Abstract
Lymphocytes from blood or tumors of patients with advanced cancer did not proliferate and produced very low levels of tumor necrosis factor and IFN-γ when cultured with autologous tumor cells. Proliferation and lymphokine production dramatically increased in the presence of beads conjugated with mAbs to CD3 plus mAbs to CD28 and/or CD40, and the lymphocytes destroyed the tumor cells. Expression density of CD3 concomitantly increased from low to normal levels. Furthermore, beads providing a CD3 signal (in combination with CD28 or CD28 plus CD40) gave partial protection against the inhibitory effect of transforming growth factor type β1 on lymphocyte proliferation and production of tumor necrosis factor and IFN-γ. MHC class I-restricted cytolytic T cells lysing autologous tumor cells in a 4-h Cr51 release assay were generated when peripheral blood leukocytes were activated in the presence of autologous tumor cells and anti-CD3/CD28 or anti-CD3/CD28/CD40 beads. Experiments performed in a model system using anti-V-β1 or anti-V-β2 mAbs to activate subsets of T cells expressing restricted T cell receptor showed that lymphocytes previously activated by anti-V-β can respond to CD3 stimulation with vigorous proliferation and lymphokine production while retaining their specificity, also in the presence of transforming growth factor type β1. Our results suggest that T lymphocytes from cancer patients can proliferate and form Th1 type lymphokines in the presence of autologous tumor cell when properly activated, and that antigen released from killed tumor cells and presented by antigen-presenting cells in the cultures facilitates the selective expansion of tumor-directed, CD8+ cytolytic T cells.
Keywords: immunotherapy, tumor vaccines, tumor immunity, transforming growth factor type β1
Immune responses do not protect against most cancers, although data were published in the 1960s indicating that the immune systems of cancer patients recognize antigens that can be targets for tumor destruction (1). Recent evidence for immunogenic, tumor-associated antigens includes the demonstration, with tetramer technology, that lymphocytes from melanoma patients recognize tumor epitopes (2), the finding of IgG antitumor antibodies by using the SEREX technique (3), and the generation of cytolytic T cells (CTL) to a large variety of tumor epitopes (4, 5). Most likely, the failure of immunological mechanisms to prevent tumor formation is due to mechanisms normally protecting against autoimmunity. For example, most neoplastic (like most normal) cells do not express key costimulatory molecules and are able to “sneak through” immunological control until their antigens have been taken up and processed by “professional” antigen-presenting cells (6–8). Furthermore, tumors make immunosuppressive factors, as can lymphoid cells in response to tumor antigens (9). Members of the transforming growth factor (TGF) type β1 family (9–15) are particularly important in this regard. Reflecting the immunosuppressed state, molecules involved in T cell signaling are down-regulated among lymphocytes from blood or tumors derived from tumor-bearing animals and human patients (16–18). Unless the immunosuppression can be overcome, it is unlikely that tumor vaccination or adoptive transfer of immune T lymphocytes will have a major impact in patients with metastatic cancer.
We now show that lymphocytes from patients with advanced cancer can proliferate, produce high levels of tumor necrosis factor (TNF) and IFN-γ, and generate tumor-destructive CTL in the presence of autologous tumor cells after polyclonal activation, via CD3, and costimulatory signal(s), via CD28, alone or together with CD40. We further show that lymphocytes stimulated via CD3 plus costimulatory signals become relatively resistant to inhibition by TGF-β1. These data are supported by experiments performed in a model system where anti-V-β1 and anti-V-β2 are used as surrogate antigens to which responses are induced or recalled by using the respective specific mAbs.
Materials and Methods
Patient Material.
Tumors were obtained at surgery or from malignant effusions (mostly ascites) of patients with stage IV carcinomas. Most studies were performed with eight patients, five of whom (1OV, 3OV, 8OV, 44OV, 48OV) had ovarian carcinoma, two (1C, 22C) had colon carcinoma, and one (1HN) had a head and neck carcinoma. Cells from an ovarian carcinoma line, 4007, also were used.
Preparation of Tumor and Blood Samples.
Solid tumors were suspended in medium, and fluids were removed from effusions after which the cells were resuspended. Erythrocytes were removed by Ficoll-Hypaque (Amersham Pharmacia), and a Percoll gradient (Sigma) was used to separate tumor cells from tumor-infiltrating lymphocytes (TIL). Lymphocyte samples were used directly or stored in liquid nitrogen for later use. Tumor samples were explanted to establish cell cultures. Peripheral blood leukocytes (PBL) were purified by using Ficoll-Hypaque. In the initial experiments, CD8+ T lymphocytes (>90% pure) were positively selected from TIL by using VarioMac magnetic beads (Miltenyi Biotech, Auburn, CA). For all other experiments, lymphoid cell populations containing T lymphocytes, monocytes, and B cells were used.
Combination of Lymphocytes and Tumor Cells.
In the initial experiments, five lymphocytes were added per tumor cell, after which the mixtures were incubated at 37°C in Costar (3513) 12-well plates (Corning) with RPMI medium (GIBCO) and 10% FCS (Atlanta Biological, Norcross, GA). They were followed by experiments in which PBL or TIL were cultured with or without autologous tumor cells in the presence of magnetic beads (Dynal, Lake Success, NY) and conjugated, using a published technique (19, 20), with mAbs to CD3, CD28, and/or CD40; beads not conjugated with mAb (or with an irrelevant mAb) were used as controls. The mAbs were 64.1 (21), 9.3 (21), and G28.5 (22), which, respectively, stimulate lymphocytes polyclonally (anti-CD3), costimulate them (anti-CD28), or activate antigen-presenting cells (anti-CD40). When autologous tumor cells were used, cells (40,000–75,000/well) were first attached by overnight incubation to Costar 24-well plates containing 2 ml Iscove's modified Dulbecco medium with 10% FBS. mAb-conjugated beads (3 × 106/ml) were then added, followed by lymphocytes (106/ml) in RPMI with 10% FBS. The plates were incubated at 37°C in a 6% CO2 in air atmosphere for 4–5 days. The beads then were removed using a magnet, and the lymphocytes were placed in new wells in medium containing 10 units/ml of IL-2 (Roche Molecular Biochemicals) and moved into flasks when their concentration had reached ≈2 × 106 cells/ml. Cultures were observed for evidence of tumor cell destruction. Lymphocyte proliferation was determined by counting. Media were sampled to measure production of TNF and/or IFN-γ, which was assayed with WEHI cells (23) and an ELISA (IFN-γ ELISA, EH-IFNG, Endogen, Woburn, MA), respectively. TGF-β1 was purchased from Sigma. In all experiments using TGF-β1, the molecule remained in the cultures, also after removal of mAb-conjugated beads.
CTL Assays.
Classical 4-h 51Cr release assays were performed. To characterize the effector cells, experiments were done to inhibit cytotoxicity by addition of mAb w6/32 (10 μg/ml), which recognizes a MHC class I framework determinant (Research Diagnostics, Flanders, NJ). mAbs to the natural killer markers CD16 and CD56 (Beckman Coulter), anti-CD8 mAb HIT8a (PharMingen), and anti-integrin-β2 (CD18) mAb 60.3 (24) also were used.
Fluorescence-Activated Cell Sorting (FACS) Analysis of Lymphocytes.
Density of CD expression was evaluated by FACS (Epics XL, Coulter), using phycoerythrin-labeled mAb and counting cells as positive when they had a preset minimum brightness. To investigate whether an increased density of CD3 expression after in vitro activation of lymphocytes was due to the selective proliferation of cells with originally high CD3 expression, PBL harvested from cancer patients were labeled with the dye CFDA (Molecular Probes). Subsequently, they were cultured in the presence of anti-CD3/CD28/CD40 beads for 5 days, after which the beads were removed and the lymphocytes were expanded in medium containing 10 units IL-2/ml. At two time points after removal of the beads (4 h and 3 days) FACS analysis was performed, in which cells were analyzed for labeling by CFDA and expression of CD3. Labeled lymphocytes that had been cultured with control beads were studied for comparison.
Use of a Model System with Anti-V-β1 and Anti-V-β2 as Surrogate Antigens.
Experiments were performed to investigate the effect of stimulation of PBL from healthy adult donors (one donor in each experiment) on the proliferation and lymphokine production of naïve lymphocytes and on lymphocytes activated by mAbs to V-β1 and V-β2 (Beckman Coulter). The well bottoms of a culture plate were coated with 1 μg/ml of anti-V-β1 or anti-V-β2 by incubation at 4°C overnight. Subsequently, PBL (2 × 106/well) were added, either together with control beads or beads conjugated with anti-CD3/anti-CD28/anti-CD40 mAbs. After 4–5 days of coculture, beads were removed and lymphocytes were expanded in medium supplemented with 10 units IL-2/ml. The number of lymphocytes/well was counted, and production of TNF and IFN-γ was measured. Lymphocytes were analyzed by FACS for expression of CD3, V-β1, and V-β2.
A second round of stimulation then was carried out to study the effects of stimulation by anti-V-β and/or mAb-coated beads on sensitized lymphocytes. In these experiments, lymphocytes sensitized in the presence of either anti-V-β were cultured for 5 days in the presence of the same or a different, immobilized anti-V-β, with or without beads conjugated with anti-CD3/CD28/CD40 mAbs.
Results
Cocultivation of PBL or TIL with Autologous Tumor Cells.
Six initial experiments were performed in which CD8+ T lymphocytes purified from TIL were cultured with tumor cells, after which the supernatants were assayed for TNF or IFN-γ. In a representative experiment, CD8+ TIL from a colon cancer patient (1C), first cultivated with 1C tumor cells for 15 days, were removed and added to either a fresh set of 1C cells or to tumor cells from a lung carcinoma patient (3L). A small amount of TNF (1.2 pg/ml) was detected when 1C lymphocytes were combined with the 1C but not with the 3L tumor, whereas TNF and IFN-γ (1.5 pg/ml) were produced when TIL from 3L were combined with 3L tumor cells but not when cultured alone. There was no evidence of lymphocyte proliferation. In subsequent experiments, TIL populations comprising monocytes, CD4+ T cells, and B cells in addition to CD8+ lymphocytes were combined with autologous tumor cells. Approximately 10 times higher levels of TNF (4.5–48 pg/ml) and up to 150 pg/ml of IFN then were detected in supernatants from cultures of eight of 13 patients. There was still no lymphocyte proliferation.
We then adapted a system in which mAb-conjugated magnetic beads are used to induce signals via various lymphocyte receptors (19, 20). PBL or TIL were combined with autologous tumor cells in the presence of beads conjugated with mAbs to CD3 and mAbs to CD28, alone or together with CD40. Similar groups were included with lymphocytes but without tumor cells. As controls, lymphocytes, with or without tumor cells, were cultivated with control beads. After 3–5 days, the beads were removed and the lymphocytes were incubated separately over 2–21 days with 10 units/ml of IL-2.
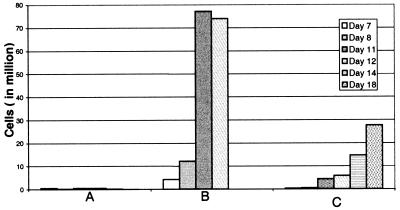
Fig. 1 shows an experiment in which TIL from OV44 proliferated vigorously when exposed for 4 days to anti-CD3/CD28/CD40-conjugated beads. Lymphocytes cultivated in the absence of a CD3 signal did not proliferate and neither did lymphocytes cultured with anti-CD28 and/or CD40 beads (data not shown). Proliferation was greater when autologous tumor cells were initially present with the beads inducing signals via CD3 (Fig. 1B). Anti-CD3/CD28-conjugated beads induced proliferation similar to that with anti-CD3/CD28/CD40-conjugated beads (data not shown).
Figure 1.
Proliferation of in vitro expanded TILs from 44 OV. (A) Autologous tumor, control beads (similar results without tumor). (B) Autologous tumor, anti-CD3/CD28/CD40-conjugated beads. (C) No tumor, anti-CD3/CD28/CD40-conjugated beads.
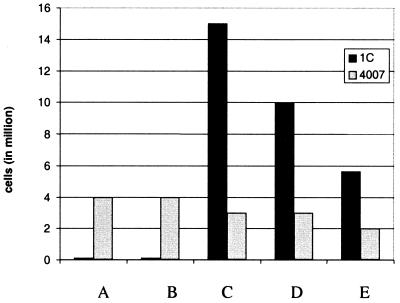
Fig. 2 shows an experiment in which PBL from patient 1C and various mAb-conjugated beads were cultivated for 5 days with either autologous tumor cells or allogeneic (4007) cells. The number of lymphocytes per culture was much higher when CD3/CD28 (Fig. 2C) or anti-CD3/CD28/CD40 (Fig. 2D) activated lymphocytes were combined with 1C tumor than with 4007 cells, a finding similar to that illustrated in Fig. 1. When, on the other hand, the beads did not provide any signal via CD3 (Fig. 2 A and B), the situation was the reverse and probably represented an immunological response to alloantigens expressed on the 4007 cells. FACS analysis showed that >90% of the activated lymphocytes expressed CD3 and ≈70% of them were CD8+, with less than 5% expressing CD16 or CD56.
Figure 2.
Proliferation of PBL from 1C after in vitro activation in the presence of autologous or allogeneic 4007 mismatched ovarian carcinoma cells. (A) Control beads. (B) Anti-CD28/CD40-conjugated beads. (C) Anti-CD3/CD28-conjugated beads. (D) Anti-CD3/CD28/CD40-conjugated beads. (E) Anti-CD3/CD40-conjugated beads.
Most of the tumor cells were destroyed within 24–48 h after exposure to autologous lymphocytes in the presence of anti-CD3/CD28- or anti-CD3/CD28/CD40-conjugated beads, often leaving cultures entirely comprising cells with lymphocyte morphology. To study whether this destruction had immunological specificity, four experiments were performed in which serial dilution of PBL (106–105/sample) from cancer patients were combined with autologous tumor cells or with either tumor cells or fibroblasts from an allogeneic donor. In two experiments, there was ≈10 times more TNF in the culture supernatants in the presence of the autologous tumor, but there was no difference in the killing of cells from autologous or allogeneic tumors or of allogeneic fibroblasts. We conclude that tumor cell destruction seen after 24–72 h in the presence of lymphocyte activation was not immunologically specific, perhaps because large amounts of activated T lymphocytes and lymphokines obscured any specific components.
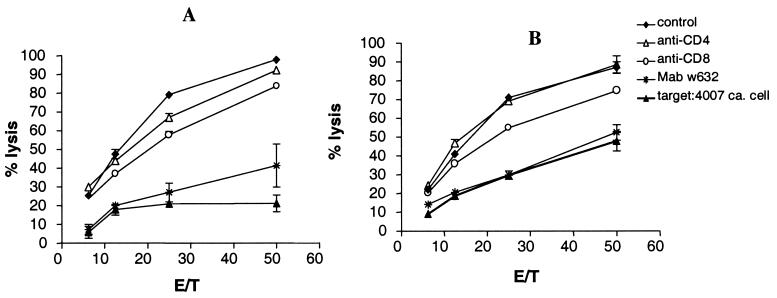
MHC-class I-restricted CTL were generated from lymphocytes activated by tumor cells plus anti-CD3/CD28 or anti-CD3/CD28/CD40 beads. Fig. 3 presents an experiment with PBL from 1C, which had been activated in the experiment shown in Fig. 2. After activation by tumor cells and mAb-conjugated beads, the beads were removed and the lymphocytes were expanded with 10 units IL-2/ml medium over 3 weeks in the absence of tumor cells and beads. PBL activated by 1C and anti-CD3/CD28 beads were strongly cytolytic to 1C cells, and lysis was inhibited by a mAb to CD8 and by anti-MHC class I framework mAb w6/32 (Fig. 2A). Allogeneic 4007 cells were killed by only 20% at an effector-to-target cell ratio of 50:1, as compared with 98% lysis of 1C cells (Fig. 2A). Fig. 2B demonstrates analogous data for PBL stimulated with anti-CD3/CD28/CD40 beads. Lysis of 4007 cells then was at the same low level as that of 1C in the presence of mAb w6/32. In contrast, PBL stimulated with anti-CD3/CD40 beads killed both 1C and 4007 cells, also in the presence of mAbs to CD8 or mAb w6/32 (data not shown). CD8+ cells enriched from the cell population used in the experiment shown in Fig. 2B lysed 25% of 1C cells at an effector-to-target cell ratio of 1:20 as compared with 0% of cells from the 4007 line and 0% of cells from an allogeneic B cell line. In this experiment, lysis of 1C cells was 5% in the presence of mAb w6/32 and 5% with the anti-CD18 mAb 60.3, and it only decreased from 25% to 18% with a combination of mAbs to CD16 and-CD56. Lymphocytes activated by cocultivation with 4007 cells and any of the beads did not selectively lyse 1C or 4007 cells (data not shown). The CTL assays were repeated twice with similar results.
Figure 3.
Cr51 release data with PBL from 1C, tested on the indicated target cells, after activation on 1C cells by anti-CD3/CD28-conjugated beads (A) or anti-CD3/CD28/CD40-conjugated beads (B). Standard deviations were <10% of cpm. E/T, effector-to-target cell ratio.
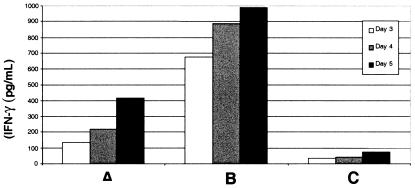
Large amounts of IFN-γ were detected in supernatants of cultures from lymphocytes activated via CD3 (Fig. 4). Fig. 4 also shows that the production of IFN-γ was higher when autologous tumor cells were present during the first 4–5 days of culture.
Figure 4.
IFN-γ produced by in vitro expanded PBL from 1HN. (A) No tumor, anti-CD3/CD28-conjugated beads. (B) Autologous tumor, anti-CD3/CD28-conjugated beads. (C) Autologous tumor, control beads (similar results without tumor).
Table 1 presents six additional representative experiments showing proliferation and lymphokine production by PBL or TIL, which were either tested upon harvest from the patients or after one round of in vitro activation with beads. Anti-CD3, anti-CD3/CD28, anti-CD3/CD40, and anti-CD3/CD28/CD40 beads strongly increased lymphocyte proliferation with no significant difference between them. In contrast, anti-CD28, anti-CD40, and anti-CD28/CD40 beads alone did not increase lymphocyte proliferation and lymphokine production over control beads (data not shown), indicating that signaling via CD3 was essential. Production of TNF and IFN-γ correlated well. It decreased to background levels when the lymphocytes were grown without tumor cells and beads for more than 3–5 days (data not shown). As in Figs. 1 and 4, CD3 signaling was required to induce vigorous lymphocyte proliferation and lymphokine production.
Table 1.
Proliferation (cell numbers × 106 per sample) and lymphokine production (pg/ml of TNF or IFN-γ) of freshly harvested PBL and TIL from patients with advanced cancer after culturing for 4–5 days ± autologous tumor cells in the presence of mAb-conjugated beads, followed by 2–4 days without beads (same time within each experiment)
| mAb-conjugated beads | PBL
|
TIL
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10V
|
80V
|
1HN*
|
480V*
|
30V
|
22C*
|
||||||||
| × 106 | TNF | IFN-γ | × 106 | TNF | × 106 | TNF | × 106 | × 106 | TNF | IFN-γ | × 106 | TNF | |
| Control | 1.1 | 30 | 479 | 4.2 | 12 | 1.3 | 3 | 0.4 | 1.2 | 0 | 271 | 1.4 | 5 |
| Anti-CD3 | 11.3 | 2,440 | 5,560 | 15.3 | 1,550 | 4.8 | 2,080 | NT | 8.2 | 950 | 3,720 | NT | NT |
| Anti-CD3/CD28 | 9.6 | 2,500 | 7,810 | 22.7 | 2,500 | 7.2 | 13,020 | NT | 7.0 | 2,100 | 4,730 | 5.8 | 1,660 |
| Anti-CD3/CD40 | 7.8 | 2,500 | 4,480 | 18.6 | >1,000 | 4.0 | 1,450 | NT | 5.4 | 660 | 3,060 | 3.6 | 1,540 |
| Anti-CD3/CD28/CD40 | NT | NT | NT | NT | NT | NT | NT | 17.1 | NT | NT | NT | 6.5 | 2,060 |
Cultures were initiated with 106 PBL or TIL/sample. NT, not tested.
Autologous tumor cells present together with the lymphocytes.
The density of CD antigen expression on lymphocyte populations was measured by FACS before and after 3- to 5-day cultivation with tumor cells and anti-CD3/CD28/CD40 beads, followed by an additional 3- to 7-day expansion without beads. To reflect changes in the density of CD receptor expression, the number of cells in each population whose brightness at least equaled the density at the chosen setting is reported (Table 2); unstimulated PBL from six healthy donors (30–65 years of age) were analyzed for comparison. Unstimulated PBL from the cancer patients had low levels of CD3, CD4 and CD28. Four of five patients also had low CD8 density, whereas the CD86 density was higher than among unstimulated PBL from the healthy donors. Culturing of PBL with control beads partially increased CD3 expression, but did not significantly increase CD28 expression. In contrast, culturing with anti-CD3/CD28/CD40 beads consistently restored the expression of CD3 and CD28 to normal levels, and it doubled the number of cells with high-density CD8 expression. Density of CD3 expression was studied with TIL from five patients. It was 2.9%, 40.2%, 96%, 42.8%, and 40.1%, respectively—i.e., it displayed more variation and was generally higher than for PBL. CD8 expression by TIL was higher than among PBL and increased from 61.4% to 87.3%. The corresponding figures for CD28 expression among TIL were 39.3% and 52.8%. Before cultivation, PBL and TIL from cancer patients comprised 10–20% CD14+ cells (most likely monocytes). At 2 to 3 days after cultivation with anti-CD3/CD28 or anti-CD3/CD28/CD40 beads, 5–20% of the lymphoid cells were CD83+/CD3−; there was less than 1% of such cells with control beads or anti-CD28/CD40 beads.
Table 2.
CD expression (mean ± SD) of PBL from six healthy adults and from cancer patients (5–8 patients per group), tested directly (unstimulated) or after culturing with mAb-conjugated beads for 4–5 days
| Marker | Healthy donors, unstimulated | Cancer
patients
|
||||
|---|---|---|---|---|---|---|
| Unstimulated | Beads
|
|||||
| Control | Anti-CD3/CD28 | Anti-CD3/CD40 | Anti-CD3/CD28/CD40 | |||
| CD3 | 72.3 ± 11 | 20 ± 24* | 52 ± 32 | 92 ± 10† | 96 ± 5† | 94 ± 5† |
| CD4 | 45.4 ± 11 | 21 ± 20 | 37 ± 24 | 42 ± 23 | 29 ± 20 | 51 ± 18 |
| CD8 | 21.7 ± 10 | 9 ± 7 | 18 ± 13 | 47 ± 26 | 70 ± 15† | 45 ± 15† |
| CD28 | 62.1 ± 12 | 33 ± 17* | 45 ± 32 | 79 ± 30 | 70 ± 36 | 93 ± 3† |
| CD56 | 2.2 ± 3 | 11 ± 12 | 25 ± 34 | 2 ± 4 | 3 ± 2 | 1.5 ± 2.4 |
| CD80 | 0.1 ± 0 | 2 ± 2 | 4 | 1 ± 1 | 3 ± 5 | 5.6 ± 9 |
| CD86 | 0.2 ± 0 | 34 ± 24* | 10 ± 13 | 10 ± 14 | 16 ± 16 | 5 ± 3 |
Five to eight samples were tested per group of cancer patients.
, P < 0.01 compared to unstimulated lymphocytes from healthy donors.
†, P < 0.01 compared to unstimulated lymphocytes from patients.
To investigate whether an increased density of CD3 expression after in vitro activation of lymphocytes was due to the selective proliferation of cells with originally high CD3 expression, experiments were performed with TIL, 40.2% of which originally expressed CD3, which were labeled with the dye CFDA (25). After activation via anti-CD3/CD28/CD40 beads, CD3 expression increased to 95%. FACS analyses, using CFDA and phycoerythrin-labeled anti-CD3 as probes, showed that there was no selective proliferation of the subpopulation of PBL that originally had higher CD3 expression.
Effect of TGF-β1 in the Presence of Activation Signals via mAb-Conjugated Beads.
Table 3 shows five representative experiments performed to investigate whether the inhibitory effect of TGF-β1 on lymphokine production and lymphocyte proliferation could be altered by coculture with beads inducing signals via CD3. With control beads, the TNF and IFN-γ levels were low, and these levels were further suppressed by TGF-β1. In contrast, with anti-CD3/CD28/CD40 beads these levels increased to levels often approaching those seen in the absence of TGF-β1. Likewise, when anti-CD3/CD28/CD40 beads were used, there was much less inhibitory effect of TGF-β1 on lymphocyte proliferation with no inhibition at all seen with patient 1HN. A relative resistance of T cell proliferation and lymphokine production was seen also when the TGF-β1 dose was increased to 20 ng/ml and when the concentration of lymphocytes was decreased to 105/sample (data not shown). Beads stimulating via CD28, CD40, alone or together, did not protect against TGF-β1 (data not shown).
Table 3.
Proliferation (cell numbers × 106 per sample) and lymphokine production (pg/ml of TNF or IFN-γ) by fresh or previously stimulated (*) PBL and TIL from cancer patients cultured for 4–5 days with tumor cells and mAb-conjugated beads ± (5 ng/ml) TGF-β1, followed by 2–3 days without beads or tumor cells but with TGF-β1 remaining
| mAb-conjugated beads | TGF-β1 present | PBL
|
TIL
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 22C
|
480V
|
1HN*
|
480V
|
22C*
|
||||||||
| × 106 | TNF | IFN-γ | × 106 | IFN-γ | × 106 | TNF | IFN-γ | × 106 | IFN-γ | × 106 | ||
| Control | − | 4.6 | 40 | 264 | 0.5 | 310 | 1.3 | 3 | 23 | 4.4 | 98 | 1.7 |
| + | 2.8 | 0 | 19 | 0.5 | 19 | 0.4 | 5 | 48 | 2.8 | 27 | 1.4 | |
| Anti-CD3/CD28/CD40 | − | 19.7 | 2,900 | >20,000 | 17.2 | >10,000 | 7.2 | 11,680 | 24,050 | 8.1 | 9,810 | 6.5 |
| + | 15.5 | 860 | 6,700 | 9.8 | 3,810 | 7.4 | 4,640 | 19,250 | 3.7 | 5,220 | 3.1 | |
Cultures were initiated with 106 PBL or TIL/sample.
Model Experiments with mAbs to V-β1 and V-β2 as Surrogate Antigens.
Because stimulation via CD3 interferes with the induction of primary immune responses, experiments were performed in a model system to investigate the effect of CD3 stimulation on lymphocytes that had been activated by an antigen. As surrogate antigen, we used mAbs to V-β1 or V-β2, which allowed both the stimulation and recognition of CD3+ T cells expressing specific T cell receptors. PBL from healthy donors were exposed to anti-V-β1 or anti-V-β2 mAbs either in the presence of control beads or beads conjugated with anti-CD3/CD28/CD40 mAbs.
The presence of anti-CD3/CD28/CD40 beads completely inhibited the induction of an immune response specific for anti-V-β1 or anti-V-β2 (data not shown). In contrast, as shown in two representative experiments (Table 4), signaling via these beads expanded the proliferation of V-β-specific T lymphocytes that had been previously activated. Lymphocytes primed by exposure to anti-V-β1 or anti-V-β2 proliferated, with retained specificity for the given anti-V-β, in response to the respective anti-V-β alone or in combination with anti-CD3/CD28/CD40 beads. Similarly, V-β specificity was retained when cells activated by anti-V-β were expanded with the anti-CD3/CD28/CD40 beads in the presence of the same anti-V-β mAb, a different one, or no such mAb. Stimulation via anti-V-β mAb together with activation signals via anti-CD3/CD28/CD40 beads led to higher production of TNF than achieved by antigen-specific stimulation or activation alone (Table 4, experiment 1). The presence of anti-CD3/CD28/CD40 beads protected against the inhibitory effect of TGF-β1 on lymphocyte proliferation and allowed the production of significant amounts of IFN-γ 2 days after removal of the beads from the lymphocytes and with TGF-β1 remaining in the culture medium (Table 4, experiment 2). With the anti-CD3/CD28/CD40 beads, >20,000 pg IFN-γ was detected in the culture medium whether or not TGF-β1 was present (data not shown). The number of T cells in groups exposed to anti-V-β1 and not receiving TGF-β1 was approximately the same whether or not anti-CD3/CD28/CD40 beads were present, whereas T cell proliferation sharply decreased when the group stimulated only via anti-V-β1 was exposed to TGF-β1. Consequently, the observed protection of CD3 engagement against inhibition by TGF-β1 was not an artifact caused by fewer molecules of TGF-β1 per T lymphocyte.
Table 4.
Secondary sensitization of PBL from two healthy donors in the presence of beads conjugated with anti-CD3/CD28/CD40 mAbs (or unconjugated beads, as controls) using anti-V-β1 or anti-V-β2 as surrogate antigens
| Exp. | 1st stimul. on anti-V-β | 2nd stimul. on anti-V-β | Anti-CD3/CD28/CD40 beads | TGF-β | Lymphocytes (×
106)
|
Lymphokine pg/ml | ||
|---|---|---|---|---|---|---|---|---|
| Total | V-β1 | V-β2 | ||||||
| 1 | 1 | 1 | − | − | 12.1 | 11.4 | 0.04 | 150 |
| 1 | 2 | − | − | 2.5 | 1.9 | 0.05 | NT | |
| 1 | None | − | − | 1 | 0.79 | 0.01 | 1 | |
| 1 | 1 | + | − | 11.9 | 9.3 | 0.12 | 1,760 | |
| 1 | 2 | + | − | 14.0 | 11.3 | 0.28 | NT | |
| 1 | None | + | − | 15.7 | 12.9 | 0.16 | 880 | |
| 2 | 1 | − | − | 0.9 | 0.03 | 0.54 | NT | |
| 2 | 2 | − | − | 3.5 | 0.03 | 2.5 | 340 | |
| 2 | None | − | − | 0.5 | 0.005 | 0.32 | 2 | |
| 2 | 1 | + | − | 14.7 | 0.15 | 7.6 | NT | |
| 2 | 2 | + | − | 11.5 | 0.23 | 5.1 | 5,040 | |
| 2 | None | + | − | 13.9 | 0.14 | 7.2 | 1,960 | |
| 2 | 1 | 1 | − | − | 12.1 | 8.0 | 0.1 | 1,220 |
| 1 | 1 | − | + | 1.1 | 0.8 | 0.1 | 194 | |
| 1 | 1 | + | − | 14.9 | 6.5 | 0.1 | 1,546 | |
| 1 | 1 | + | + | 11.0 | 8.2 | 0.3 | 405 | |
| 1 | 2 | − | − | 4.4 | 3.7 | 0.3 | 47 | |
| 1 | 2 | − | + | 1.8 | 1.6 | 0.2 | 47 | |
| 1 | 2 | + | − | 19.1 | 12.1 | 0.2 | 536 | |
| 1 | 2 | + | + | 16.9 | 12.8 | 0.3 | 161 | |
TGF-β1 (5 ng/ml) was added as indicated in exp. 2. Lymphocyte numbers (× 106) and lymphokine production (TNF in exp. 1 and IFN-γ in exp. 2) was measured 2 days after removal of the beads. NT, not tested.
>90% of the lymphocytes are CD3-positive according to FACS analysis.
Discussion
Although initial experiments showed that PBL and TIL from patients with stage IV cancer secreted TNF and IFN-γ when cocultivated with autologous tumor cells, the lymphokine levels were extremely low (particularly in cultures lacking monocytes), and there was no lymphocyte proliferation. In contrast, lymphokine production was dramatically increased, and there was vigorous lymphocyte proliferation when we used a procedure (19, 20) in which beads conjugated with a mAb to CD3 in combination with mAbs to CD28 and/or CD40 were added to the cultures. Tumor-selective CTL, which were MHC class I-restricted and CD8+, could be generated from lymphocytes that had been activated over 4–5 days by anti-CD3/CD28 or anti-CD3/CD28/CD40 beads in the presence of autologous tumor cells (and monocytes) and then expanded in the absence of tumor cells and beads. We conclude that stimulation of T lymphocytes via CD3 (and costimulatory signals) expands all T lymphocytes and facilitates the generation of CTL in the presence of autologous tumor cells and antigen-presenting cells in the cultures. The low proliferation and lymphokine production of unstimulated PBL harvested from cancer patients correlated with their low expression of CD3, CD28, CD4, and CD8. Exposure to anti-CD3/CD28 or anti-CD3/CD28/CD40 beads up-regulated lymphocyte expression of CD3 and CD28, as it increased their ability to proliferate and form lymphokines. Increased expression of CD3 was not the result of a preferential expansion of lymphocytes that originally had high density of CD3 expression.
Our data indicate that at least some patients with advanced cancers have T lymphocytes that can mount tumor-destructive immune reactions but are inhibited from doing so in vivo, and that signals mediated via CD3 in the presence of tumor antigens can activate these reactions. Because the view that polyclonal stimulation via CD3 can activate antitumor immunity challenges the current concept that such stimulation prevents or overrides recognition of antigen by the T cell receptor, experiments were performed in a model, using mAbs to V-β1 or V-β2 as surrogate antigens. These experiments demonstrated that signals mediated via CD3 dramatically expanded the proliferation of already primed T lymphocytes without loss of their V-β specificity whereas they prevented the de novo induction of a specific response. Furthermore, exposure of T lymphocytes to the specific anti-V-β together with anti-CD3/CD28/CD40 beads was optimal in inducing the production of TNF.
Tumor cells exposed to lymphocytes and stimulated via CD3 in combination with CD28 and/or CD40 were regularly destroyed within 24–48 h by a mechanism that had no detectable antigen specificity, although TNF production appeared to be greater in the presence of autologous tumor cells than allogeneic tumor cells or fibroblasts. Most likely, polyclonal stimulation of CD3 plus costimulation via CD28 and CD40 produced lymphokines that activated natural killer cells and monocytes and also (like TNF) had a direct toxic effect. We hypothesize that lymphocyte activation, accompanied by tumor cell killing, causes the release of antigen, which is taken up and processed by monocytes in the cultures that differentiate into dendritic cells and present epitopes for the selective expansion of tumor-reactive T cells. Therapeutic vaccines may be based on the same principle to activate and expand suppressed lymphocytes in tumor-bearing individuals and also may facilitate the generation of immune responses to subdominant epitopes. It is noteworthy that treatment of tumor-bearing mice with anti-CD3 mAb has been shown to have antitumor activity under certain circumstances (26).
The procedures we have used make possible the generation of CD83+ dendritic cells and CD3+ lymphocytes, which continue to expand over >10 weeks of in vitro culturing and should lend themselves to adoptive immunotherapy. This finding may be because costimulation via CD28 decreases the probability for lymphocytes to undergo apoptosis (27, 28), providing them with a long lifespan in vitro (19). Costimulated lymphocytes also have survived for a long time after transfer back to autologous patients (29) as opposed to lymphocytes expanded in the presence of high doses of IL-2.
Most of the patients died within a year of donating PBL or TIL, despite our evidence that their T cell repertoire at least in some cases included lymphocyte clones that could recognize the tumors as antigenically foreign. Most likely, the failure of these clones to expand in vivo and differentiate into effector cells was due to the production by the tumor and/or the host of molecules that down-regulated or terminated T cell reactivity. Many such molecules are known (9) with members of the TGF-β1 family being among the most powerful. The effects of TGF-β1 were thus investigated. It is encouraging that T cell stimulation via CD3 in combination with CD28 alone or together with CD40 protected against ≈50% of its inhibitory effect on lymphocyte proliferation and production of TNF and IFN-γ, even when the TGF-β1 was used at saturation levels of 20 ng/ml in the cultures. Likewise, T cell stimulation via anti-CD3/CD28/CD40 beads protected against the inhibitory effect of TGF-β1 in the anti-V-β model.
Acknowledgments
This work was supported by National Institutes of Health Grants 1 P50 CA83636–01, 1 R01 CA90143–01, and 5 R01 CA79490, as well as by the Pacific Northwest Research Institute.
Abbreviations
- CTL
cytolytic T cells
- FACS
fluorescence-activated cell sorting
- PBL
peripheral blood leukocytes
- TGF
transforming growth factor
- TIL
tumor-infiltrating lymphocytes
- TNF
tumor necrosis factor
References
- 1.Hellström K E, Hellström I. Adv Cancer Res. 1969;12:167–223. doi: 10.1016/s0065-230x(08)60331-0. [DOI] [PubMed] [Google Scholar]
- 2.Cassian Y, Savage P A, Lee P P, Davis M M, Greenberg P D. J Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- 3.Old L J, Chen Y-T. J Exp Med. 1998;187:1163–1167. doi: 10.1084/jem.187.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boon T, Coulie P G, Van den Eynde B. Immunol Today. 1997;18:267–268. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg S A. J Clin Oncol. 1992;10:180–199. doi: 10.1200/JCO.1992.10.2.180. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Ashe S, Brady W A, Hellström I, Hellström K E, Ledbetter J A, McGowan P, Linsley P S. Cell. 1992;71:1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 7.Huang A Y, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 8.Yang G, Mizuno M T, Hellström K E, Chen L. J Immunol. 1997;158:851–858. [PubMed] [Google Scholar]
- 9.Kiessling R, Wasserman K, Horiguchi S, Kono K, Sjöberg J, Pisa P, Petersson M. Cancer Immunol Immunother. 1999;48:353–362. doi: 10.1007/s002620050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bright J J, Kerr L D, Sriram S. J Immunol. 1997;159:175–183. [PubMed] [Google Scholar]
- 11.D'Orazio T J, Niederkorn J Y. J Immunol. 1998;160:2089–2098. [PubMed] [Google Scholar]
- 12.Ebert E C. Clin Exp Immunol. 1999;115:415–420. doi: 10.1046/j.1365-2249.1999.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kehrl J H, Wakefield L M, Roberts A B, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn M B, Fauci A S. J Exp Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranges G E, Figari I S, Espevik T, Palladino M A., Jr J Exp Med. 1987;166:991–998. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torre-Amione G, Beauchamp R D, Koeppen H, Park B H, Schreiber H, Moses H L, Rowley D A. Proc Natl Acad Sci USA. 1990;87:1486–1490. doi: 10.1073/pnas.87.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizoguchi H, O'Shea J J, Longo D L, Loeffler C M, McVicar D W, Ochoa A C. Science. 1992;258:1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 17.Nakagomi H, Petersson M, Magnusson I, Juhlin C, Matsuda M, Mellstedt H, Taupin J-L, Vivier E, Anderson P, Kiessling R. Cancer Res. 1993;53:5610–5612. [PubMed] [Google Scholar]
- 18.Kono K, Reissing M E, Brandt R M, Melief C J, Potkul R K, Andersson B, Petersson M, Kast W M, Kiessling R. Clin Cancer Res. 1996;2:1825–1828. [PubMed] [Google Scholar]
- 19.Levine B L, Bernstein W B, Connors M, Craighead N, Lindsten T, Thompson C B, June C H. J Immunol. 1997;159:5921–5930. [PubMed] [Google Scholar]
- 20.Garlie N K, LeFever A V, Siebenlist R E, Levine B L, June C H, Lum L G. J Immunother. 1999;22:336–345. doi: 10.1097/00002371-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Martin P J, Ledbetter J A, Morishita Y, June C H, Beatty P G, Hansen J A. J Immunol. 1986;136:3282–3287. [PubMed] [Google Scholar]
- 22.Ledbetter J A, Shu G, Gallagher M, Clark E A. J Immunol. 1987;138:788–794. [PubMed] [Google Scholar]
- 23.Espevik T, Nissen-Meyer J. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 24.Beatty P G, Ledbetter J A, Martin P J, Price T H, Hansen J A. J Immunol. 1983;131:2913–2918. [PubMed] [Google Scholar]
- 25.den Haan J M M, Lehar S M, Bevan M J. J Exp Med. 2000;192:1685–1695. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellenhorn J D, Hirsch R, Schreiber H, Bluestone J A. Science. 1988;242:569–571. doi: 10.1126/science.2902689. [DOI] [PubMed] [Google Scholar]
- 27.Boise L H, Minn A J, Noel P J, June C H, Accavitti M A, Lindsten T, Thompson C B. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 28.Daniel P T, Kroidl A, Cayeux S, Bargou R, Blankenstein T, Dorken B. J Immunol. 1997;159:3808–3815. [PubMed] [Google Scholar]
- 29.Ranga U, Woffendin C, Verma S, Xu L, June C H, Bishop D K, Nabel G J. Proc Natl Acad Sci USA. 1998;95:1201–1206. doi: 10.1073/pnas.95.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]