Abstract
Monoamine oxidase A (MAO-A) is the key enzyme for the degradation of brain serotonin (5-hydroxytryptamine, 5-HT), norepinephrine (NE) and dopamine (DA). We recently generated and characterized a novel line of MAO-A hypormorphic mice (MAO-ANeo), featuring elevated monoamine levels, social deficits and perseverative behaviors as well as morphological changes in the basolateral amygdala and orbitofrontal cortex. Here we showed that MAO-ANeo mice displayed deficits in motor control, manifested as subtle disturbances in gait, motor coordination, and balance. Furthermore, magnetic resonance imaging of the cerebellum revealed morphological changes and a moderate reduction in the cerebellar size of MAO- ANeo mice compared to wild type (WT) mice. Histological and immunohistochemical analyses using calbindin-D-28k (CB) expression of Purkinje cells revealed abnormal cerebellar foliation with vermal hypoplasia and decreased in Purkinje cell count and their dendritic density in MAO- ANeo mice compared to WT. Our current findings suggest that congenitally low MAO-A activity leads to abnormal development of the cerebellum.
Keywords: Monoamine oxidase A, Hypomorphism, Serotonin, Cerebellum, Purkinje cells
1. Introduction
Monoamine neurotransmitters, including serotonin (5-hydroxytryptamine, 5-HT) and norepinephrine (NE), are known to exert a profound impact on early brain development through the regulation of neurogenesis, migration, differentiation, plasticity and other key morphogenetic processes (Azmitia, 2001; Gaspar et al., 2003; Schultz, 2007; Thompson and Stanwood, 2009). Monoamine oxidase A (MAO-A) is the key enzyme that catalyzes the oxidative degradation of 5-HT and NE (Bortolato et al., 2008; Shih et al., 1999). Congenital deficiency of MAO-A results in high brain levels of 5-HT and NE (Cases et al., 1995), neurodevelopmental abnormalities of the cortex and other brain regions (Cases et al., 1996; Kim et al., 1997; Upton et al., 1999), as well as overt aggression and other emotional alterations (Cases et al., 1995).
We recently generated MAO-ANeo mice, a line of MAO-A hypomorphic mutants. These transgenic animals harbor a neomycin resistance cassette in intron 12 of the Maoa gene, which by alternative splicing of the Maoa mRNA, leads to a reduction in the levels of the functional MAO-A enzyme. MAO-ANeo mice showed undetectable MAO-A enzymatic activity in the hippocampus and midbrain that was accompanied by high levels of 5-HT and NE, while low levels of MAO-A enzymatic activity in the prefrontal cortex and amygdala was accompanied by normal 5-HT levels and high NE levels. Notably, these animals exhibited a unique behavioral phenotype, different than their wild type (WT) and MAO-A knockout (KO) counterparts (Bortolato et al., 2011). In particular, we found that these mice exhibited social deficits and perseverative responses (Bortolato et al., 2011), two common traits observed in autism-spectrum disorder (ASD). The possibility that MAO-A hypomorphism may induce ASD-related alterations is in line with previous findings documenting monoaminergic dysregulations that have been implicated in these and other neurodevelopmental disorders (Simpson et al., 2011; Whitaker-Azmitia, 2005; Winter et al., 2008).
Neuropathological alterations of the cerebellum are among the most consistent morphological aberrances found in ASD subjects (Bailey et al., 1998; Bauman and Kemper, 1985; Kemper and Bauman, 1993; Ritvo et al., 1986; Whitney et al., 2008a). In particular, 72% of ASD cases reported in the literature exhibited a decreased number of Purkinje Cells (PCs) in the cerebellar cortex (Palmen et al., 2004). Furthermore, reports have also shown a decrease in the size of cerebellar vermis (Scott et al., 2009; Webb et al., 2009). Interestingly, anatomical and immunohistochemical studies have shown that the cerebellum is innervated by an extensive plexus of both 5-HT and NE fibers (Bishop and Ho, 1985; Fillenz, 1990; Hoffer et al., 1971; Takeuchi et al., 1982) and they modulate the firing rate of PC and cerebellar nuclei (Bickford-Wimer et al., 1991; Hoffer et al., 1973; Mitoma et al., 1994; Murano et al., 2011; Strahlendorf et al., 1991). Disruption of these monoamine systems during development could alter the microcircuitry within the cerebellum as well as their extra-cerebellar connections.
Based on these lines of information, the aim of the present study was to investigate the morphological and functional characteristics of the cerebellum in MAO-ANeo mice. A portion of the data have been presented in an abstract form (Alzghoul et al., 2011).
2. Experimental Procedure
2.1. Animals
All animals were treated in accordance with respective universities approved by the animal care and use committee and complied with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health (NIH) guidelines. Briefly, mice were generated from 129S6 mice (WT) by insertion of a floxed neomycin-resistance cassette in intron-12 of the Maoa gene, which by alternative splicing produces a truncated enzyme at the C-terminus. This leads to a reduction in the amount of functional transcript of the MAO-A enzyme and resulted in higher 5-HT and NE levels in many brain regions (Bortolato et al., 2011). A total of 47 male mice were used in this study (24 WT and 23 MAO-ANeo). Among them, 24 mice (12 WT and 12 MAO-ANeo) were used for behavioral studies, 10 mice (5 from each group) were used for the MR imaging study, and 7 WT and 6 MAO-ANeo mice were used for histological and immunohistochemical analyses.
2.2. Behavioral testing
2.2.1. Footprint assay
Gait was assessed as described elsewhere (Carter et al., 1999). Briefly, we used a Plexiglas runway (1000 × 6 cm) lined with white paper. Mouse forepaws and hindpaws were dipped in blue and orange non-toxic tempera paint. Mice were placed on the runaway and allowed to traverse it. For each set of footprints, we measured the distance between paw axes (stride-width) for both forepaws and hindpaws. A minimum of six measurements were taken per mouse. Gait pattern was also analyzed by calculating the ratio between the hindpaw and forepaw stride-widths, as well as the overall stride length (forepaws and hindpaws) for each genotype.
2.2.2. Rotarod
Motor coordination was measured using a rotarod apparatus (Med-associates, St. Albans VT, USA). Mice were placed on a rotating rod (6 cm in length and 3 cm in diameter at 25 rpm) for 5 min. Mice that fell were quickly returned to the rotarod and the total number of falls was recorded.
2.2.3. Balance beam
To test for balance, we used a steel beam (40 cm in length × 1 cm in diameter) suspended 20 cm above a padded surface. Mice were placed on the beam and the latency to fall was recorded. A total of three trials were performed per mouse.
2.3. Behavioral data analysis
Normality and homoscedasticity of data distribution were verified by using Kolmogorov-Smirnov and Bartlett’s tests. Parametric and non-parametric analyses were performed with one-way ANOVAs and Mann-Whitney tests, respectively. Significance threshold was set at 0.05.
2.4. Neuroimaging
Five adult WT and 5 adult MAO-ANeo mice were transcardially perfused with 4% paraformaldehyde solution and T2-weighted magnetic resonance images (MRI) of the heads were acquired with a 21.1T/900 MHz/105 mm magnet, at 60 um isotropic resolution, echo time (TE)=10.5 msec, repetition time (TR)=150 msec.
Brain MR images were aligned, with rigid transformations, to an average mouse brain MR scan (Ma et al., 2008), with the use of the freely available image registration software RView (http://www.colin-studholme.net/), and then analyzed using commercially available image analysis software (Amira, 4.1.1). Regions of Interest (ROI) included cerebral cortex, olfactory bulb, striatum, nucleus accumbens-olfactory tubercle, thalamus, hypothalamus, hippocampus, amygdala, midbrain, pons, medulla oblongata, pontine nuclei, inferior olive, cerebellum, and total brain. ROIs were manually highlighted from cross-sections at the coronal, horizontal, and sagittal planes and volumetric measurements were made. ROI volumes were compared between WT and MAO-ANeo mice with independent t-tests, followed by the Benjamini-Hochberg correction for multiple comparisons (Thissen et al., 2002). Results from histo-anatomical analysis of the cerebellum prompted us to further analyze the cerebellar vermis. The thickness of the vermis was defined at 1400 μm along the midline of the cerebellum, and did not include any paravermal tissue in the rostral and caudal areas of the structure. Lobules I–III, IV–V, VI–VIII, and IX–X were segmented and their volumes were assessed using analysis of covariance (ANCOVA) with total brain volume as a covariate, followed by Bonferroni tests when appropriate.
2.5. Histological and immunohistochemical analysis
Adult mice (7 WT and 6 MAO-ANeo) were perfused with saline followed by 3.5% buffered paraformaldehyde in 0.1 M phosphate buffer solution (PBS). The brains were postfixed in the same fixing solution for 24 hours, followed by incubation overnight in 30% sucrose in 0.1 M PBS at 4°C. Each brain was assigned a code number, and raters were unaware of the genotype in order to limit the risk of introducing bias by rater expectation. Later, 40 μm sagittal sections were made with a freezing microtome, and serial sections throughout the vermis of the cerebellum (200 μm increments, which produced an average of 8–9 sections per case) were selected and either Nissl stained for anatomical and cytoarchitecture measurements or processed for immunohistochemistry.
For immunohistochemical analysis, sections were incubated in 1% bovine serum albumin with 0.3% Triton-X100 in PBS containing Calbindin-D-28K (CB) antibody (1:1,000; Sigma-Aldrich Corp. St. Louis, MO, USA) overnight at room temperature. The next day, sections were rinsed three times in PBS for 10 minutes and then incubated in biotinylated anti-mouse IgG (1:100; ABC kit; Vector) for 1 hour at room temperature. Sections were rinsed again and then linked with Cy3-conjugated streptavidin (1:200 dilution, Jackson ImmunoResearch Laboratories) for 1 h at room temperature. Sections were then mounted on gelatin coated slides, allowed to air dry, and later coverslipped using the mountant DPX (a mixture of distyrene, a plasticizer, and xylene). Tissue from MAO-ANeo and WT mice were always processed together in pairs during immunostaining procedures in order to minimize variability of immunoreactivity among the experimental subjects.
2.6. Cerebellar morphology and cytoarchitecture
Cross-sectional area of the cerebellar vermis was estimated from Nissl stained sections using unbiased design-based stereology by performing the Cavalieri’s principle (Cavalieri and Lombardo-Radice, 1966) as described previously (Gundersen and Jensen, 1987; Gundersen et al., 1999; West et al., 1991). Briefly, an array of rectangular lattice points were superimposed in random positions and those points that lie over the structure of interest were indicated with a mouse-click. The area of the structure was later estimated from the total number of points counted. Point size and distances were optimized to achieve a coefficient of error of less than 5%. Standard stereological equipment was used, which include a Nikon Eclipse E600 light microscope, Rolera™ Thunder camera (QImaging, Surrey, BC Canada), Mac 5000 motor stage control unit (Ludl electronic Products, Ltd., Hawthrone, NY, USA) and Stereo Investigator 10 software (MicroBrightField, Williston, VT, USA).
The relative area of the cerebellar molecular layer, granular layer and white matter in reference to total cerebellum area was estimated using the Area Fraction Fractionator probe (AFF), which is essentially a Cavalieri point counting probe used to estimate the fraction of a region occupied by subregions. AFF sampling method is a modification of the Optical Fractionator probe, hence, the results obtained are independent of any kind of tissue changes or deformation (Gundersen and Jensen, 1987; Gundersen et al., 1999; West et al., 1991).
2.7. Purkinje cell count
The PCs count was performed using Nikon E800 epifluorescent microscope equipped with Microfire camera (Optronics, Goleta, CA, USA), Mac 5000 motor stage control unit (Ludl electronic Products, Ltd., Hawthrone, NY, USA) and Stereo Investigator 8 software (MicroBrightField, Williston, VT, USA). Since PCs are arranged in a single layer, PCs density in the cerebellum is often calculated in relation to layer length rather than area (Herrup and Wilczynski, 1982; Todorov et al., 2012; Whitney et al., 2008b). A stereology based adaptation from this approach was used (Gundersen, 1986; West et al., 1991). Briefly, random points were overlaid on CB-stained sagittal sections using the optical fractionator option of the stereoinvestigator, and then the number of PC through 500 μm span of PCs layer was counted. The optical dissector method was used to avoid overestimation and lost caps (Mayhew and Gundersen, 1996; Sterio, 1984).
2.8. Purkinje cell size and dendritic density
For further analysis of CB immunostained PCs, both soma size and dendritic density were analyzed. Cell size was measured in parallel with PCs number count, i.e. every fifth cell counted was used to measure the soma size using the Nucleator Probe (Gundersen, 1988). For PCs dendritic density, digital photomicrographs in the molecular layer of cerebellar sections were acquired by MetaMorph image software (Molecular Devices) using a Nikon E800 microscope. A 300 μm wide region that spans the entire width of the molecular layer was chosen and the images were flattened/skeletonized to minimize the background distortion. An inclusive threshold analysis was applied to delineate the dendrites and the percentage of the threshold area was recorded as the measurement for dendritic density.
2.9. Cerebellar morphology and immunohistochemistry statistical analysis
Data were then analyzed with t-tests (SPSS software), and P values < 0.05 were considered significant.
3. Results
3.1. Gait and coordination analyses
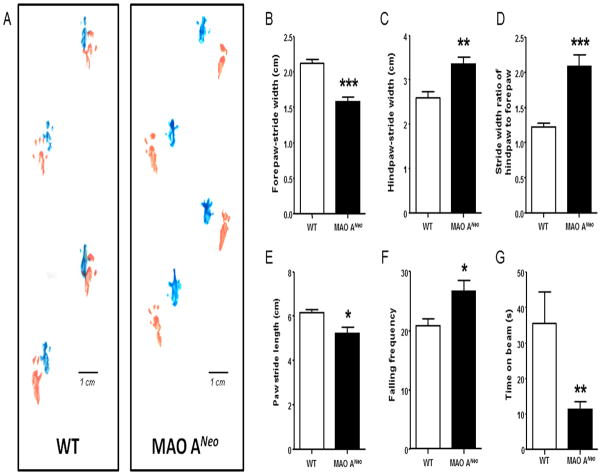
MAO-ANeo mice showed abnormal gait patterns in the footprint assay (Fig. 1A). MAO-ANeo mice exhibited a significant decrease in the forepaw stride-width (Fig. 1B) [F(1,13) = 29.59; p <0.001], but an increase in the hindpaw stride-width (Fig. 1C) [F(1,13) = 13.64; p <0.01] compared to their WT counterparts. Similarly, in comparison to WT mice, the markedly higher ratio between the stride-width of the hindpaws and the forepaws in MAO-ANeo mice (Fig. 1D) [U(7,8) = 0.00; p <0.001] suggests an abnormal gait pattern. MAO-ANeo mice also showed a significant reduction in total paw stride length (Fig. 1E) [U(16,16) = 71.0; p <0.05]. To examine whether this alteration in gait resulted in motor coordination deficits, we tested MAO-ANeo mice on the rotarod and the balance beam. MAO-ANeo mice displayed a significantly higher frequency of falling in the rotarod test (Fig. 1F) [F(1,22) = 5.79; p <0.05] and a significant reduction of the time spent on the balance beam (Fig. 1G) [U(8,10) = 11; p <0.01] compared to WT mice.
Fig. 1.
MAO-ANeo mice exhibit abnormalities in gait and deficits in balance and motor coordination. (A) Typical example of footprint patterns for each genotype. Forepaw prints are represented in blue; hindpaw prints are represented in orange. (B–D) MAO-ANeo mice display a significant lower stride-width between forepaws, but a significantly higher stride-width between hindpaws, resulting in a significantly greater stride-width ratio between hindpaws and the forepaws. (E) Similarly, MAO-ANeo mice have a shorter overall stride length. (F–G) In line with disturbances in gait, MAO-ANeo mice exhibited an increase in the total number of falls in the rotarod assay and a reduction in the time spent on the balance beam in comparison to their WT counterparts. Data are shown as mean ± SEM. ***P<0.001, **P<0.01, and *P<0.05 compared to WT mice.
3.2. Neuroimaging of the cerebellum
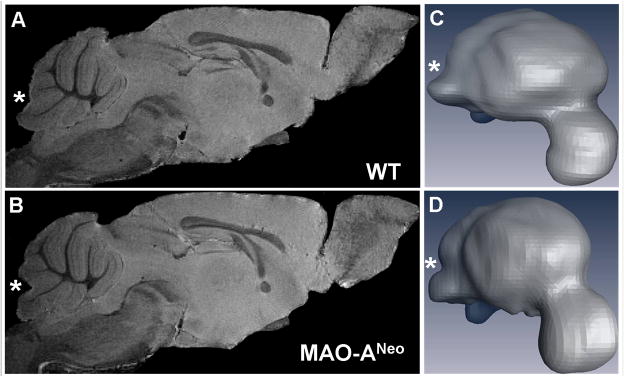
Our volumetric analysis from MRI data revealed a slight decrease in total cerebellar volume in MAO-ANeo mice [61.49 mm3 ± 1.75 SEM] compared to WT [64.49 mm3 ± 1.43 SEM], which did not reach statistical significance [t(8) = 1.326 p = 0.221], and a slight decrease in the relative volumes of the cerebellum as a proportion of total brain volume, that did not reach significance [WT = 14.36 % ± 0.3 SEM, MAO-ANeo = 13.72 % ± 0.11 SEM] [t(8) = 2.008, p = 0.079]. Interestingly, MRI revealed gross morphological differences of the cerebellum between WT and MAO-ANeo mice (Fig. 2). In particular, the caudal vermis of the cerebellum was rather smooth in the WT but a groove/furrow was noted in the vermis of MAO-ANeo mice. This indentation between lobule 8 and 9 was very prominent in 4 out of 5 MAO-ANeo mice but was not present in any of the 5 WT mice. Volumetric analysis of the vermal lobules showed a significant 14.5% decrease in the volume of lobules VI-VIII in MAO-ANeo mice, compared to WT [F (1, 7) = 7.68, p = 0.02] (Fig. 3). No other morphological or volumetric differences were observed between WT and MAO-ANeo mice.
Fig. 2.
Left: Magnetic resonance images of WT (A) and MAO-ANeo mice (B) brain. Note the indentation in the caudal vermis of the cerebellum, between lobules 8 and 9. Right: Three-dimensional re-construction of the entire brain including the cerebellum of a WT (C) and MAO-ANeo mice (D). Note the deeper groove (marked with *) in the caudal vermis of the MAO-ANeo mice, compared to WT.
Fig. 3. Lobular changes in the cerebellar vermis of MAO-ANeo mice compared to WT.
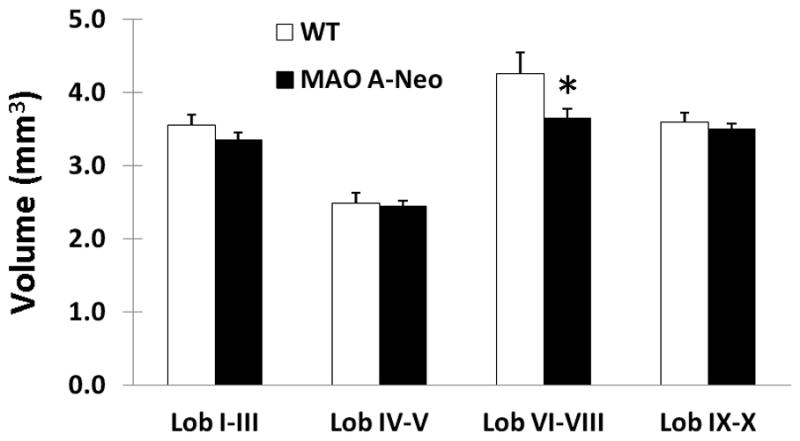
The bars represent the mean (+SEM) volume of the vermal cerebellar lobules. In lobules VI–VIII, MAO ANeo mice show a significant, 14.5%, volumetric decrease, compared to WT. * (p=0.02).
3.3. Morphological assessment of the cerebellum
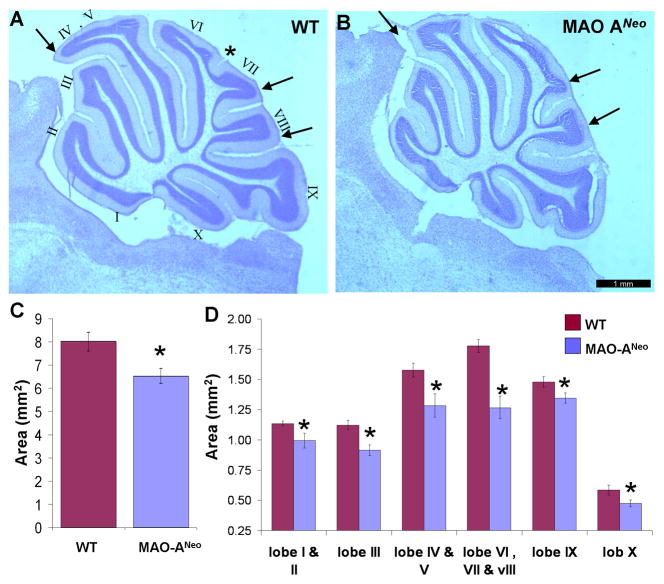
To further substantiate morphological changes at the histological level, Nissl stained sections revealed abnormal foliation in the cerebellum of MAO-ANeo mice compared to WT. Fig. 4 demonstrates these changes with sagittal sections through the vermis of the WT (A) and MAO-ANeo (B) mice. In general, a hypoplasia of lobules IV-VIII along with a failure in the development of the fissure that separates lobule VI from VII was observed in all five MAO-ANeo mice examined, but not in any WT mice (Fig. 4A, B).
Fig. 4. Decrease in cerebellar sagittal sectional area and change of individual lobules in MAO-ANeo mice.
Photomicrographs of Nissl stained sagittal sections of the cerebellum of WT (A) and MAO-ANeo mice (B) mice. The lobules are marked by Romanic numbers (I through X) in (A). Arrows indicate lobule IV, VII and VIII in WT (A) which are reduced in MAO-ANeo (B) mice. The fissure between lobule VI and VII is indicated by * in WT and its totally absent in MAO-ANeo mice. (C) Bar graph represents the mean area of the sagittal section of cerebellum of wild type and MAO-ANeo mice showing a 12% decrease. The differences in the mean area of major foliations of the cerebellum, though all lobules show a decrease as shown in (D), but superior and posterior lobules (IV to VIII) show the greatest decrease in size. Bars show the standard error. * P < 0.05.
The area analysis revealed a significant reduction (~18.5%) in the sagittal cross-sectional area of cerebellum in MAO ANeo mice [6.53 mm2 ± 0.803 SD] compared to WT [8.01 mm2 ± 1.08 SD] [t(11) = 2.77, p = .018] (Fig. 4C). Analysis of individual lobules revealed the greatest reduction in lobules IV-VIII (Fig. 4D).
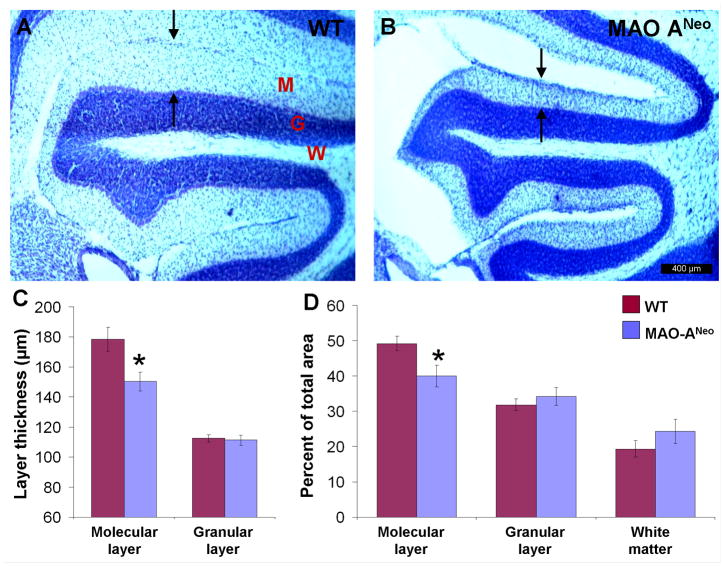
We further examined layer-specific cerebellar alterations (Fig. 5A, B). Our data showed a significant reduction in the thickness of the molecular layer, but not the granular layer in all cerebellar lobules of MAO-ANeo mice [molecular layer, WT = 178.6 μm ± 21.14 SD, MAO-ANeo = 150.41 μm ± 15.25 SD, t(11) = 2.71, p =0.02; granular layer, WT= 112.52 μm ± 6.24 SD MAO-ANeo = 111.19 ± 8.46 SD, t(11) = 0.325, p =0.75] (Fig. 5C). Because there was a reduction of area within the entire vermis of the cerebellum, we also compared the percentage of each layer with the total cerebellum area. The molecular layer remained significantly reduced in MAO-ANeo mice [40.13% ± 4.969 SD] compared to WT [49.46% ± 3.069 SD] [t(11) =17.1, p = .002], but not the granular layer or the white matter [granular layer, WT= 31.36% ± 1.0 SD MAO-ANeo = 32.45% ± 1.95 SD, t(11)= −1.29, p =0.22; white matter, WT= 19.63% ± 1.08 SD MAO-ANeo = 21.51% ± 1.47 SD, t(11)= −2.64, p =0.12] (Fig. 5D).
Fig. 5. Laminar changes in the cerebellum of MAO-ANeo mice compare to WT.
Photomicrographs of Nissl stained lobule II of the cerebellum in WT mice (A), and MAO-ANeo (B). The arrows highlight the thickness of the molecular layer in (A) & (B). (C) Note the reduction in thickness of the molecular layer but not granular layer in MAO-ANeo mice compare to WT. Further, to overcome the reduction in the cerebellar area of MAO-ANeo, the mean percentage area the molecular layer, granular layer and white matter in relation to the entire cerebellum area was also measured (D), reduction only in the percentage area of the molecular layer was noted in MAO-ANeo mice compare to WT (D). Bars show the standard error. Different cerebellar layers are marked by red letters (M= molecular layer, G= granular layer, and W= white matte).* P < 0.05.
3.4. Purkinje cell count and dendritic density measurements
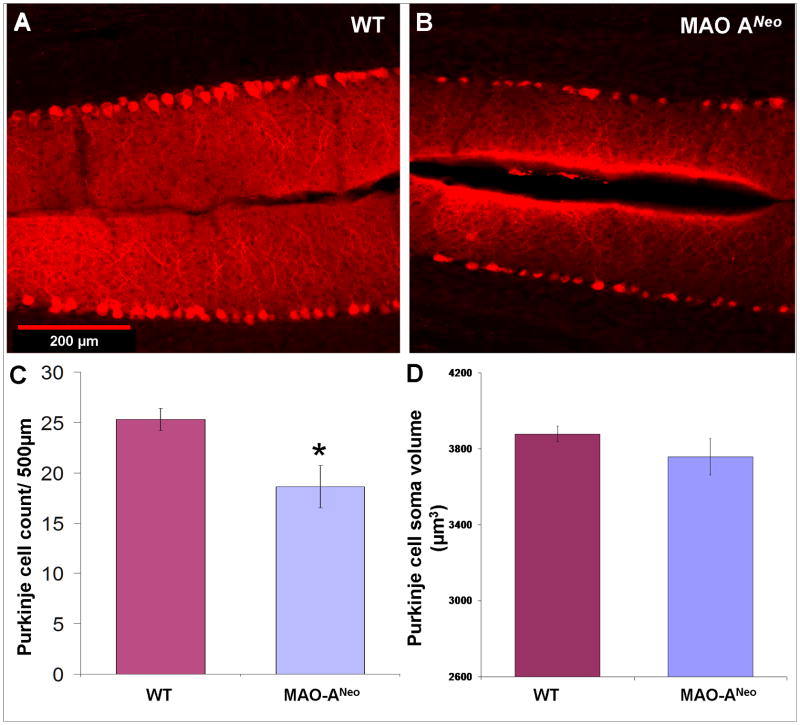
Using CB immunostained sections as a PCs cell marker, we examined PCs and their dendritic profile. The results of our analysis showed a 26% reduction in PCs density throughout the cerebellum of MAO-ANeo mice [18.6 cells/500 μm ± 2.09 SD] compared to WT [25.26 cells/500 μm ± 1.298 SD] [(t(11) = 7.1, p < 0.001] (Fig. 6). CB negative gaps of PCs were apparent in all cerbellar lobules of MAO-ANeo mice and at variable width (FIig. 6B), giving a distinct “patchy” appearance of PCs loss. Nevertheless, our data did not show any difference in PCs density between various cerebellar lobules in MAO-ANeo or WT mice (data not shown). Additionally, individual PCs soma size was not different between the two groups [WT = 3864.6 μm3/cell ± 22.7.919 SD, MAO ANeo = 3758.47 μm3/cell ± 226.458 SD]; [t(11) = 839, p =0.42] (Fig. 6D).
Fig. 6. Loss of Purkinje cells (PC) in the cerebellum of MAO-ANeo mice.
Photomicrographs of CB immunostained PCs in the cerebellum of WT (A) and MAO-ANeo (B) mice. (C) Bar graph represents the mean PC count in the cerebellum and showing a 26% decrease in MAO-ANeo mice compare to WT. (D) Bar graph represent no difference in the mean PCs soma volume in WT and MAO-ANeo. Bars show the standard error. * P < 0.05
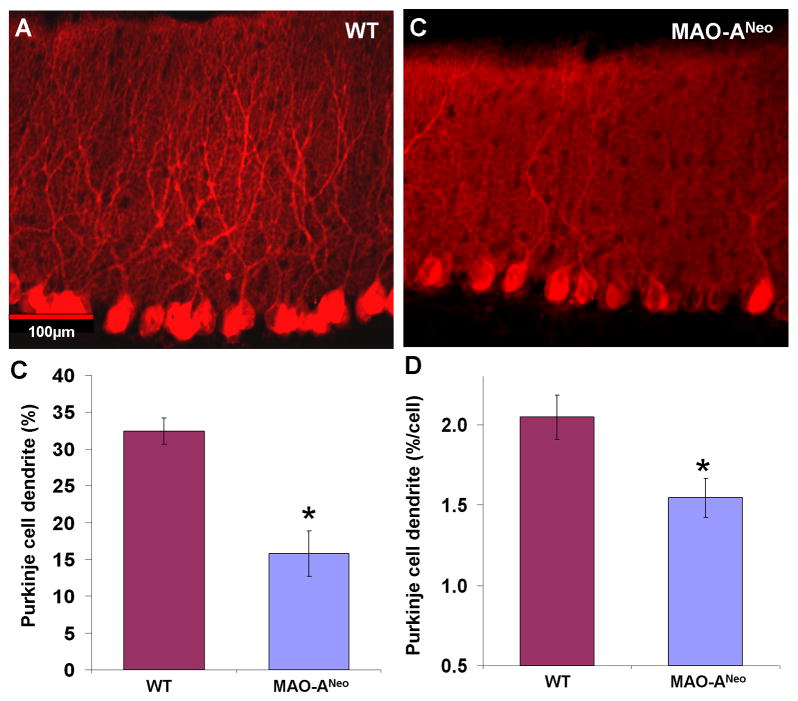
To further evaluate the integrity of PCs, we also quantified CB immunostained dendritic density of PCs in the molecular layer (Fig. 7A,B). A substantial reduction (~51.1%) of PC dendritic processes was noted in MAO-ANeo [15.826% ± 4.464% SD] mice compared to WT [32.38% ± 1.63% SD] [t(11) = 9.178, p <0.001] (Fig. 7C). Since this reduction could have been due to either a decrease in PCs density or a decrease in dendritic arborization per cell, we further analyzed the percentage threshold area per PC. Again, our data revealed a 24.5% reduction in the dendritic density per cell in MAO-ANeo mice [1.546% ± 0.301% SD] compared to WT [2.047% ± 0.365% SD] [t(11) = 2.67, p = 0.022] (Fig. 7D).
Fig. 7. Decrease of calbindin (CB) immunostained dendritic profile of Purkinje cells (PC) in MAO-ANeo mice compare to wild type (WT).
Photomicrographs of CB immunostained PCs and their dendrites in the cerebellum of WT (A), and MAO-ANeo mice (B). (C) Bar graph represents the mean PC dendrite density within the molecular layer of WT and MAO-ANeo mice showing a 51% decrease in MAO-ANeo mice compare to WT. To analyze the dendritic density independently from PC density changes, the dendritic density per PC count was measured (D), reduction in the overall dendritic profiles per cell was noted in PCs of MAO- ANeo mice compare to WT (D). Bars show the standard error. * P < 0.05.
4. Discussion
Our data revealed that MAO-ANeo mice exhibited gross morphological abnormalities in the cerebellum with reduced cerebellar foliation and generalized volume reduction. Using stereological techniques, a thinning of the molecular cell layer was found in MAO-ANeo mice compared to WT, as well as a loss of PCs and their dendritic density. In parallel to these findings, we showed that MAO-ANeo mice exhibited disturbances in gait and motor coordination.
4.1. Monoamines and cerebellar development
Although 5-HT innervation is less prominent in the cerebellum than in the neocortex, 5-HT is critical for cerebellar development and its proper function in the mature state. During early development, the cerebellum exhibits a transient expression of 5-HT1 and 5-HT3 receptors, which play an important role in the formation of the cerebellar neuronal network (Miquel et al., 1994; Oostland et al., 2011). This may offer the possible mechanism behind a compromised serotonergic system during development and subsequent cerebellar abnormalities in adulthood. Moreover, the cerebellum exhibits postnatal neurogenesis (Bonfanti and Ponti, 2008), up to postnatal day 21 in mice (Fujita, 1967), which makes it more vulnerable to postnatal disruption. It is worth noting that, while 5-HT increases cell proliferation and differentiation of cerebellar neural progenitor cells at low concentrations, administration of high concentrations of 5-HT or fluoxetine, (a serotonin reuptake inhibitor; SSRI) significantly decreased cerebellar cell proliferation (Zusso et al., 2008). Thus, it is not surprising to notice a decrease in cerebellar size, abnormal foliation, as well as molecular cell layer thinning in MAO-ANeo mice who have abnormally high levels of brain 5-HT throughout early development. Furthermore, the patchy appearance of the PCs noticed in MAO-ANeo mice suggested a late onset of PC degeneration. The existence of CB negative gaps in the cerebellum of MAO-ANeo mice suggests that PCs were generated migrated to their proper location in the PC layer and some of them subsequently degenerated. Pervious studies have reported that establishing functional synaptic contacts is crucial for the survival of cerebellar cells (Sotelo and Triller, 1979), hence, dysregulation of 5-HT levels and its effect on proper synaptic formation could account for PCs loss noticed in MAO-ANeo mice. Needless to say, further studies are required to examine the precise timing and potential mechanisms for PC density changes noted in Mao-ANeo mice.
Moreover, our data revealed a greater decrease in size and abnormal foliation in IV to VIII lobules. While cerebellar lobules have different times in the peak of neurogenesis (Altman and Bayer, 1997), which make variable hypoplasia developmentally plausible, increased 5-HT levels can provide an additional explanation, as serotonergic fiber density is not homogeneous across cerebellum lobules. For example, serotonergic fibers have a greater density in vermal lobules VII to X in rats (Bishop and Ho, 1985), while the greatest density in opossum was noted in VIII to IX and lowest in lobules II to V (Bishop et al., 1985). Even during development, the postnatal transient expression of 5-HT3 receptors is relatively high in lobules I to VI and lesser in lobules VII to X (Oostland et al., 2011). These lines of information suggest that individual cerebellar lobules may have a varying sensitivity to 5-HT homeostasis, and could be the mechanism behind the lobule-specific effects we observed in MAO-ANeo mice.
In addition to elevated 5-HT levels, NE levels were significantly higher in all brain regions tested in MAO-ANeo mice (Bortolato et al., 2011). While the role of NE in the cerebellum is less documented, studies have shown that NE can modulate neuronal circuitry within the cerebellum through a direct inhibitory effect on PCs (Bickford-Wimer et al., 1991; Woodward et al., 1991). Over activation of the NE system in early life by the β2 agonist terbutaline leads to PC degeneration and a thinning of the molecular and granular Layer (Rhodes et al., 2004). These converging lines of evidences suggest that the observed changes in the cerebellum of MAO- ANeo mice could be attributed to the increased levels of either 5-HT and/or NE. Further studies are need to be conducted to better understand the suspected integrative role of various monoamines in cerebellar development.
4.2. Functional considerations
Our findings suggest that MAO-ANeo mice displayed vermal hypoplasia and a loss of PCs, which were accompanied by alterations in behavioral paradigms known to test cerebellar function. Specifically, our mice exhibited abnormalities in gait as well as deficits in motor coordination and balance tasks. Although previous studies on MAO-A deficient mice reported altered beam-walking and other motor problems, the intrinsic visual impairments of the C3H background strain severely limited any conclusive result (Cases et al., 1995; Cazalets et al., 2000). Disturbances of the cerebellar serotonergic system have been linked to many types of ataxia (Trouillas and Fuxe, 1993). In addition, cocaine-induced ataxia and PCs degeneration have been suggested as a consequence of the early-life modification to the cerebellar serotonergic system (Arpin-Bott et al., 2006; Summavielle et al., 2004). There is also growing evidence that early selective 5-HT reuptake inhibition may induce motor deficits later in life in both humans (Albertini et al., 2004; Casper et al., 2003) and rodents (Bairy et al., 2007; Lisboa et al., 2007; Maciag et al., 2006). Moreover, clinical studies demonstrate improved cerebellar ataxia in response to treatment with 5-HT precursor (Trouillas et al., 1988; Trouillas et al., 1995) as well as 5-HT1a agonist (Lou et al., 1995; Trouillas et al., 1997). Taken together, these data indicate the importance of 5-HT homeostasis and its relation to the pathophysiology of cerebellum related disorders.
Previous studies reported that cerebellar lesions lead to inaccurate, irregular, and variable movements (Bradshaw and Mattingley, 1995). Decreased forepaw stride, but increased hindpaw stride as well as decreased motor coordination noted in MAO-ANeo mice suggest a cerebellar origin of motor deficits. Yet, the role of the fronto-striatal basal ganglia region still needs to be considered. In MAO-ANeo mince, 5-HT and NE levels were also increased in many brain regions that involved in motor circuitry. We have previously documented high levels of serotonin and NE in motor and prefrontal cortex as well as the striatum (Bortolato et al., 2011). Since these structures are engaged in the motor control loop and heavily connected to cerebellum, therefore, the motor deficits seen in our animals may be due to disturbances in the inter-connection between these areas and the cerebellum. Further studies to test the involvment of these structures in the motor deficits seen in MAO ANeo mice are still needed.
In addition to motor deficits, increasing evidence suggestes that cerebellar abnormalities are also implicated in cognitive deficits (Akshoomoff and Courchesne, 1992; Gordon, 2007; Steinlin, 2008) and other disorders, such as schizophrenia and ASD (Bauman and Kemper, 1985; Gaffney et al., 1987; Heath et al., 1979; Heath et al., 1982; Weinberger et al., 1979). Postmortem studies have shown a reduction in the number of granule cells and PCs as well as reduction in vermis size of the cerebellum in ASD subjects (Allen, 2005; Amaral et al., 2008). Interestingly, the vermis has anatomical and physiological interactions with limbic related structures throughout the brain (Anand et al., 1959; Buckner et al., 2011; Haines et al., 1997; Heath and Harper, 1974; Snider and Maiti, 1976). Clinical and experimental studies have provided empirical support for the role of the cerebellar vermis in the modulation of emotion, attention, cognition and social interaction (Berman, 1997; Bobee et al., 2000; Limperopoulos et al., 2007; Steinlin, 2008). Likewise, vermal excision has been shown to result in affective deficits and ASD-related alterations (Levisohn et al., 2000; Riva and Giorgi, 2000). Importantly, our recently published data highlighted that MAO-ANeo mice showed a significant reduction in social interactions and preservative responses, as well as morphological changes in the orbitofrontal cortex (Bortolato et al., 2011). In this report, our data report a vermal hypoplasia and loss of PCs. In light of these data and the new findings, we suggest that these cerebellar changes have a role in the abnormal behaviors seen in the MAO-ANeo mice and can be extended to similar patterns of behavioral and anatomical changes seen in ASD subjects.
These conclusions are in line with previous observations of many genetic models that demonstrated vermal hypoplasia as well as lesion studies that target the cerebellar vermis and report pervasive behavioral abnormalities that are similar to the abnormal behaviors detected in MAO ANeo mice (Bortolato et al., 2011). For example, knockout mice that lack the neural cell adhesion molecule L1Cam displayed a vermal hypoplasia, which is most prominent in lobule VI. These animals demonstrated a decreased motor activity and impaired open field exploration (Fransen et al., 1998). Bobee et al. (2000) have also reported that early life vermal lesions in rats lead to pervasive behaviors and decreased attention to environmental cues (Bobee et al., 2000). Moreover, many studies have reported that decreased cerebellar cortex inhibition in the form of reduction in cerebellar PCs number (Whitney et al., 2008a), size (Fatemi et al., 2002a), or even GAD 65 and 67 enzymes (Fatemi et al., 2002b; Yip et al., 2007) would likely lead to a disruption to the modulatory function of the cerebellum for all brain systems for which it projects, and may contribute to the manifestation of motor, cognitive and socio-emotional deficits (Allen, 2005; Belmonte et al., 2004; Blatt, 2005; Blatt and Fatemi, 2011; Fatemi et al., 2012).
4.3. Technical considerations
In this study, both neuroimaging and histo-anatomical analyses were used to assess the cerebellar integrity in MAO-ANeo mice. While MRI provides a practical alternative to histology in vivo and has the advantage of three-dimensional (3D) imaging for morphology assessments, but it has the disadvantage of low spatial resolution. As MRI analysis usually has a millimeter-scale resolution, compare to the micrometer-scale achieved by histo-anatomical analysis (Osechinskiy and Kruggel, 2009). The use of both techniques did provide a great advantage for this study. For example, MRI analysis revealed gross morphological differences in the cerebellum of MAO-ANeo mice, but fail to suggest a decrease in the cerebellum volume in MAO-ANeo mice, as it did not reach significance (with p = 0.079). Nevertheless, it gives us a rational to further analysis utilizing the hiso-anatomical analysis. In addition, the histo-anatomical data also motivate us to go back and further analyze the different lobules of the vermis, again the data from these tywo approaches leads to same results when MRI data did provide a reduction in the lobule VI & VIII which were the most reduced (Fig. 3), but fail to find significant change in other segments that noted in histo-anatomical analysis.
4. Conclusion
In summary, our present findings using MAO-ANeo mice support the involvement of MAO-A in the proper development of the cerebellum and subsequent cerebellar-linked behaviors. Although the specific role of cerebellar neuropathology in these deficits has yet to be determined, the observed decreased in PCs density and vermal hypoplasia may be implicated in the motor control disturbances as well as the impaired social interaction and pervasive behaviors noted in MAO-ANeo mice. Future studies will be needed to elucidate the potential validity of these mutant mice as animal models of ASD and neurodevelopmental disorders.
Highlights.
The cerebellar organization of hypomorphic MAO-A mutant mice was examined
Behavioral analyses revealed gait and postural disturbances
Cerebellar vermis hypoplasia and decreased Purkinje cell density was also noted
Cerebellar neuropathology implicated in motor and non-motor abnormal behaviors
Acknowledgments
The present study was supported by National Institute of Health grants R01MH39085 (to JCS), EUREKA R01MH084194 (to CSL), R21HD070611 (to MB), and RR017701 (to image core of CPN), the Boyd and Elsie Welin Professorship (to JCS), as well as the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program (AA 11034, AA07574, and AA07611 (to NDV).
Abbreviations
- 5-HT
serotonin
- ASD
Autism spectrum disorder
- DA
Dopamine
- CB
Calbindin
- MAO-A
Monoamine Oxidase A
- MAO-ANeo
MAO-A hypomorphic mice
- NE
Norepinephrine
- PC
Purkinje cell
- SSRI
selective serotonin reuptake inhibitor
- WT
wild type
Footnotes
Author contribution: L. Alzghoul, Junlin Zhang, and R.D. Darling: perform, analyzed the anatomical and immunhistochenistry data and prepared the manuscript.
K.L. Simpson and R.C.S. Lin: provided the experimental design and prepared the manuscript.
M. Bortolato, S.C. Godar, K. Chen, and J.C. Shih: generated the animals, conducted and analyzed the behavioral experiments as well as prepared the manuscript.
F. Delis, P.K. Thanos, S. Grant, G.J. Wang, and N.D. Volkow: acquired and analyzed the MRI data, and prepared the manuscript.
References
- Akshoomoff NA, Courchesne E. A new role for the cerebellum in cognitive operations. Behav Neurosci. 1992;106:731–8. doi: 10.1037//0735-7044.106.5.731. [DOI] [PubMed] [Google Scholar]
- Albertini G, et al. Oral dyskinesia induced by fluoxetine therapy for infantile autism. Pediatr Neurol. 2004;31:76. doi: 10.1016/j.pediatrneurol.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Allen G. The Cerebellum in Autism. CLINICAL NEUROPSYCHIATRY. 2005;2:321–337. [Google Scholar]
- Altman J, Bayer SA. Development of the cerebellar system: in relation to its evolution, structure, and functions. CRC Press; 1997. [Google Scholar]
- Alzghoul L, et al. Altered cerebellum organization in monoamine oxidase A deficient mice. Program No. 151.13/V11. 2011 Neuroscience Meeting Planner; 2011; Washington, DC: Society for Neuroscience; 2011. Online Vol., ed.^eds. [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–45. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Anand BK, et al. Cerebellar projections to limbic system. J Neurophysiol. 1959;22:451–7. doi: 10.1152/jn.1959.22.4.451. [DOI] [PubMed] [Google Scholar]
- Arpin-Bott MP, et al. Induction by cocaine of the serotonergic 5-HT3 receptor in rat cerebellum. Ann N Y Acad Sci. 2006;1074:382–9. doi: 10.1196/annals.1369.038. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56:413–24. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- Bailey A, et al. A clinicopathological study of autism. Brain. 1998;121(Pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Bairy KL, et al. Developmental and behavioral consequences of prenatal fluoxetine. Pharmacology. 2007;79:1–11. doi: 10.1159/000096645. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–74. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, et al. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–31. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman AJ. Amelioration of aggression: response to selective cerebellar lesions in the rhesus monkey. Int Rev Neurobiol. 1997;41:111–9. [PubMed] [Google Scholar]
- Bickford-Wimer P, et al. Electrically-evoked release of norepinephrine in the rat cerebellum: an in vivo electrochemical and electrophysiological study. Brain Res. 1991;558:305–11. doi: 10.1016/0006-8993(91)90782-q. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Ho RH. The distribution and origin of serotonin immunoreactivity in the rat cerebellum. Brain Res. 1985;331:195–207. doi: 10.1016/0006-8993(85)91545-8. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Ho RH, King JS. Localization of serotonin immunoreactivity in the opossum cerebellum. J Comp Neurol. 1985;235:301–21. doi: 10.1002/cne.902350303. [DOI] [PubMed] [Google Scholar]
- Blatt GJ. GABAergic cerebellar system in autism: a neuropathological and developmental perspective. Int Rev Neurobiol. 2005;71:167–78. doi: 10.1016/s0074-7742(05)71007-2. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Fatemi SH. Alterations in GABAergic biomarkers in the autism brain: research findings and clinical implications. Anat Rec (Hoboken) 2011;294:1646–52. doi: 10.1002/ar.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobee S, et al. Effects of early midline cerebellar lesion on cognitive and emotional functions in the rat. Behav Brain Res. 2000;112:107–17. doi: 10.1016/s0166-4328(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Ponti G. Adult mammalian neurogenesis and the New Zealand white rabbit. Vet J. 2008;175:310–31. doi: 10.1016/j.tvjl.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Chen K, Shih JC. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv Drug Deliv Rev. 2008;60:1527–33. doi: 10.1016/j.addr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, et al. Social deficits and perseverative behaviors, but not overt aggression, in MAO-A hypomorphic mice. Neuropsychopharmacology. 2011;36:2674–88. doi: 10.1038/npp.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw JL, Mattingley JB. Clinical neuropsychology: behavioral and brain science. Academic Press; 1995. [Google Scholar]
- Buckner RL, et al. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–45. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RJ, et al. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J Neurosci. 1999;19:3248–57. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–6. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, et al. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Casper RC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142:402–8. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- Cavalieri B, Lombardo-Radice L. Geometria degli indivisibili di Bonaventura Cavalieri. Unione Tipografico-Editrice Torinese; Torino: 1966. [Google Scholar]
- Cazalets JR, Gardette M, Hilaire G. Locomotor network maturation is transiently delayed in the MAOA-deficient mouse. J Neurophysiol. 2000;83:2468–70. doi: 10.1152/jn.2000.83.4.2468. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, et al. Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol Neurobiol. 2002a;22:171–5. doi: 10.1023/A:1019861721160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, et al. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002b;52:805–10. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, et al. Consensus Paper: Pathological Role of the Cerebellum in Autism. Cerebellum. 2012 doi: 10.1007/s12311-012-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillenz M. Noradrenergic Neurons. Cambridge University Press; 1990. [Google Scholar]
- Fransen E, et al. L1 knockout mice show dilated ventricles, vermis hypoplasia and impaired exploration patterns. Hum Mol Genet. 1998;7:999–1009. doi: 10.1093/hmg/7.6.999. [DOI] [PubMed] [Google Scholar]
- Fujita S. Quantitative analysis of cell proliferation and differentiation in the cortex of the postnatal mouse cerebellum. J Cell Biol. 1967;32:277–87. doi: 10.1083/jcb.32.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney GR, et al. Cerebellar structure in autism. Am J Dis Child. 1987;141:1330–2. doi: 10.1001/archpedi.1987.04460120096044. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–12. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gordon N. The cerebellum and cognition. Eur J Paediatr Neurol. 2007;11:232–4. doi: 10.1016/j.ejpn.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. The nucleator. J Microsc. 1988;151:3–21. doi: 10.1111/j.1365-2818.1988.tb04609.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, et al. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Haines DE, et al. The cerebellar-hypothalamic axis: basic circuits and clinical observations. Int Rev Neurobiol. 1997;41:83–107. doi: 10.1016/s0074-7742(08)60348-7. [DOI] [PubMed] [Google Scholar]
- Heath RG, Harper JW. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies in monkeys and cats. Exp Neurol. 1974;45:268–87. doi: 10.1016/0014-4886(74)90118-6. [DOI] [PubMed] [Google Scholar]
- Heath RG, Franklin DE, Shraberg D. Gross pathology of the cerebellum in patients diagnosed and treated as functional psychiatric disorders. J Nerv Ment Dis. 1979;167:585–92. doi: 10.1097/00005053-197910000-00001. [DOI] [PubMed] [Google Scholar]
- Heath RG, et al. Cerebellar vermal atrophy in psychiatric patients. Biol Psychiatry. 1982;17:569–83. [PubMed] [Google Scholar]
- Herrup K, Wilczynski SL. Cerebellar cell degeneration in the leaner mutant mouse. Neuroscience. 1982;7:2185–96. doi: 10.1016/0306-4522(82)90129-4. [DOI] [PubMed] [Google Scholar]
- Hoffer BJ, Siggins GR, Bloom FE. Studies on norepinephrine-containing afferents to Purkinje cells of rat cerebellum. II. Sensitivity of Purkinje cells to norepinephrine and related substances administered by microiontophoresis. Brain Res. 1971;25:523–34. doi: 10.1016/0006-8993(71)90458-6. [DOI] [PubMed] [Google Scholar]
- Hoffer BJ, et al. Activation of the pathway from locus coeruleus to rat cerebellar Purkinje neurons: pharmacological evidence of noradrenergic central inhibition. J Pharmacol Exp Ther. 1973;184:553–69. [PubMed] [Google Scholar]
- Kemper TL, Bauman ML. The contribution of neuropathologic studies to the understanding of autism. Neurol Clin. 1993;11:175–87. [PubMed] [Google Scholar]
- Kim JJ, et al. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proc Natl Acad Sci U S A. 1997;94:5929–33. doi: 10.1073/pnas.94.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123(Pt 5):1041–50. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–93. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- Lisboa SF, et al. Behavioral evaluation of male and female mice pups exposed to fluoxetine during pregnancy and lactation. Pharmacology. 2007;80:49–56. doi: 10.1159/000103097. [DOI] [PubMed] [Google Scholar]
- Lou JS, et al. Use of buspirone for treatment of cerebellar ataxia. An open-label study. Arch Neurol. 1995;52:982–8. doi: 10.1001/archneur.1995.00540340074015. [DOI] [PubMed] [Google Scholar]
- Ma Y, et al. In Vivo 3D Digital Atlas Database of the Adult C57BL/6J Mouse Brain by Magnetic Resonance Microscopy. Front Neuroanat. 2008;2:1. doi: 10.3389/neuro.05.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag D, et al. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew TM, Gundersen HJ. If you assume, you can make an ass out of u and me’: a decade of the disector for stereological counting of particles in 3D space. J Anat. 1996;188(Pt 1):1–15. [PMC free article] [PubMed] [Google Scholar]
- Miquel MC, et al. Postnatal development and localization of 5-HT1A receptor mRNA in rat forebrain and cerebellum. Brain Res Dev Brain Res. 1994;80:149–57. doi: 10.1016/0165-3806(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Mitoma H, et al. Enhancement by serotonin of GABA-mediated inhibitory synaptic currents in rat cerebellar Purkinje cells. Neurosci Lett. 1994;173:127–30. doi: 10.1016/0304-3940(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Murano M, Saitow F, Suzuki H. Modulatory effects of serotonin on glutamatergic synaptic transmission and long-term depression in the deep cerebellar nuclei. Neuroscience. 2011;172:118–28. doi: 10.1016/j.neuroscience.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Oostland M, Sellmeijer J, van Hooft JA. Transient expression of functional serotonin 5-HT3 receptors by glutamatergic granule cells in the early postnatal mouse cerebellum. J Physiol. 2011;589:4837–46. doi: 10.1113/jphysiol.2011.217307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osechinskiy S, Kruggel F. Quantitative comparison of high-resolution MRI and myelin-stained histology of the human cerebral cortex. Conf Proc IEEE Eng Med Biol Soc. 2009:85–9. doi: 10.1109/IEMBS.2009.5334695. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, et al. Neuropathological findings in autism. Brain. 2004;127:2572–83. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Rhodes MC, et al. Terbutaline is a developmental neurotoxicant: effects on neuroproteins and morphology in cerebellum, hippocampus, and somatosensory cortex. J Pharmacol Exp Ther. 2004;308:529–37. doi: 10.1124/jpet.103.060095. [DOI] [PubMed] [Google Scholar]
- Ritvo ER, et al. Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC Autopsy Research Report. Am J Psychiatry. 1986;143:862–6. doi: 10.1176/ajp.143.7.862. [DOI] [PubMed] [Google Scholar]
- Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123(Pt 5):1051–61. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–88. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Scott JA, et al. A comprehensive volumetric analysis of the cerebellum in children and adolescents with autism spectrum disorder. Autism Res. 2009;2:246–57. doi: 10.1002/aur.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KL, et al. Perinatal antidepressant exposure alters cortical network function in rodents. Proc Natl Acad Sci U S A. 2011;108:18465–70. doi: 10.1073/pnas.1109353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider RS, Maiti A. Cerebellar contributions to the Papez circuit. J Neurosci Res. 1976;2:133–46. doi: 10.1002/jnr.490020204. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Triller A. Fate of presynaptic afferents to Purkinje cells in the adult nervous mutant mouse: a model to study presynaptic stabilization. Brain Res. 1979;175:11–36. doi: 10.1016/0006-8993(79)90511-0. [DOI] [PubMed] [Google Scholar]
- Steinlin M. Cerebellar disorders in childhood: cognitive problems. Cerebellum. 2008;7:607–10. doi: 10.1007/s12311-008-0083-3. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–36. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Strahlendorf JC, Lee MH, Strahlendorf HK. Serotonin modulates muscimol- and baclofen-elicited inhibition of cerebellar Purkinje cells. Eur J Pharmacol. 1991;201:239–42. doi: 10.1016/0014-2999(91)90352-q. [DOI] [PubMed] [Google Scholar]
- Summavielle T, et al. Abnormal immunoreactivity to serotonin in cerebellar Purkinje cells after neonatal cocaine exposure. Ann N Y Acad Sci. 2004;1025:630–7. doi: 10.1196/annals.1316.078. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Kimura H, Sano Y. Immunohistochemical demonstration of serotonin-containing nerve fibers in the cerebellum. Cell Tissue Res. 1982;226:1–12. doi: 10.1007/BF00217077. [DOI] [PubMed] [Google Scholar]
- Thissen D, Steinberg L, Kuang D. Quick and Easy Implementation of the Benjamini-Hochberg Procedure for Controlling the False Positive Rate in Multiple Comparisons. Journal of Educational and Behavioral Statistics. 2002;27:77–83. [Google Scholar]
- Thompson BL, Stanwood GD. Pleiotropic effects of neurotransmission during development: modulators of modularity. J Autism Dev Disord. 2009;39:260–8. doi: 10.1007/s10803-008-0624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov B, et al. Purkinje Cell-Specific Ablation of Ca(V)2.1 Channels is Sufficient to Cause Cerebellar Ataxia in Mice. Cerebellum. 2012;11:246–58. doi: 10.1007/s12311-011-0302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillas P, Brudon F, Adeleine P. Improvement of cerebellar ataxia with levorotatory form of 5-hydroxytryptophan. A double-blind study with quantified data processing. Arch Neurol. 1988;45:1217–22. doi: 10.1001/archneur.1988.00520350055016. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Fuxe K. Serotonin, the cerebellum, and ataxia. Raven Press; 1993. [Google Scholar]
- Trouillas P, et al. Levorotatory form of 5-hydroxytryptophan in Friedreich’s ataxia. Results of a double-blind drug-placebo cooperative study. Arch Neurol. 1995;52:456–60. doi: 10.1001/archneur.1995.00540290042016. [DOI] [PubMed] [Google Scholar]
- Trouillas P, et al. Buspirone, a 5-hydroxytryptamine 1A agonist, is active in cerebellar ataxia. Results of a double-blind drug placebo study in patients with cerebellar cortical atrophy. Arch Neurol. 1997;54:749–52. doi: 10.1001/archneur.1997.00550180059013. [DOI] [PubMed] [Google Scholar]
- Upton AL, et al. Excess of serotonin (5-HT) alters the segregation of ispilateral and contralateral retinal projections in monoamine oxidase A knock-out mice: possible role of 5-HT uptake in retinal ganglion cells during development. J Neurosci. 1999;19:7007–24. doi: 10.1523/JNEUROSCI.19-16-07007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, et al. Cerebellar vermal volumes and behavioral correlates in children with autism spectrum disorder. Psychiatry Res. 2009;172:61–7. doi: 10.1016/j.pscychresns.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, et al. Structural abnormalities in the cerebral cortex of chronic schizophrenic patients. Arch Gen Psychiatry. 1979;36:935–9. doi: 10.1001/archpsyc.1979.01780090021002. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int J Dev Neurosci. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Whitney ER, et al. Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum. 2008a;7:406–16. doi: 10.1007/s12311-008-0043-y. [DOI] [PubMed] [Google Scholar]
- Whitney ER, et al. Calbindin-D28k is a more reliable marker of human Purkinje cells than standard Nissl stains: a stereological experiment. J Neurosci Methods. 2008b;168:42–7. doi: 10.1016/j.jneumeth.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Winter C, et al. Dopamine and serotonin levels following prenatal viral infection in mouse--implications for psychiatric disorders such as schizophrenia and autism. Eur Neuropsychopharmacol. 2008;18:712–6. doi: 10.1016/j.euroneuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward DJ, et al. The cerebellar norepinephrine system: inhibition, modulation, and gating. Prog Brain Res. 1991;88:331–41. doi: 10.1016/s0079-6123(08)63820-0. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol. 2007;113:559–68. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Zusso M, et al. Fluoxetine-induced proliferation and differentiation of neural progenitor cells isolated from rat postnatal cerebellum. Biochem Pharmacol. 2008;76:391–403. doi: 10.1016/j.bcp.2008.05.014. [DOI] [PubMed] [Google Scholar]