Abstract
Cu2O p-type semiconductor hollow porous microspheres have been prepared by using a simple soft-template method at room temperature. The morphology of as-synthesized samples is hollow spherical structures with the diameter ranging from 200 to 500 nm, and the surfaces of the spheres are rough, porous and with lots of channels and folds. The photocatalytic activity of degradation of methyl orange (MO) under visible light irradiation was investigated by UV-visible spectroscopy. The results show that the hollow porous Cu2O particles were uniform in diameters and have an excellent ability in visible light-induced degradation of MO. Meanwhile, the growth mechanism of the prepared Cu2O was also analyzed. We find that sodium dodecyl sulfate acted the role of soft templates in the synthesis process. The hollow porous structure was not only sensitive to the soft template but also to the amount of reagents.
Keywords: Cu2O, Hollow porous microspheres, Photocatalytic, Visible light
Background
Much attention has been focused on fabricating high-efficiency photocatalytic materials, which is one of the most potential routes to mitigating environmental pollution [1]. Among them, metal oxide semiconductors, such as ZnO and TiO2, have attracted much attention owing to their high efficiency in the degradation of wide-ranged pollutants in which electron–hole pairs are generated under irradiation and degrade the pollutants absorbed on the surface of the photocatalytic materials [2-5]. Most of the metal oxide semiconductors have large band gaps, for example, 3.2 eV for ZnO [6] and 3.0 eV for TiO2[7], which are in the range of an UV spectrum. For this kind of materials, it is hard to generate electron–hole pairs under visible light irradiation because of the low photo energy, which therefore leads to lower photocatalytic efficiency and limits their large-scale applications. Therefore, in recent years, great efforts have been devoted to develop new photocatalytic materials with high efficiency under visible light irradiation.
As a typical p-type semiconducting material, cuprous oxide (Cu2O) possesses a stable, direct band gap of about 2.17 eV and a higher hall mobility up to 60 cm2/V·s [8], which has wide-scale applications in gas sensors, solar cells and lithium-ion batteries [7-9] owing to its unique optical and magnetic properties [9-15]. In 1998, Hara et al. [16] found that Cu2O can split water under visible light due to its low band gap. From then on, as a candidate of photocatalytic materials, Cu2O has attracted much research interests due to its important applications in degrading industrial dyeing wastewater, nitrogen-containing pesticides, etc. under visible light energy. Cu2O particles with different shapes, such as cube, octahedral, multipod, nanowire, hollow structure and porous spheres, have been synthesized [17-22]. It was found that the properties varied with the specific structure in different shapes [23]. Especially, hollow-structured particles with large specific areas have widespread potential applications in photocatalysts [24], drug delivery carriers, lightweight fillers and gas sensors [25,26]. Among lots of preparation approaches, the soft-template method is commonly used to synthesize Cu2O hollow-structured particles. So far, some polymer, such as EDTA-4Na [27], gelatin [28], PEG [29], PVP [30] and Oleic [31], were used as soft templates to fabricate hollow structures. Moreover, most reported photocatalytic studies related to Cu2O materials are mainly focused on UV-visible light irradiation [24,32,33]. In this paper, we used sodium dodecyl sulfate (SDS) as a soft template accompanied with gas bubble processes [34] to synthesize hollow porous Cu2O microspheres. The optical properties and photocatalytic activities under visible light irradiation were also investigated.
Methods
All of the chemical reagents used in the experiment were of analytical reagent grade and were directly used without further purification. To prepare the hollow porous Cu2O particles, CuSO4 was used as the Cu2+ source with SDS as the soft template. In a typical procedure, 180 mg SDS was dissolved into 45 ml deionized water under magnetic stirring for more than 20 min to form a stable micellar solution. Then, 1 ml (0.1 g/ml) CuSO4 solution, 0.04 ml (13 M) ammonia and 0.15 ml (5 M) NaOH solution were added in sequence to the above solution every 20 min. The color of the solution then turned to light turbid blue from clarification. After fully stirred, 0.18 ml (50 wt.%) N2H4·H2O solution was added dropwise as the reducing agent. As the reaction proceeded with constant stirring, the solution produced a lot of bubbles. The whole experiment process was under the condition of 20 °C for 40 min. When the reaction finished, the final products were separated by centrifugation and cleaned several times by filtration with plenty of deionized water and ethanol. Finally, the products were dried at 50 °C for 6 h in a vacuum oven.
The crystal structures of the as-prepared samples were identified by X-ray powder diffraction (XRD) using an advanced X-ray diffractometer (D8 ADVANCE, Bruker, Bremen, Germany) with a Cu-Kα rotating anode point source operating at 40 kV and 40 mA. The morphology and size were investigated by field emission scanning electron microscopy (SEM; Zeiss Ultra 55, Carl Zeiss Microscopy GmbH, Hamburg, Germany) at an accelerating voltage of 5 kV. The inner microstructure of the as-synthesized samples was studied by transmission electron microscopy (TEM; JEM-2100, JEOL, Tokyo, Japan). The optical absorption properties of the as-prepared Cu2O microspheres were characterized by UV-visible absorption spectroscopy with a He-Cd laser line of 325 nm as an excitation source (Lab-RAM HR 800 UV, HORIBA Jobin Yvon, Kyoto, Japan). The photocatalytic activity was analyzed by using methyl orange (MO) as a model pollutant molecule. The photocatalytic absorbance measurements were performed on a UNIC7000 spectrophotometer (Unic Company, USA) at 464 nm.
Results and discussion
The SEM and TEM images of the as-prepared Cu2O hollow microspheres are shown in Figure 1a,b. The morphology of the sample has been identified as hollow spherical structures, and the surfaces of the spheres are rough, porous and with lots of channels and folds. Most of them are uniform, and the diameter ranges from 200 to 500 nm. The TEM result and SEM images of several unclosed particles (inset in Figure 1a) confirm the hollow structure further.
Figure 1.
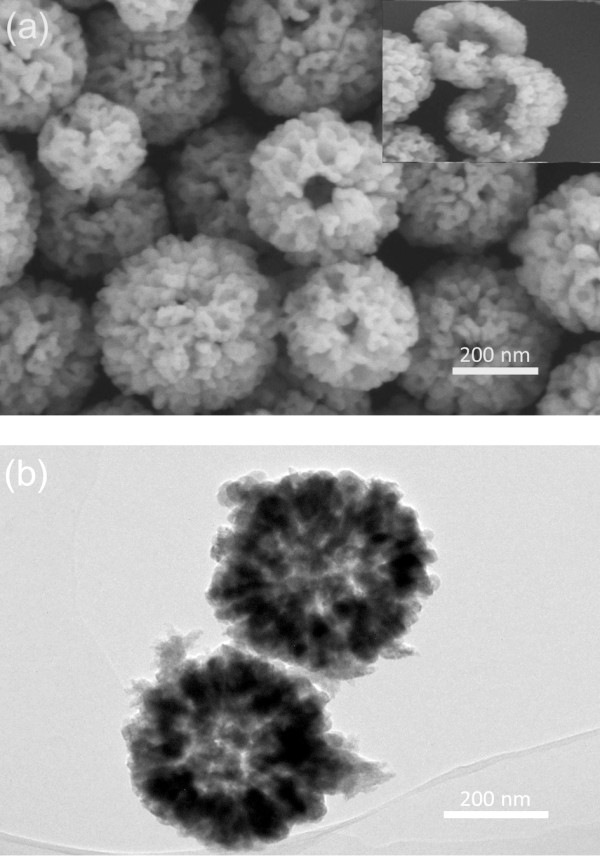
SEM (a) and TEM (b) images of the as-prepared Cu2O hollow porous microspheres. The inset in Figure 1a is an SEM image of several unclosed hollow spheres.
The typical XRD patterns of the as-synthesized hollow microspheres are shown in Figure 2. All the diffraction peaks of the samples are labeled and can be indexed very well according to the standard cubic phase Cu2O with a space group Pn3m (JCPDS file no. 05–0667). From the XRD patterns, no other characteristic diffraction peaks, such as CuO or Cu, can be detected, indicating that the pure Cu2O was obtained using this simple soft-template method at room temperature. The average crystalline grain size of the sample was calculated from the XRD patterns according to the Scherrer formula (Dhkl = kλ / βcosθ, where D is the average crystalline grain size, k is the Scherrer constant related to the shape and index (hkl) of the crystals, λ is the wavelength (0.154056 nm) of the X-ray, θ is the Blagg diffraction angle, and β is the full-width at half-maximum). The average crystalline grain size of the as-synthesized nanoparticle was estimated to be about 26 nm.
Figure 2.
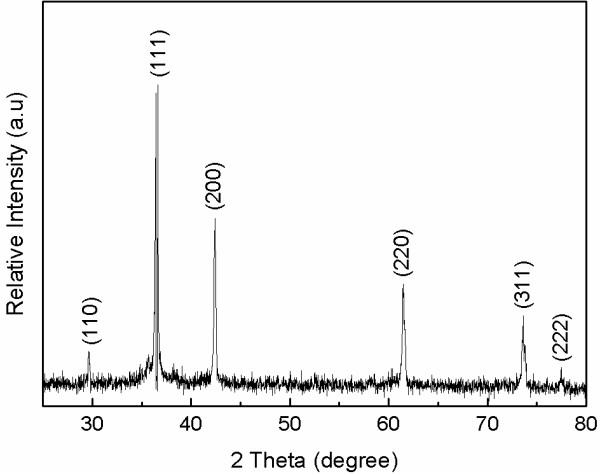
XRD pattern of the Cu2O hollow porous microspheres.
Optical absorption behavior is one of the very important fundamental properties in revealing the energy structures and applications in photocatalysis. Figure 3 shows the UV-visible absorbance spectra of the as-prepared hollow porous Cu2O spheres by ultrasonically dispersing in absolute ethanol. Two strong absorption peaks in the UV region are observed at wavelengths of about 434 and 325 nm for the as-synthesized samples. The broader one around 434 nm should attribute to the intrinsic band gap absorption, and the sharp one at 325 nm may result from the residual SDS absorption peak. The direct optical band gap energy (Eg) of the Cu2O microspheres can be calculated from the 434-nm absorption peak. The inset in Figure 3 shows the curve of (αEphoton)2 versus the photon energy Ephoton, where α and Ephoton (hν) are respectively the absorption coefficient and the discrete photon energy. The Eg was determined by extrapolating the linear portion of the curve to zero, and the calculated value of Eg is 2.22 eV, which is a little larger than 2.17 eV for bulk materials. This may be attributed to the size effects since the microspheres are composed of nanoscaled particles, according to the SEM and TEM results.
Figure 3.
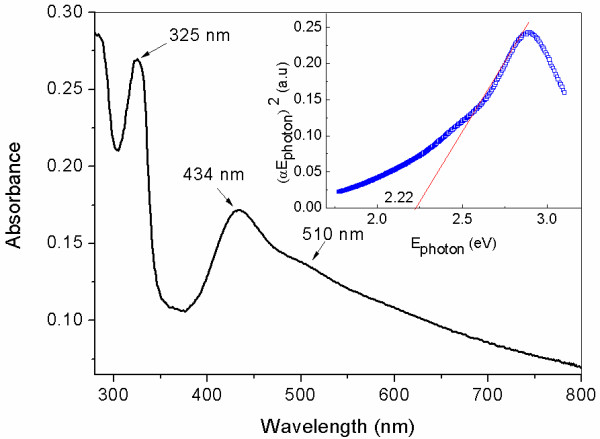
UV–vis absorbance spectra of as-prepared hollow porous Cu2O microspheres. The inset is the plot of (αEphoton)2 versus Ephoton to evaluate the band gap of Cu2O microspheres.
The photocatalytic activity of the Cu2O microspheres under visible light irradiations was also investigated. A cutoff filter was added under the Xe lamp to filter out the UV part (λ < 400 nm) to form a visible light source. The hollow porous Cu2O microspheres were dispersed in a MO aqueous solution and stirred for 10 min in the dark to establish absorption-desorption equilibrium. Then, the beaker containing this mixed solution was placed in the visible light source. An amount (3 ml) of the solution was removed from the beaker every 10 min to test the absorbance properties at 464 nm after filtrating the solid spheres. As shown in Figure 4, although the higher concentration initially degraded fast, two samples with different concentrations of 0.2 and 0.4 g/l were almost completely degraded in 1 h; especially for the sample with 0.2 g/l concentration, the degradation reached 80 % within 30 min. To confirm the results further, self-degradation experiment without hollow porous Cu2O microspheres was also performed, that is, only the MO solution was degraded under the same visible light source. Obviously, its degradation effect can be neglected. Compared with other reported results [35,36], hollow porous Cu2O microspheres possess higher degradation efficiency, that is, it has excellent visible light photocatalytic activity.
Figure 4.
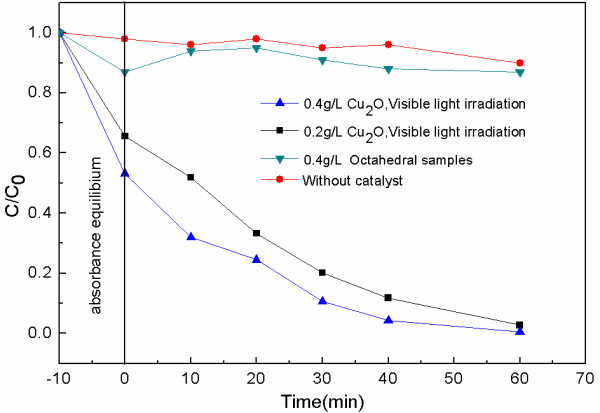
Photocatalytic activity of the Cu2O microspheres under visible light irradiations in different concentrates.
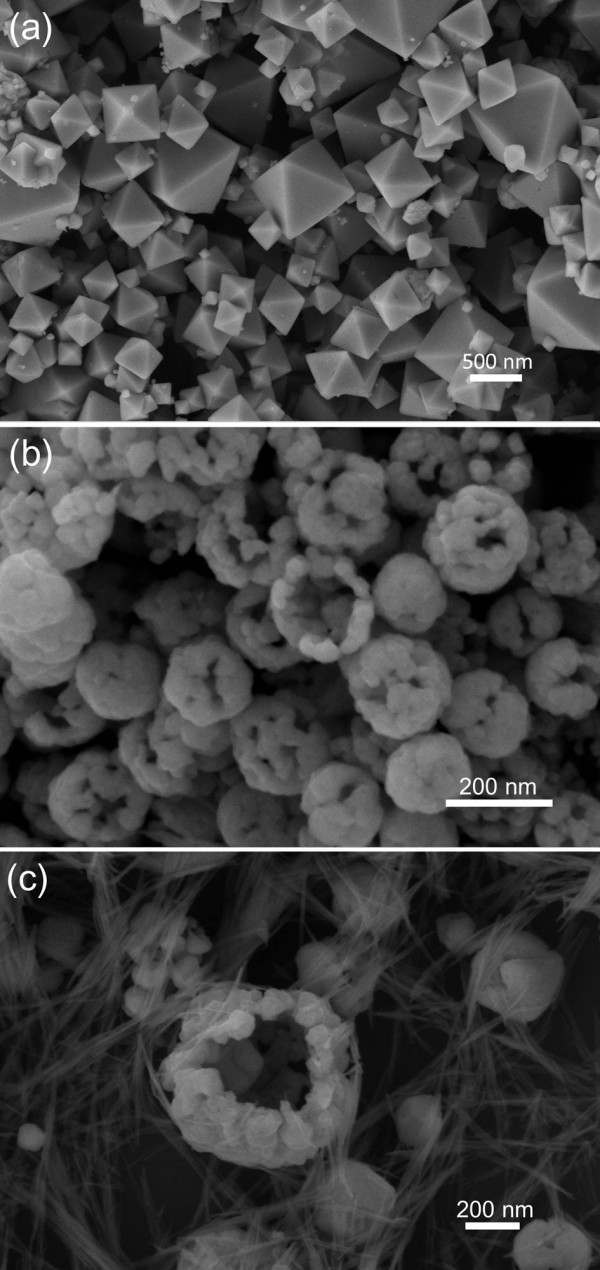
This higher degradation efficiency of hollow porous Cu2O microspheres under visible light is mainly attributed to its peculiar structure with large specific areas as well as its outstanding visible light optical absorption characteristics. A hollow porous structure can provide more surface contact and space to absorb the pollutant molecule to make the excited electron arrive more easily at the surface [37]. As a comparison, the photocatalytic property of Cu2O particles with smooth octahedral surface (the SEM image is shown in Figure 5a) was also investigated. As shown in the result in Figure 4, its degradation efficiency is much less than those of the hollow porous samples, which indicates that the hollow porous structure played an important role for its higher degradation efficiency.
Figure 5.
SEM images. (a) Products without SDS, (b) with ammonia amount increased from 0.04 to 0.08 ml, and (c) with NaOH amount increased from 0.15 to 0.3 ml.
In order to confirm the above explanations, experiment without SDS was also performed. As shown in Figure 5a, some well-crystallized particles in octahedral shapes were formed instead of hollow porous microspheres, which indicate the soft-template role of SDS in the reaction.
The effects of ammonia and NaOH amount were also investigated. When the ammonia amount was increased from 0.04 to 0.08 ml (see Figure 5b) or the NaOH amount was increased from 0.15 to 0.3 ml (see Figure 5c), the morphology of the products changed a lot, and the hollow structure became imperfect. Especially, there formed many small nanoparticles and nanowires at the third condition (see Figure 5c). Summarily, SDS and the amount of reagents all played very important roles in the synthesis of novel hollow porous Cu2O microspheres.
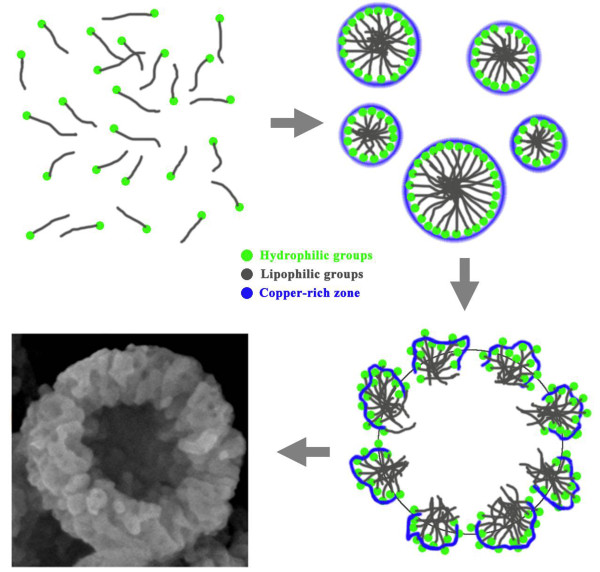
The proposed growth process of hollow porous microspheres may be understood by the schematic shown in Figure 6. The anionic surfactant SDS acts the role of soft template and is a stable micelle in the solution. The inside lipophilic groups intertwined together in aqueous solution, whereas the outside hydrophilic groups containing sulfates attract Cu2+ to form the copper-rich zone. When ammonia and NaOH were added to the solution, Cu(OH)2 precursor formed firstly at the outside of the micelle and, finally, was reduced to Cu2O by hydrazine hydrate, which is a common reduction agent. At the same time, N2 was generated in the redox reaction process and produced lots of bubbles. Under the effects of SDS and bubbles, both of which acted as soft templates, the hollow porous Cu2O microspheres would be formed finally. The related chemical reactions should be as follows:
Figure 6.
Growth schematic of hollow porous Cu2O microspheres.
| (1) |
| (2) |
| (3) |
Conclusions
We have successfully synthesized hollow porous Cu2O microspheres with high purity by using a soft-template method at room temperature. This material has excellent photocatalytic activity under visible light irradiation in the degradation of MO owing to its unique optical properties and special morphology. The band gap was calculated to be 2.22 eV from its UV-visible absorbance spectrum. It was also found that the SDS acted as the soft template, and the amounts of ammonia and NaOH had important effects on the morphology of the products. These Cu2O microspheres with hollow porous structures may be a good candidate of photocatalytic materials under visible light irradiation.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
YY performed the experiment and measured the SEM and TEM data. LYZ designed the experiments and wrote the manuscript. ZY helped in the technical support for the experiments. JW and NTH participated in the measurements. MCL provided useful suggestions. YFZ supervised all of the study and provided financial support. All authors read and approved the final manuscript.
Contributor Information
Yuan Yu, Email: yuyuan@sjtu.edu.cn.
Liying Zhang, Email: liyingzhang@sjtu.edu.cn.
Jian Wang, Email: wangjian09@sjtu.edu.cn.
Zhi Yang, Email: zhiyang@sjtu.edu.cn.
Mingce Long, Email: long_mc@sjtu.edu.cn.
Nantao Hu, Email: hunantao@sjtu.edu.cn.
Yafei Zhang, Email: yfzhang@sjtu.edu.cn.
Acknowledgments
This work is supported by the National High-Tech R & D Program of China (863, no. 2011AA050504), National Natural Science Foundation of China (no. 61006002), the U-M/SJTU Collaborative Research Program and the Analytical and Testing Center of SJTU, Shanghai Pujiang Program (no. 11PJD011), Natural Science Foundation of Shanghai (no. 09ZR1414800), and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning.
References
- Hagfeldt A, Gratzel M. Light-induced redox reactions in nanocrystalline systems. Chem Rev. 1995;95:49–68. doi: 10.1021/cr00033a003. [DOI] [Google Scholar]
- Ma C, Zhou Z, Wei H, Yang Z, Wang Z, Zhang Y. Rapid large-scale preparation of ZnO nanowires for photocatalytic application. Nanoscale Res Lett. 2011;6:536. doi: 10.1186/1556-276X-6-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Irie H, Fujishima A. TiO2 photocatalysis: a historical overview and future prospects. Jpn J Appl Phys. 2005;44:8269–8285. doi: 10.1143/JJAP.44.8269. [DOI] [Google Scholar]
- Wei H, Wang L, Li Z, Ni S, Zhao Q. Synthesis and photocatalytic activity of one-dimensional CdS@TiO2 core-shell heterostructures. Nano-Micro Lett. 2011;3:6–11. [Google Scholar]
- Zhao W, Fu W, Yang H, Tian C, Li M, Ding J, Zhang W, Zhou X-A, Zhao H, Li Y. Synthesis and photocatalytic activity of Fe-doped TiO2 supported on hollow glass microbeads. Nano-Micro Lett. 2011;3:20–24. [Google Scholar]
- Srikant V, Clarke DR. On the optical band gap of zinc oxide. J Appl Phys. 1998;83:5447–5451. doi: 10.1063/1.367375. [DOI] [Google Scholar]
- Lee H-S, Woo C-S, Youn B-K, Kim S-Y, Oh S-T, Sung Y-E, Lee H-I. Bandgap modulation of TiO2 and its effect on the activity in photocatalytic oxidation of 2-isopropyl-6-methyl-4-pyrimidinol. Top Catal. 2005;35:255–260. doi: 10.1007/s11244-005-3832-2. [DOI] [Google Scholar]
- Ishizuka S, Maruyama T, Akimoto K. Thin-film deposition of Cu2O by reactive radio-frequency magnetron sputtering. Jpn J Appl Phys. 2000;39:L786–L788. doi: 10.1143/JJAP.39.L786. [DOI] [Google Scholar]
- Zhang J, Liu J, Peng Q, Wang X, Li Y. Nearly monodisperse Cu2O and CuO nanospheres: preparation and applications for sensitive gas sensors. Chem Mater. 2006;18:867–871. doi: 10.1021/cm052256f. [DOI] [Google Scholar]
- Wanga R-C, Lin H-Y. Simple fabrication and improved photoresponse of ZnO–Cu2O core–shell heterojunction nanorod arrays. Sens Actuators B. 2010;149:94–97. doi: 10.1016/j.snb.2010.06.025. [DOI] [Google Scholar]
- Liu W, Chen G, He G, Zhang W. Synthesis of starfish-like Cu2O nanocrystals through γ-irradiation and their application in lithium-ion batteries. J Nanopart Res. 2011;13:2705–2713. doi: 10.1007/s11051-011-0422-z. [DOI] [Google Scholar]
- Das K, De SK. Optical and photoconductivity studies of Cu2O nanowires synthesized by solvothermal method. J Lumin. 2009;129:1015–1022. doi: 10.1016/j.jlumin.2009.04.019. [DOI] [Google Scholar]
- Hafez M, Al-Marzouki F, Mahmoud WE. Single crystalline quasi aligned one dimensional P-type Cu2O nanowire for improving Schottky barrier characteristics. Mater Lett. 2011;65:1868–1870. doi: 10.1016/j.matlet.2011.03.093. [DOI] [Google Scholar]
- Yue Y, Chen M, Yang J, Zhang L. Stress-induced growth of well-aligned Cu2O nanowire arrays and their photovoltaic effect. Scripta Mater. 2012;66:81–84. doi: 10.1016/j.scriptamat.2011.09.041. [DOI] [Google Scholar]
- Liu Y, Zhou H, Li J, Chen H, Li D, Zhou B, Cai W. Enhanced photoelectrochemical properties of Cu2O loaded short TiO2 nanotube array electrode prepared by sonoelectrochemical deposition. Nano-Micro Lett. 2010;2:277–284. [Google Scholar]
- Hara M, Kondo T, Komoda M, Ikeda S, Shinohara K, Tanaka A, Kondoa JN, Domen K. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chem Commun. 1998;3:357–358. [Google Scholar]
- Jimenez-Cadena G, Comini E, Ferroni M, Sberveglieri G. Synthesis of Cu2O bi-pyramids by reduction of Cu(OH)2 in solution. Mater Lett. 2010;64:469–471. doi: 10.1016/j.matlet.2009.11.051. [DOI] [Google Scholar]
- Jiasheng X, Xue D. Five branching growth patterns in the cubic crystal system: A direct observation of cuprous oxide microcrystals. Acta Mater. 2007;55:2397–2406. doi: 10.1016/j.actamat.2006.11.032. [DOI] [Google Scholar]
- Chang Y, Zeng H. Manipulative synthesis of multipod framework for self-organization and self-amplification of Cu2O microcrystals. Cryst Growth Des. 2004;4:273–278. doi: 10.1021/cg034146w. [DOI] [Google Scholar]
- Tan Y, Xue X, Peng Q, Zhao H, Wang T, Li Y. Controllable fabrication and electrical performance of single crystalline Cu2O nanowires with high aspect ratios. Nano Lett. 2007;7:3723–3728. doi: 10.1021/nl0721259. [DOI] [Google Scholar]
- Liu X, Renzhi H, Xiong S, Liu Y, Chai L, Bao K, Qian Y. Well-aligned Cu2O nanowire arrays prepared by an ethylene glycol-reduced process. Mater Chem Phys. 2009;114:213–216. doi: 10.1016/j.matchemphys.2008.09.009. [DOI] [Google Scholar]
- Zhang L, Wang H. Cuprous oxide nanoshells with geometrically tunable optical properties. ACS Nano. 2011;5:3257–3267. doi: 10.1021/nn200386n. [DOI] [PubMed] [Google Scholar]
- Yang Z, Chiang C-K, Chang H-T. Synthesis of fluorescent and photovoltaic Cu2O nanocubes. Nanotechnology. 2008;19:025604. doi: 10.1088/0957-4484/19/02/025604. [DOI] [PubMed] [Google Scholar]
- Shi J, Li J, Huang X, Tan Y. Synthesis and enhanced photocatalytic activity of regularly shaped Cu2O nanowire polyhedra. Nano Res. 2011;4:448–459. doi: 10.1007/s12274-011-0101-5. [DOI] [Google Scholar]
- Wang X, Liu Z, Qian Y. Synthesis, transformation to octahedron-like crystals, and gas-sensing property of six-horned nanospheres of Cu2O. Cryst Res Technol. 2011;46:967–972. [Google Scholar]
- Conghua L, Qi L, Yang J, Wang X, Zhang D, Xie J, Ma J. One-pot synthesis of octahedral Cu2O nanocages via a catalytic solution route. Adv Mater. 2005;17:2562–2567. doi: 10.1002/adma.200501128. [DOI] [Google Scholar]
- Zhao X, Bao Z, Sun C, Xue D. Polymorphology formation of Cu2O: a microscopic understanding of single crystal growth from both thermodynamic and kinetic models. J Cryst Growth. 2009;311:711–715. doi: 10.1016/j.jcrysgro.2008.09.081. [DOI] [Google Scholar]
- Xu L, Chen X, Wu Y, Chen C, Li W, Pan W, Wang Y. Solution-phase synthesis of single-crystal hollow Cu2O spheres with nanoholes. Nanotechnology. 2006;17:1501–1505. doi: 10.1088/0957-4484/17/5/056. [DOI] [Google Scholar]
- Gou L, Murphy CJ. Controlling the size of Cu2O nanocubes from 200 to 25 nm. J Mater Chem. 2004;14:735–738. doi: 10.1039/b311625e. [DOI] [Google Scholar]
- Chen S, Chen X, Xue Z, Li L, You X. Solvothermal preparation of Cu2O crystalline particles. J Cryst Growth. 2002;246:169–175. doi: 10.1016/S0022-0248(02)01902-4. [DOI] [Google Scholar]
- Liang X, Gao L, Yang S, Sun J. Facile synthesis and shape evolution of single-crystal cuprous oxide. Adv Mater. 2009;21:2068–2071. doi: 10.1002/adma.200802783. [DOI] [Google Scholar]
- Li J, Liu L, Ying Y, Tang Y, Li H, Feipeng D. Preparation of highly photocatalytic active nano-size TiO2–Cu2O particle composites with a novel electrochemical method. Electrochem Commun. 2004;6:940–943. doi: 10.1016/j.elecom.2004.06.008. [DOI] [Google Scholar]
- Haolan X, Wang W, Zhu W. Shape evolution and size-controllable synthesis of Cu2O octahedra and their morphology-dependent photocatalytic properties. J Phys Chem B. 2006;110:13829–13834. doi: 10.1021/jp061934y. [DOI] [PubMed] [Google Scholar]
- Peng Q, Dong Y, Li Y. ZnSe semiconductor hollow microspheres. Angew Chem Int Ed. 2003;42:3027–3030. doi: 10.1002/anie.200250695. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Deng B, Zhang T, Gao D, Anwu X. Shape effects of Cu2O polyhedral microcrystals on photocatalytic activity. J Phys Chem C. 2010;114:5073–5079. doi: 10.1021/jp9110037. [DOI] [Google Scholar]
- Ahmed A, Gajbhiyea NS, Joshi AG. Shape controlled synthesis and characterization of Cu2O nanostructures assisted by composite surfactants system. Mater Chem Phys. 2011;129:740–745. doi: 10.1016/j.matchemphys.2011.04.042. [DOI] [Google Scholar]
- Duan X, Gao R, Zhang Y, Jian Z. Synthesis of sea urchin-like cuprous oxide with hollow glass microspheres as cores and its preliminary application as a photocatalyst. Mater Lett. 2011;65:3625–3628. doi: 10.1016/j.matlet.2011.07.104. [DOI] [Google Scholar]