Summary
Aggregatibacteractinomycetemcomitansis a gram-negative facultative capnophile involved in pathogenesis of aggressive forms of periodontal disease. In the present study, we interrogated the ability of A. actinomycetemcomitans to stimulate innate immune signaling and cytokine production then established that A.actinomycetemcomitans causes bone loss in a novel rat calvarial model. In vitro studies indicated that A. actinomycetemcomitansstimulated considerable production of soluble cytokines, TNF-α, IL-6, and IL-10 in both primary bone marrow derived macrophages and NR8383 macrophages. Immunoblot analysis indicated that A. actinomycetemcomitansexhibits sustained activation of all major mitogen activated protein kinase (MAPK) pathways, as well as the negative regulator of MAPK signaling, MAPK phosphatase-1 (MKP-1),for at least 8 hours. In a rat calvarial model of inflammatory bone loss, high and low doses of formalin fixed A. actinomycetemcomitanswere microinjected into the supraperiostealcalvarial space for 1 to 2 weeks.Histological staining and micro-computed tomography (μCT) of rat calvariae revealed a significant increase of inflammatoryand fibroblast infiltrate and increased bone resorptionas measured by total lacunar pit formation.From these data, we provide new evidence thatfixed whole cell A. actinomycetemcomitansstimulation elicits a pro-inflammatory host response through sustained MAPK signaling leading to enhanced bone resorption within the rat calvarial bone.
Keywords: periodontal disease, host immune response, cytokines, bone loss
Introduction
Periodontal disease is a chronic inflammatory disease marked by inflammation in gingival tissue and alveolar bone resorption that commonly lead to tooth loss. In humans, a subgingival biofilm containing Aggregatibacter actinomycetemcomitans, a gram-negative capnophile,is associated with aggressive forms of periodontal disease in juveniles and adults (Kamma et al., 2004; Lopez et al., 1996; Slots and Ting, 1999; Takeuchi et al., 2003; Tinoco et al., 1997).In a susceptible host, A. actinomycetemcomitans endotoxin stimulates the release of pro-inflammatory mediators such as cytokines and prostaglandins that lead to destruction of periodontal tissues.
Bacterial constituents activate the innate immune response through toll-like receptors (TLRs) and promote subsequent release of cytokines. A. actinomycetemcomitansderived lipopolysaccharide (LPS), a gram-negative cell wall component, induces the mitogen-activated protein kinase (MAPK) pathway. MAPKs, a family of serine/threonine proteases, includep38, extracellular signal-related kinases (ERK1/2), and c-Jun N-terminal kinases (JNK). Specifically, MAPK-activated protein kinase 2 (MK2) and MAPK phosphatase-1 (MKP-1) regulate inflammatory bone loss in periodontal disease(Li et al., 2011; Yu et al., 2011a).Moreover,MK2 is targeted and phosphorylated by p38 MAPK. In vivo, silencing MK2 attenuates LPS-induced inflammatory bone loss in a rat periodontal disease model(Li et al., 2011). Contrary to MK2, MKP-1 regulates MAPKs by dephosphorylating proteins activated upon TLR signaling. MPK-1 is anti-inflammatory in A. actinomycetemcomitans LPS-induced bone loss in a periodontal disease rat model(Yu et al., 2011a).
Stress-induced activation of the MAPK pathway causes downstream regulation of host-derived inflammatory cytokinesand prostanoidssuch as tumor necrosis factor (TNF)-α, interleukin(IL)-6, IL-1β,and prostaglandin E2(PGE2) that directly or indirectlycause subsequent bone loss(Chen et al., 1998; Ishimi et al., 1990; Rogers et al., 2007; Tsai et al., 1995; Zou and Bar-Shavit, 2002). These pro-inflammatory mediators lead to activation of matrix metalloproteinases (MMPs) that destroy the matrix in tissue surrounding the tooth (Rogers et al., 2007).
Inflammation in periodontal disease markedly enhances osteoclastogenesis in the alveolar bone(Hall and Chambers, 1996). Alveolar bone turnover that is normally coupled, is indisequilibrium in the periodontal disease state leading to net bone loss(Rogers et al., 2007). Interestingly, IL-1 and TNF-α can induce osteoclastogenesis directly by nuclear factor-kappa B ligand (RANKL), a TNF family cytokine, or indirectly through IL-6(Rogers et al., 2007). Osteoblasts respond to IL-1β, PGE2 and TNF-α to upregulate RANKL and stimulate osteoclast differentiation(Roux and Orcel, 2000).
In order to experimentally model the osteoimmunological host response in periodontal disease, small animal models are commonly used.For example, A. actinomycetemcomitans inoculation byoral gavage, A. actinomycetemcomitans LPS palatal injections, oral A. actinomycetemcomitans inoculation,or liveA. actinomycetemcomitansin feeding, mimic a periodontal disease host inflammatory response in rats or mice(Fine et al., 2009; Garlet et al., 2006; Yu et al., 2011a). Although A. actinomycetemcomitans LPS elicits a host response when injected into the palatal maxillae of rats,A. actinomycetemcomitans contains other virulence factors associated with aggressive periodontitis, such as cytolethal distending toxin that can contribute to pathogenesis by inhibiting both cellular and humoral immunity via apoptosis of immune response cells(Rogers et al., 2007; Tan et al., 2002).Therefore, whole A. actinomycetemcomitasstimulation is necessary to obtain a more comprehensive appreciation for the bacterial-induced host response.
In this study, we addressthe MAPK host intracellular signaling pathway, through stimulation with formalin-fixed A. actinomycetemcomitansin rat macrophages andin vivo using a rat calvarial model.A. actinomycetemcomitansexpounds thecontribution of the host immune response to periopathogenicbacterial stimulation and osteoimmunological impact on bone destruction.
Methods
Bacterial Preparation
A. actinomycetemcomitans Strain Y4 was grown on 5% Sheep Blood Agar plates (Becton Dickinson, Franklin Lakes, NJ USA) for 36-48 hours at 37°C with 10% CO2 with large liquid culture prepared in NIH thioglycollate(Becton Dickinson, Franklin Lakes, NJ USA) under the same conditions. Harvested bacteria were centrifuged and fixed in 4% paraformaldehyde for 1 hour and then washed extensively with 5% glycerol in PBS. Final bacterial suspension in PBS was at a concentration of 4 × 109 cells/ml. A. actinomycetemcomitansLPS was extracted from strain Y4 by the hot phenol-water method as previously described (Rogers et al., 2007). The LPS used in this study contained <0.001% nucleic acid by spectrophotometry and approximately 0.7% protein by bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL USA) as described previously(Rogers et al., 2007).
Macrophage Culture
NR8383 cells (rat macrophage) were purchased from the American Type Culture Collection (ATTC, Manassas, VA USA). This rat macrophage cell line was established from a normal rat alveolar macrophage cells that were obtained by lung lavage. Cells were maintained in Ham’s F-12 medium with 15% FBS. Primary bone marrow-derived macrophages (BMMϕ) were harvested from adult male Sprague-Dawley rats (Harlan Laboratories, Inc., Indianapolis, IN USA). The femurs and tibia were flushed with αMEM (Invitrogen Life Technologies, Grand Island, NY USA). Total bone marrow cultures were incubated at in αMEM containing 10% fetal bovine serum, 2mM glutamine, 100μl/ml penicillin and 100 μg/ml of streptomycin overnight. Non-adherent cells post 24 hour culture were plated for macrophage differentiation using DMEM containing 10% fetal bovine serum, 2mM glutamine, 50 ng/ml murine macrophage-colony stimulating factor (R&D Systems, Minneapolis, MN USA), 100μl/ml penicillin and 100 μg/ml of streptomycin. BMMϕ were established after 7 days under these conditions as described previously(Yu et al., 2011b).
Immunoblot and ELISA Analysis
Activation of the select MAPK/MKP/NF-κB signaling pathways were evaluated using total protein extracted from samples harvested at indicated time pointsfollowing stimulation with A. actinomycetemcomitans LPS (100 ng/ml). Protein samples were obtained using a commercially available extraction buffer, supplemented with PMSF, protease inhibitors in SDS-PAGE buffer (BioRad, Hercules, CA USA). Total cellular lysate (25μg) was electrophoresed on 10% denatured SDS-PAGE gels and transferred to nitrocellulose membranes (BioRad). Antibodies against phosphorylated and non-phosphorylated forms of p38, JNK, ERK MAPK, MK2,NF-kB and NF-kB p65 (Cell Signaling Technologies, Danvers, MA USA), along with MKP-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) were used as primary antibodies in these studies. Primary antibodies were detected using Horseradish peroxidase (HRP)-conjugated secondary antibodies and exposure development against radiographic film using the chemiluminescence system (LumiGlo, Cell Signaling). Radiographic images were obtained on a Gel-Doc XR system and densitometric analysis was performed using Quantity One Software (Bio-Rad, Hercules, CA).Cytokine expression in cell culture supernatants was measured using the ELISA protocol DuoSet ELISA Development Systems to determine IL-6, TNF-α, and IL-10 (R&D Systems) according to manufacturer’s instructions.
Calvarial Model
The Institutional Animal Care and Use Committee (IACUC) at the Medical University of South Carolina approvedall animal protocols. Adult male Sprague-Dawley rats (Harlan Laboratories) weighing ~250–274 g were used for in vivo studies. Rats were maintained under specific pathogen-free conditions in pairs with food and tap water ad libitum. Once weekly, animals were routinely weighed to ensure proper growth and nutrition. Following anesthesia with 5% isoflurane, Sprague-Dawley rats were injected once with 100μl of formalin-fixed A. actinomycetemcomitans at 1×109 and 1×108cells/ml, A. actinomycetemcomitans LPS (250 μgin 25 μl), or PBS control in the mid-sagittal suture area near the supraperiosteal region of the rat calvarium via a 26-gauge needle through aHamilton syringe. PBS + vehicle controls consisted of an empty 50ml tube treated under the same formalin fixation conditions without bacteria.
Micro-computed tomography imaging
Harvested calvarial samples were fixed for 24 hours in 10% formalin and then changed to 70% ethanol. Samples were scanned by micro-computed tomography (μCT) (GE Healthcare, Chalfont St. Giles,Buckinghamshire, UK). Each scan was reconstructed at a mesh size of 18 μm3, and three-dimensional digitized images were generated for each sample using GEHealthcare MicroView software (version viz. + 2.0 build 0029). By using μCT analysis it is possible to view the scans from virtually any angle or cross-section in order to retrieve an accurate bone volumetric fraction (BVF) analysis. Therefore, a standardized orientation of the images was necessary prior to measurement. Region of interest (ROI) landmarks for resorption pit enumeration were set using the coronal sutures adjacent to the occipital bone; the coronal sutures adjacent to the nasofrontal area;and the lambdoid sutures creating a 4-point connected square from the superior view. All μCT scans were measured and assessed by an independent examiner in a blinded manner.
Histological Examination
Formalin-fixed, decalcified maxillae were paraffin embedded and serial sagittal sections (7 μm) prepared. Some slides were stained routinely with H&E for descriptive histology. Histological images were acquired using an Olympus BX61 Research (Center Valley, PA USA) microscope outfitted with a DP71 digital camera.The inflammatory cell infiltrateand fibroblast scores were quantified using a pathological scoring system ranging from 0 to 4. A score of 0 indicated no significant inflammatory infiltrate, 1 was mild (<500), 2 was moderate (500-1000), 3 was severe (1001-1500), and 4 was extremely severe (>1501). A fibroblast score of 0 indicated no proliferation with no fibrotic band, 1 was mild with a thin band, 2 was moderate with a moderately thick band, 3 was severe with a thick band, and 4 was extremely significant fibroblast number with a extremely thick band.
Osteoclast Measurement
To quantitate osteoclasts, tartrate-resistant acid phosphatase (TRAP) staining was performed in tissue sections using a leukocyte acid phosphatase kit (Sigma Aldrich). Mature osteoclasts were counted as TRAP-positive multinucleated (≥3 nuclei) in contact with the bone surface. Slides from serial sagittal sections were used to enumerate TRAP-positive cells. The tissue sections were counterstained with hematoxylin post TRAP staining.
Statistical Analysis
Pairwise comparisons between experimental groups were performed using the student t-test with Welch’s correction for unequal variances or identified by one-way analysis of variance (ANOVA). Post-hoc power analysis confirmed in vivo experimental trials. P-values less than 0.05 were considered significant. All calculations were performed using Prism 4 software (GraphPad, Inc.).
Results
Induced Cytokine Expression post A. actinomycetemcomitans stimulation
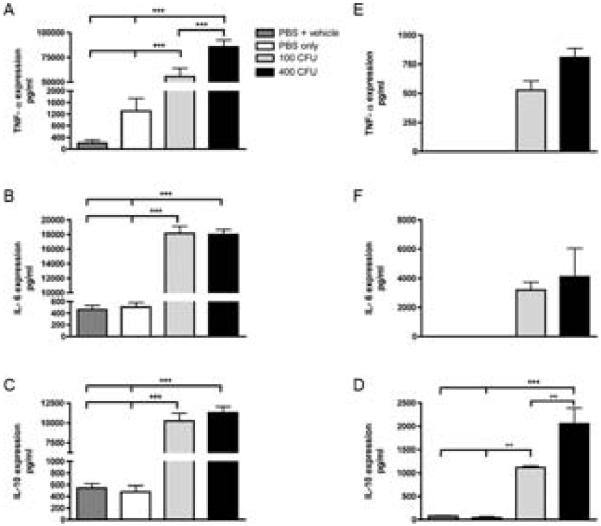
For these studies we chose to elucidate activated signaling pathways by stimulating (NR8383) rat macrophage cell lines (1 × 106 cells/well) with formalin-fixed, whole A. actinomycetemcomitans bacteria at a high and low-dosage. Cells were harvested after 24 hours following microbial stimulation. Expression of candidate targets were tested independently enzyme-linked immunobsorbent assay analysis (ELISA) using cell culture supernatant. Exposure to the periopathogenic stimulus resulted in significantly increased levels of TNF-α (p<0.0001), IL-6 (p<0.0001), and IL-10 (p<0.0001) in NR8383 cell lines, in comparison to controls (Figure 1A-C). To verify that these stimulatory effects were not limited to cell lines, primary rat BMMϕ were treated in the same manner. As shown in Figure 1D-F, TNF-α, IL-6, and IL-10 (p<0.0001) cytokine levels were all significantly elevated post A. actinomycetemcomitans exposure.
Figure 1. Pronounced expression of inflammatory cytokines was observed in A. actinomycetemcomitans stimulated rat macrophage cells and primary BMMϕ cells.
NR8383 cells were stimulated with A. actinomycetemcomitans for 24 hrs and cytokine expression measured via ELISA for TNF-α (A), IL-6 (B), and IL-10 (C).Following isolation and culturing for 7 days, BMMϕ cells were stimulated with A. actinomycetemcomitans for 24 hrs and ELISA analysis used to measure TNF-α (D), IL-6 (E), and IL-10 (F). Representative data of 5 separate experiments is presented. (**p<0.001, ***p<0.0001)
Activation of MAPK Signaling Pathway
Production of inflammatory cytokines, including TNF-α and IL-6 are closely related to the activation of the MAPK pathway and NF-κB(Rogers et al., 2007; Soloviev et al., 2002). NR8383rat macrophage and primary BMMϕcells were exposed to 400 CFU of formalin-fixed A. actinomycetemcomitans or controls and harvested at indicatedtime pointspost-bacterialexposure. Previously, our group hasshown that endotoxin stimulation activates protein kinases including, ERK, JNK and p38 MAPK in a manner consistent with formalin-fixed A. actinomycetemcomitans stimulation (Li et al., 2011; Yu et al., 2011a). Remarkably, immunoblotting analysis also revealed activation of targeted protein kinases, ERK, JNK, and p38 MAPK as indicated by an increase inphosphorylated expression starting at 30 minutes post-stimulation in tissue culturein both a rat macrophage cell line as well as primary cultured macrophages (Figure 2). In addition, these studies indicate that MKP-1, a negative regulator of MAPK signaling, and MK2, a downstream substrate ofp38 MAPK has sustained activation up to 8 hours of bacterial exposure (Figure 2, Figure S1). This unexpected prolonged response was in contrast to the previous findings where cells displayed a significant increase in protein levels between 60 and 300 minutes following LPS stimulation, peaking from 30 to 120 minutes with subsequent downregulation (Li et al., 2011; Yu et al., 2011a). Results using primary macrophages indicate results are not cell line specific(Figure 2B).
Figure 2. Sustained activation of MAPK signaling pathway in rat macrophages and BMMϕ cells following A. actinomycetemcomitans stimulation.
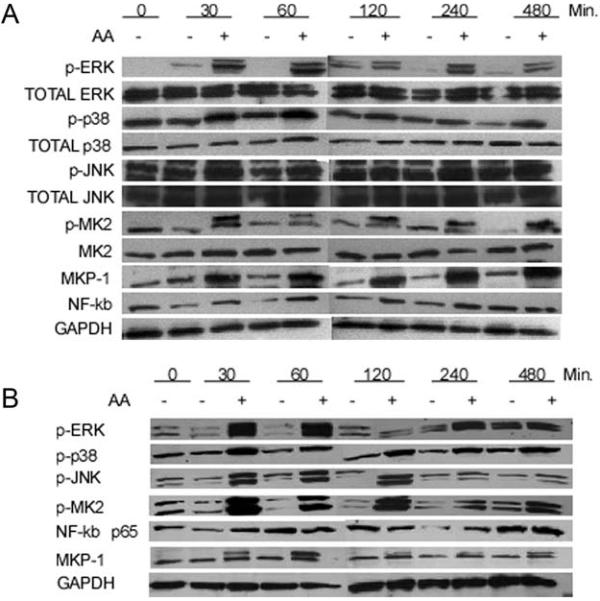
Sustained activation of intracellular signaling NFκB and MAPK/MKP pathways is observed post stimulation with A. actinomycetemcomitans in NR8383 rat macrophage cells (A) and BMMϕs (B). Western blot data representative of 3 separate experiments for panel A and 2 separate experiments in panel B.
Induction of Bone Resorption
Inflammatory cytokine release can result in bone resorption as a consequence of the elicited host immune response to oral pathogens. In most cases this signaling activity is for protection, but if uncontrolled this may ultimately lead to alveolar bone loss. In the present study, A. actinomycetemcomitanswith control samples was delivered to the mid-sagittal suture area near the supraperiosteal region of rat calvaria. Animals were sacrificed 1 or 2 weeks post injection andharvested calvarie and surrounding tissues were initially scanned by μCT to examine changes in calvarial bone resorption within the defined landmarks. Areas of resorption were identified as engraved pits (Figure 3A). We observed that bacterial stimulated rat calvarial tissues displayed greater lacunar formation in comparison to LPS and vehicle control samples (Figure 3B). Quantitatively, we found that areas of lacunar resorption dominated areas of the A. actinomycetemcomitans (high dosage) micro-delivery within the rat calvariae within specified landmarks of interest, in comparison to controls or LPS at the 2-week harvest (Figure 3B). The decrease in lacunae from 1 to 2 weeks with the low dosage of A. actinomycetemcomitans compared to the high dosage at week 2 suggests that inflammation resolution and bone coupling is occurring. After 2 weeks post-injection the 400 CFUdosageA. actinomycetemcomitans is still sufficient to sustain an osteoclastic predominated event to maintain resorption craterscompared with 100 CFU dosage A. actinomycetemcomitans.
Figure 3. Induction of bone resorption following A. actinomycetemcomitansmicroinjection.

Computer rendered micro-CT (μCT) images of representative calvaria sample post 2 weeks exposure to A. actinomycetemcomitans. Magnification indicates bone resorption lacunae(A). Enumeration of bone resorptionareasat 1 (upper panel) and 2 weeks (lower panel)post bacterial stimulation(B). Data is expressed as mean number of resorption lacunae (n=6 for controls and n=8 for A. actinomycetemcomitans injected samples; ***p<0.0001, **p<0.001, *p<0.05).
Inflammatory Cell Infiltration, Fibroblast Proliferation, and Osteoclastogenesis
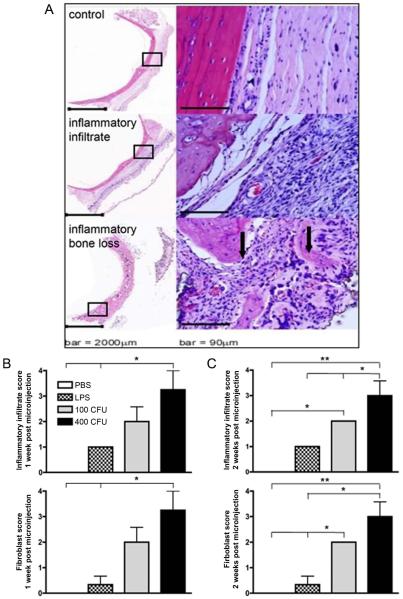
Rat calvarial tissues were fixed and embedded for histological staining by hematoxylin and eosin (H&E). An influx of inflammatory cell infiltrate following A. actinomycetemcomitans microinjection was observed in representative sections from the harvested rat calvarie (Figure 4A). Followingblinded pathologicalenumeration, cell types and tissue environment included robust inflammatory infiltratenumbers within the connective tissues, and numerous capillaries. In contrast, H&E staining indicated low levels of inflammatory cell infiltrate in control samples (Figure 4A). The inflammatory score was significantly increased in LPS (p<0.05) and 400 CFUdosage A. actinomycetemcomitans(p<0.05) treated rats 1-week post-injection when compared to the PBS (Figure 4B). Two weeks post-injection, the 100 CFU (p<0.05) and 400 CFU dosages of A. actinomycetemcomitans (p<0.001) produced a significant increase in inflammatory infiltrate compared to the control. The inflammatory infiltrate in the 400 CFU dosage group was also significantly different than LPS (p<0.05) and 100 CFU dosage groups (p<0.05) (Figure 4C). Upon microinjection, the fibroblast score increased in the 400 CFU dosage group compared to LPS during week 1 and 2 (p<0.05) and control groups at week 1 (p<0.05) and week 2 (p<0.001) post-stimulation (Figure 4B and C). By the second week, the 100 CFUamount of A. actinomycetemcomitansalso produced an increase in the fibroblast proliferation score compared to the PBS and LPS treated groups (p<0.05) (Figure 2C). Inflammatory and fibroblast scores were dependent on potency of stimulus. Therefore, increased host response and healing was necessary during increased dosages. We also show that LPS and A. actinomycetemcomitanshave a trend towards increased osteoclast formation (TRAP positive cells with ≥ 3 nuclei) (Figure 5A).
Figure 4. Pronounced inflammatory cell and fibroblast infiltration following A. actinomycetemcomitans stimulation.
Histological examination of rat calvarial tissues was used to evaluate the extent of inflammatory infiltrate and fibroblast infiltrate. Representative H&E samples are presented indicating control (PBS) injected (upper panel), and A. actinomycetemcomitans injected samples (middle and lower panels) showing inflammation and bone resorption respectively. Inflammatory cell infiltrate and fibroblast proliferation was enumerated by a pathological scoring system (B and C).Results are expressed as mean with S.E.(n=6 for controls and n=8 for A. actinomycetemcomitans injected samples; **p<0.001, *p<0.05).
Figure 5. Increased osteoclast formation following LPS and A. actinomycetemcomitans stimulation.

Graphic representation of enumerated TRAP+ multinucleated formation in calvarial tissue section is presented from indicated groups in after 1week (A) or 2 weeks post stimulation (B). Horizontal bar indicates mean TRAP+ cell counts.
Discussion
The hallmark of destructive periodontal disease is the overproduction of cytokines and other inflammatory mediators (reviewed in (Kirkwood and Rossa, 2009)). Production of cytokines and inflammatory mediators is usually a tightly controlled process that is initiated by external stimuli that are rapidly transduced through the cytoplasm and into the nucleus. Gene expression initiated within the nucleus is accompaniedby a significant component of regulation occurring within the cytosol where signaling mechanisms help to regulate mRNA stability and translation thus amplifying and sustaining expression of immune cytokines. In the present study A. actinomycetemcomitans was able to robustly stimulate pro-inflammatory (TNF-α and IL-6) as well as the anti-inflammatory cytokine IL-10 consistent with previous studies using A. actinomycetemcomitans-derived LPS (Patil et al., 2006; Patil et al., 2004; Yu et al., 2011a; Yu et al., 2011b).
Following TLR engagement, pathogen-associated molecular patterns (PAMPs) stimulate NF-κB and MAPK signaling. Proximal signaling through the p38 MAPK pathway is a well-established cascade essential for inflammatory cytokine expression through regulation of mRNA stability (Patil et al., 2004; Zhao et al., 2011). Attenuation of MAPK signaling occurs through dephosphorylation of activated MAPKs by MKPs thus suppressing signalingand MAPK-dependent cytokine expression. Recently, loss- and gain-of-function studies have indicated that MKP-1 is a major signaling protein in A. actinomycetemcomitans LPS-induced alveolar loss(Sartori et al., 2009; Yu et al., 2011a). As part of these studies, a kinetic analysis indicated that LPS activated p38 occurs as early as 10 minutes but was quickly attenuated at the same time when MKP-1 expression was increased (Sartori et al., 2009). Results from the present study using whole A. actinomycetemcomitans indicate a very different scenario. In contrast to the reciprocal regulation previously observed, data herein suggests that whole A. actinomycetemcomitans stimulates NF-κB and multiple MAPKs including p38, JNK, and ERK along with MK2, an immediate downstream kinase substrate of p38, with sustained MAPK activation for up to 8 hours in both NR8383 cell lines and primary BMMϕ cell populations. We speculate that this sustained MAPK activation may be due to continued TLR activation with multiple PAMPs over time in culture, but additional experiments addressing TLR proximal activation and internalization are needed to substantiate these observations.
In addition to the sustained MAPK activation, we also observed that NF-κB is activated over the same time frame post whole A. actinomycetemcomitans exposure. TLR signaling requires a different set of adaptors, including myeloid differentiation factor 88 (MyD88) and TIR domain-containing adaptor protein (TIRAP), which will recruit interleukin 1 receptor-associated kinase 1 (IRAK) or TRAF6. It is currently believed that the kinase TGF β-activated kinase 1 (TAK1) would link TRAF6 to the IKK complex, which is a critical regulator for the activation of NF-κB(Garcia de Aquino et al., 2009; Hayden and Ghosh, 2004; Tergaonkar, 2006). Thus, we suspect that activation of NF-κB canonical pathway occurs post A. actinomycetemcomitans exposure in macrophage populations.
A limitation of LPS-induced models of experimental periodontitis is that significant bone loss occurs in mice and rats only after multiple LPS injections (Li et al., 2011; Patil et al., 2006; Rogers et al., 2007; Rossa et al., 2006). In addition, some reports indicate difficulty in rodent colonization with the human pathogensincluding A. actinomycetemcomitans(Schreiner et al., 2011).Although some investigators have been successful using the oral gavage approach(Garlet et al., 2005; Garlet et al., 2008; Garlet et al., 2006; Gelani et al., 2009; Lima et al., 2010)success may vary depending on specific strains in rat models (Graves et al., 2008; Schreiner et al., 2011). Previous studies inducing periodontal disease require a combination of repeated oral inoculation and palatal injection of A. actinomycetemcomitans to elicit a host immune response and bone loss in mice(Garlet et al., 2005).
Thus, in the present study, formalin-fixed A. actinomycetemcomitans was used short-term following a single exposure of the periodontal pathogen to address host-bacteria interactions versus A. actinomycetemcomitans virulence.An innate immune response was observed in this model because the rats were naïve to the A. actinomycetemcomitans prior to inoculation making it less likely for an adaptive immune response to occur within the duration of the experiment (Graves et al., 2001). We noticed a sustained inflammatory infiltrate up to two weeks, which is inconsistent with prior findings using P. gingivalis calvarial injections that allowed forinflammation to decrease starting at day 5 post injection (Liu et al., 2004).In additionour modelusesSprague-Dawleyrats withA. actinomycetemcomitans. Sprague-Dawley rats are highly susceptible to repeated inoculation of A. actinomycetemcomitans LPS that caused inflammatory bone loss in a periodontal disease model (Rogers et al., 2007). However, it is also possible that we missed peak inflammation at an earlier time-point and that the infiltrate seen at one and two weeks in our study is resolving. Therefore, it is possible that in this model rats have a diminished ability to resolve inflammation and continue to have sustained infiltrate 2weeks post injection.
Following 1 to 2 weeks, we observed an increased number of resorption lacunae in rat calvariae after microinjection of A. actinomycetemcomitans in comparison to controls via μCT and TRAP staining for osteoclast formation. It is worth noting that TRAP staining only indicateda trend towards increased osteoclast number that was decreasing in week 2. This may be the result of the osteoclasts undergoing apoptosis at the 2-week time frame and thus not detectable at this time point(Tanaka et al., 2006).
Thisin vivostudy has identified the consequences of whole bacterial stimulation in rat calvariaein the context of innate immune response. Previously, A. actinomycetemcomitans LPS was found to stimulate RANKL, IL-6, and MMP-13 expression in several periodontal relevant cell types, including periodontal ligament fibroblasts, osteoblasts, and macrophages through transcriptional and post-transcriptional mechanisms(Patil et al., 2006; Rossa et al., 2006; Rossa et al., 2005). Since the calvarial boneis formed via intramembranous ossification, similar to alveolar bone, this is a suitable model to study short-term consequences of A. actinomycetemcomitansin the host immune interactions as a surrogate for a periodontal disease models similar to other studies(Assuma et al., 1998). In summary, we observed sustained MAPK activation and bone resorption in response to activated signaling pathways relevant to cytokine driven-inflammation and bone loss in response to A. actinomycetemcomitans stimulation.
Supplementary Material
Supplemental Figure 1: Sustained activation of MAPK signaling pathway in rat BMMϕ cells following A. actinomycetemcomitans stimulation. Relative fold change to unstimulatedBMMϕcells at 0 hours was quantified for p-ERK (A), p-p38 (B), p-JNK (C), p-MK2 (D), NF-κB, and MKP-1 (F). Graphic representation of densitometry for 2 independent experiments of immunoblotdata was normalized to GAPDH.
Acknowledgements
This work was conceived by KLK, laboratory and animal procedures performed by JD, QL, HY, and BH. Manuscript was initially written by JD and revised by KLK and BH. The authors would like to acknowledge the support of JaclynnKrieder from the Orthopedics Research Core at the University of Michigan. This work was supported from the National Institutes of Health (NIH): 1R01DE018290 (KLK), 1R01DE021423 (KLK), 2P20 RR017696 (KLK), and T32 DE017551(BH).
References
- Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- Chen CC, Chang KL, Huang JF, Huang JS, Tsai CC. Interleukin-6 production by human gingival fibroblasts following stimulation with Actinobacillus actinomycetemcomitans. Kaohsiung J Med Sci. 1998;14:367–378. [PubMed] [Google Scholar]
- Fine DH, Schreiner H, Nasri-Heir C, Greenberg B, Jiang S, Markowitz K, Furgang D. An improved cost-effective, reproducible method for evaluation of bone loss in a rodent model. J Clin Periodontol. 2009;36:106–113. doi: 10.1111/j.1600-051X.2008.01353.x. [DOI] [PubMed] [Google Scholar]
- Garcia de Aquino S, Manzolli Leite FR, Stach-Machado DR, Francisco da Silva JA, Spolidorio LC, Rossa C., Jr. Signaling pathways associated with the expression of inflammatory mediators activated during the course of two models of experimental periodontitis. Life Sci. 2009;84:745–754. doi: 10.1016/j.lfs.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Garlet GP, Avila-Campos MJ, Milanezi CM, Ferreira BR, Silva JS. Actinobacillus actinomycetemcomitans-induced periodontal disease in mice: patterns of cytokine, chemokine, and chemokine receptor expression and leukocyte migration. Microbes Infect. 2005;7:738–747. doi: 10.1016/j.micinf.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Garlet GP, Cardoso CR, Campanelli AP, Garlet TP, Avila-Campos MJ, Cunha FQ, Silva JS. The essential role of IFN-gamma in the control of lethal Aggregatibacter actinomycetemcomitans infection in mice. Microbes Infect. 2008;10:489–496. doi: 10.1016/j.micinf.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Garlet GP, Cardoso CR, Silva TA, Ferreira BR, Avila-Campos MJ, Cunha FQ, Silva JS. Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol Immunol. 2006;21:12–20. doi: 10.1111/j.1399-302X.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- Gelani V, Fernandes AP, Gasparoto TH, Garlet TP, Cestari TM, Lima HR, Ramos ES, de Souza Malaspina TS, Santos CF, Garlet GP, da Silva JS, Campanelli AP. The role of toll-like receptor 2 in the recognition of Aggregatibacter actinomycetemcomitans. J Periodontol. 2009;80:2010–2019. doi: 10.1902/jop.2009.090198. [DOI] [PubMed] [Google Scholar]
- Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves DT, Oskoui M, Volejnikova S, Naguib G, Cai S, Desta T, Kakouras A, Jiang Y. Tumor necrosis factor modulates fibroblast apoptosis, PMN recruitment, and osteoclast formation in response to P. gingivalis infection. J Dent Res. 2001;80:1875–1879. doi: 10.1177/00220345010800100301. [DOI] [PubMed] [Google Scholar]
- Hall TJ, Chambers TJ. Molecular aspects of osteoclast function. Inflamm Res. 1996;45:1–9. doi: 10.1007/BF02263497. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe E, Nakamura Y, Yamaguchi A, Yoshiki S, Matsuda T, Hirano T, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–3303. [PubMed] [Google Scholar]
- Kamma JJ, Nakou M, Gmur R, Baehni PC. Microbiological profile of early onset/aggressive periodontitis patients. Oral Microbiol Immunol. 2004;19:314–321. doi: 10.1111/j.1399-302x.2004.00161.x. [DOI] [PubMed] [Google Scholar]
- Kirkwood KL, Rossa C., Jr. The potential of p38 MAPK inhibitors to modulate periodontal infections. Curr Drug Metab. 2009;10:55–67. doi: 10.2174/138920009787048347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yu H, Zinna R, Martin K, Herbert B, Liu A, Rossa C, Jr., Kirkwood KL. Silencing mitogen-activated protein kinase-activated protein kinase-2 arrests inflammatory bone loss. J Pharmacol Exp Ther. 2011;336:633–642. doi: 10.1124/jpet.110.172395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima HR, Gelani V, Fernandes AP, Gasparoto TH, Torres SA, Santos CF, Garlet GP, da Silva JS, Campanelli AP. The essential role of toll like receptor-4 in the control of Aggregatibacter actinomycetemcomitans infection in mice. J Clin Periodontol. 2010;37:248–254. doi: 10.1111/j.1600-051X.2009.01531.x. [DOI] [PubMed] [Google Scholar]
- Liu R, Desta T, He H, Graves DT. Diabetes alters the response to bacteria by enhancing fibroblast apoptosis. Endocrinology. 2004;145:2997–3003. doi: 10.1210/en.2003-1601. [DOI] [PubMed] [Google Scholar]
- Lopez NJ, Mellado JC, Leighton GX. Occurrence of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia in juvenile periodontitis. J Clin Periodontol. 1996;23:101–105. doi: 10.1111/j.1600-051x.1996.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34:235–249. [PubMed] [Google Scholar]
- Patil C, Rossa C, Jr., Kirkwood KL. Actinobacillus actinomycetemcomitans lipopolysaccharide induces interleukin-6 expression through multiple mitogen-activated protein kinase pathways in periodontal ligament fibroblasts. Oral Microbiol Immunol. 2006;21:392–398. doi: 10.1111/j.1399-302X.2006.00314.x. [DOI] [PubMed] [Google Scholar]
- Patil C, Zhu X, Rossa C, Jr., Kim YJ, Kirkwood KL. p38 MAPK regulates IL-1beta induced IL-6 expression through mRNA stability in osteoblasts. Immunol Invest. 2004;33:213–233. doi: 10.1081/imm-120034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JE, Li F, Coatney DD, Rossa C, Bronson P, Krieder JM, Giannobile WV, Kirkwood KL. Actinobacillus actinomycetemcomitans lipopolysaccharide-mediated experimental bone loss model for aggressive periodontitis. J Periodontol. 2007;78:550–558. doi: 10.1902/jop.2007.060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossa C, Ehmann K, Liu M, Patil C, Kirkwood KL. MKK3/6-p38 MAPK signaling is required for IL-1beta and TNF-alpha-induced RANKL expression in bone marrow stromal cells. J Interferon Cytokine Res. 2006;26:719–729. doi: 10.1089/jir.2006.26.719. [DOI] [PubMed] [Google Scholar]
- Rossa C, Jr., Liu M, Patil C, Kirkwood KL. MKK3/6-p38 MAPK negatively regulates murine MMP-13 gene expression induced by IL-1beta and TNF-alpha in immortalized periodontal ligament fibroblasts. Matrix Biol. 2005;24:478–488. doi: 10.1016/j.matbio.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Roux S, Orcel P. Bone loss. Factors that regulate osteoclast differentiation: an update. Arthritis Res. 2000;2:451–456. doi: 10.1186/ar127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori R, Li F, Kirkwood KL. MAP kinase phosphatase-1 protects against inflammatory bone loss. J Dent Res. 2009;88:1125–1130. doi: 10.1177/0022034509349306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner H, Markowitz K, Miryalkar M, Moore D, Diehl S, Fine DH. Aggregatibacter actinomycetemcomitans-induced bone loss and antibody response in three rat strains. J Periodontol. 2011;82:142–150. doi: 10.1902/jop.2010.100250. [DOI] [PubMed] [Google Scholar]
- Slots J, Ting M. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontol. 1999;2000(20):82–121. doi: 10.1111/j.1600-0757.1999.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Soloviev A, Schwarz EM, Kuprash DV, Nedospasov SA, Puzas JE, Rosier RN, O’Keefe RJ. The role of p105 protein in NFkappaB activation in ANA-1 murine macrophages following stimulation with titanium particles. J Orthop Res. 2002;20:714–722. doi: 10.1016/S0736-0266(01)00180-2. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Umeda M, Ishizuka M, Huang Y, Ishikawa I. Prevalence of periodontopathic bacteria in aggressive periodontitis patients in a Japanese population. J Periodontol. 2003;74:1460–1469. doi: 10.1902/jop.2003.74.10.1460. [DOI] [PubMed] [Google Scholar]
- Tan KS, Song KP, Ong G. Cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Occurrence and association with periodontal disease. J Periodontal Res. 2002;37:268–272. doi: 10.1034/j.1600-0765.2002.01618.x. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Miyazaki T, Fukuda A, Akiyama T, Kadono Y, Wakeyama H, Kono S, Hoshikawa S, Nakamura M, Ohshima Y, Hikita A, Nakamura I, Nakamura K. Molecular mechanism of the life and death of the osteoclast. Ann N Y Acad Sci. 2006;1068:180–186. doi: 10.1196/annals.1346.020. [DOI] [PubMed] [Google Scholar]
- Tergaonkar V. NFkappaB pathway: a good signaling paradigm and therapeutic target. Int J Biochem Cell Biol. 2006;38:1647–1653. doi: 10.1016/j.biocel.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Tinoco EM, Beldi MI, Loureiro CA, Lana M, Campedelli F, Tinoco NM, Gjermo P, Preus HR. Localized juvenile periodontitis and Actinobacillus actinomycetemcomitans in a Brazilian population. Eur J Oral Sci. 1997;105:9–14. doi: 10.1111/j.1600-0722.1997.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Ho YP, Chen CC. Levels of interleukin-1 beta and interleukin-8 in gingival crevicular fluids in adult periodontitis. J Periodontol. 1995;66:852–859. doi: 10.1902/jop.1995.66.10.852. [DOI] [PubMed] [Google Scholar]
- Yu H, Li Q, Herbert B, Zinna R, Martin K, Junior CR, Kirkwood KL. Anti-inflammatory effect of MAPK phosphatase-1 local gene transfer in inflammatory bone loss. Gene Ther. 2011a;18:344–353. doi: 10.1038/gt.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Sun Y, Haycraft C, Palanisamy V, Kirkwood KL. MKP-1 regulates cytokine mRNA stability through selectively modulation subcellular translocation of AUF1. Cytokine. 2011b;56:245–255. doi: 10.1016/j.cyto.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Liu M, D’Silva NJ, Kirkwood KL. Tristetraprolin regulates interleukin-6 expression through p38 MAPK-dependent affinity changes with mRNA 3′ untranslated region. J Interferon Cytokine Res. 2011;31:629–637. doi: 10.1089/jir.2010.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Bar-Shavit Z. Dual modulation of osteoclast differentiation by lipopolysaccharide. J Bone Miner Res. 2002;17:1211–1218. doi: 10.1359/jbmr.2002.17.7.1211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Sustained activation of MAPK signaling pathway in rat BMMϕ cells following A. actinomycetemcomitans stimulation. Relative fold change to unstimulatedBMMϕcells at 0 hours was quantified for p-ERK (A), p-p38 (B), p-JNK (C), p-MK2 (D), NF-κB, and MKP-1 (F). Graphic representation of densitometry for 2 independent experiments of immunoblotdata was normalized to GAPDH.