10.1177/1933719108316391. [DOI] [PubMed] [Google Scholar]
- Kumazaki K, Nakayama M, Yanagihara I, Suehara N, Wada Y. Immunohistochemical distribution of Toll-like receptor 4 in term and preterm human placentas from normal and complicated pregnancy including chorioamnionitis. Hum Pathol. 2004;35:47–54. doi: 10.1016/j.humpath.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol. 2007;179:2501–2508. doi: 10.4049/jimmunol.179.4.2501. [DOI] [PubMed] [Google Scholar]
- MacDorman MF, Martin JA, Mathews TJ, Hoyert DL, Ventura SJ. Explaining the 2001–2002 infant mortality increase in the United States: data from the linked birth/infant death data set. Int J Health Serv. 2005;35:415–442. doi: 10.2190/TJ2N-DADV-1EP5-5C7F. [DOI] [PubMed] [Google Scholar]
- Macuch PJ, Tanner AC. Campylobacter species in health, gingivitis, and periodontitis. J Dent Res. 2000;79:785–792. doi: 10.1177/00220345000790021301. [DOI] [PubMed] [Google Scholar]
- Madianos PN, Lieff S, Murtha AP, Boggess KA, Auten RL, Jr, Beck JD, Offenbacher S. Maternal periodontitis and prematurity. Part II: Maternal infection and fetal exposure. Ann Periodontol. 2001;6:175–182. doi: 10.1902/annals.2001.6.1.175. [DOI] [PubMed] [Google Scholar]
- Mor G. Inflammation and Pregnancy: The Role of Toll-like Receptors in Trophoblast-Immune Interaction. Ann N Y Acad Sci. 2008;1127:121–128. doi: 10.1196/annals.1434.006. [DOI] [PubMed] [Google Scholar]
- ms Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines, and prostaglandins in rhesus monkeys. Reprod Sci. 2008;15:121–127. doi: 10.1177/1933719107310992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan AM, Dore CJ, Boyle S, Coid CR, Johnson AP. The effect of campylobacter lipopolysaccharide on fetal development in the mouse. J Med Microbiol. 1988a;26:101–105. doi: 10.1099/00222615-26-2-101. [DOI] [PubMed] [Google Scholar]
- O’Sullivan AM, Dore CJ, Coid CR. Campylobacters and impaired fetal development in mice. J Med Microbiol. 1988b;25:7–12. doi: 10.1099/00222615-25-1-7. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Riche EL, Barros SP, Bobetsis YA, Lin D, Beck JD. Effects of maternal Campylobacter rectus infection on murine placenta, fetal and neonatal survival, and brain development. J Periodontol. 2005;76:2133–2143. doi: 10.1902/jop.2005.76.11-S.2133. [DOI] [PubMed] [Google Scholar]
- Ogura N, Matsuda U, Tanaka F, Shibata Y, Takiguchi H, Abiko Y. In vitro senescence enhances IL-6 production in human gingival fibroblasts induced by lipopolysaccharide from Campylobacter rectus. Mech Ageing Dev. 1996;87:47–59. doi: 10.1016/0047-6374(96)01701-0. [DOI] [PubMed] [Google Scholar]
- Ogura N, Shibata Y, Matsuda U, Oikawa T, Takiguchi H, Izumi H, Abiko Y. Effect of Campylobacter rectus LPS on plasminogen activator-plasmin system in human gingival fibroblast cells. J Periodontal Res. 1995;30:132–140. doi: 10.1111/j.1600-0765.1995.tb01262.x. [DOI] [PubMed] [Google Scholar]
- Patni S, Flynn P, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. An introduction to Toll-like receptors and their possible role in the initiation of labour. BJOG. 2007;114:1326–1334. doi: 10.1111/j.1471-0528.2007.01488.x. [DOI] [PubMed] [Google Scholar]
- Pattillo RA, Gey GO. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968;28:1231–1236. [PubMed] [Google Scholar]
- Rams TE, Feik D, Slots J. Campylobacter rectus in human periodontitis. Oral Microbiol Immunol. 1993;8:230–235. doi: 10.1111/j.1399-302x.1993.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- Ruma M, Boggess K, Moss K, Jared H, Murtha A, Beck J, Offenbacher S. Maternal periodontal disease, systemic inflammation, and risk for preeclampsia. Am J Obstet Gynecol. 2008;198:389–5. doi: 10.1016/j.ajog.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shoji T, Yoshida S, Mitsunari M, Miyake N, Tsukihara S, Iwabe T, et al. Involvement of p38 MAP kinase in lipopolysaccharide-induced production of pro-and anti-inflammatory cytokines and prostaglandin E(2) in human choriodecidua. J Reprod Immunol. 2007;75:82–90. doi: 10.1016/j.jri.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Takiguchi H, Yamaguchi M, Mochizuki K, Abiko Y. Effect of in vitro aging on Campylobacter rectus lipopolysaccharide-stimulated PGE2 release from human gingival fibroblasts. Oral Dis. 1996;2:202–209. doi: 10.1111/j.1601-0825.1996.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med. 2006;84:712–725. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod. 2003;69:1957–1963. doi: 10.1095/biolreprod.103.019620. [DOI] [PubMed] [Google Scholar]
- Yeo A, Smith MA, Lin D, Riche EL, Moore A, Elter J, Offenbacher S. Campylobacter rectus mediates growth restriction in pregnant mice. J Periodontol. 2005;76:551–557. doi: 10.1902/jop.2005.76.4.551. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Hinode D, Yoshioka M, Fukui M, Tanabe S, Grenier D, Ito HO. Relationship between Campylobacter rectus and periodontal status during pregnancy. Oral Microbiol Immunol. 2008;23:55–59. doi: 10.1111/j.1399-302X.2007.00391.x. [DOI] [PubMed] [Google Scholar]


