Abstract
Background
The levels and immunohistochemical localization of muscle carbonic anhydrase III (CA-III) in healthy chickens and in muscular dystrophia affected (DA) chickens show that the muscles of diseased animal undergo a progressive increase of enzyme activity.
Methods
An enzyme-linked immunoassay was used to assess the CA-III levels in the muscles and other tissues from eight normal White Leghorn chickens and in two chickens with muscular dystrophy. Immunohistochemical localization of the enzyme in the muscles of these animals was also determined.
Results
The levels of CA-III in the tensor fasciae latae and the superficial pectoral muscles of the DA chickens were higher than the level in normal chickens. The concentrations of CA-III in erythrocytes and plasma from diseased chickens were approximately 15-fold and 1.4-fold higher than in the normal chickens, respectively. In the superficial pectoral and the tensor fasciae latae muscles of diseased chickens, the numbers of strongly stained and weakly stained fibers were greater than that in the normal chickens.
Conclusion
The levels of CA-III in the superficial pectoral muscle, the tensor fasciae latae muscle, plasma and erythrocytes from the chickens with muscular dystrophy were higher than found in normal chickens.
Keywords: Chicken, carbonic anhydrase III; Muscular dystrophy; Avian disease; ELISA; Immunohistochemistry
Background
Avian muscular dystrophy is inherited as a simple autosomal recessive defect [1]. In dystrophia affected (DA) chickens, the fast-twitch pectoralis superficialis muscle undergoes a progressive loss of function. Along with the muscular weakness, histopathological changes [2], ultrastructual abnormalities [3] and large elevations in certain serum enzymes that escape from the affected muscles occur [4]. These changes are similar to those found in human Duchenne dystrophy. Hoffman et al.[5] reported that dystrophin, a large membrane-associated cytoskeletal protein, was absent in the affected muscles of Duchenne dystrophy. However, dystrophin is present in both normal and DA (line 413) chicken muscle indicating that this protein cannot be implicated in the pathogenesis of avian muscle dystrophy [6]. Although the DA chicken has been established as an animal model of muscular dystrophy in humans, the mechanism leading to muscle cell degeneration remains unknown.
Mizuno [7] and Park et al.[8] reported that superoxide dismutase activity in the superficial pectoral muscle of DA chickens was significantly elevated. Chown et al.[9] reported that the carbonic anhydrase III (CA-III) level in the pectoral muscle of DA chickens was approximately three fold higher than in normal birds. CA-III may have a role in scavenging oxygen radicals and thereby protecting cells from oxidative damage [10,11]. However, the immunohistochemical localization of CA-III in the muscles of DA chicken has not been reported.
The aim of the present study was to determine the concentrations and localization of CA-III in normal and DA chickens.
Methods
Animals and tissue samples
Normal male White Leghorn (WL) chickens, (Line M) 59 weeks old (n = 5) (Nisseiken Co., Ltd., Tokyo, Japan) and normal female WL chickens (LOHMAN LSL-LITE) 25 weeks old (n = 3) (Isogaya Yokeien, Tochigi, Japan) were used. Two male chickens with muscular dystrophy (A and B: 16 weeks old; New Hampshire line 413) were provided by Nagoya University Avian Bioscience Research Center (Nagoya, Aichi, Japan).
All experiments were performed according to the guidelines of the Laboratory Animal Care Committee of Azabu University and complied with the Japanese Animal Welfare Guide. The animals were euthanized by an overdose of pentobarbital (Nembutal®, Dainippon Sumitomo Pharma, Osaka, Japan) at the end of the study.
Sampling
Before euthanasia, lithium heparin treated blood samples were taken and centrifuged at 4500 × g for 15 min at 4°C. The erythrocytes obtained were lysed with an equal volume of distilled water and centrifuged at 27000 × g for 30 min at 4°C. Hemolysate and plasma samples were stored at −20°C until analyzed. Hemolysate samples (0.1 mL) diluted from 1:4,000 to 1:16,000 and plasma samples diluted from 1:5 to 1:10 in 50 mM Tris–HCl (pH 7.5) containing 0.3% bovine serum albumin (BSA), 0.9% NaCl, 0.01% thimerosal, and 10 mM EDTA (buffer A) were subjected to duplicate ELISA.
Samples of the superficial pectoral, the tensor fasciae latae and the obliquus externus abdominis muscles, and of the kidneys, liver, lung and cardiac muscles taken at necropsy and one gram of each of these tissues were homogenized with 1 volume of 0.01 M Tris–HCl (pH 8.0) and then centrifuged at 4°C for 30 min at 27000 × g. The soluble fractions were used for analysis. Also, samples of the three muscles were immediately fixed in neutralized 10% formalin and Bouin’s solution and later dehydrated with a graded series of alcohols, cleared with xylene and then embedded in paraffin wax blocks. Four μm sections were used for immunohistochemistry.
Hemoglobin assay
The hemoglobin concentrations in the hemolysates were measured by the sodium lauryl sulfate-hemoglobin method using a hemoglobin B test (Wako Pure Chemical Industries Ltd., Tokyo, Japan).
Electrophoretic procedures and western blotting
Western blotting was performed as previously described [12]. Briefly, adequate volumes of the hemolysate and purified chicken CA-III were separated by using the PhastSystem (Pharmacia Biotech, Uppsala, Sweden) and transferred to Immobilon PVDF transfer membrane (Millipore Corp, Bedford, Mass, USA) by means of a commercially available transfer system. The buffer used in the electrophoretic transfer contained 25 mM Tris (pH 8.3), 192 mM glycine, 0.1% sodium dodecyl sulfate (SDS), and 15% methanol. CA-III was detected using rabbit antiserum to chicken CA-III previously produced in our laboratory [13]. This antiserum in 5 mL of buffer A was diluted to 1:2000. After incubation with the primary antibody, the membranes were washed in 0.15 M phosphate-buffered saline (PBS) containing 0.05% Tween (PBS-Tween) and then incubated with 1:4,000 peroxidase-conjugated goat anti-rabbit IgG (Kirkegaard & Perry Laboratories Inc., Gaithersburg, MD, USA) in 5 mL of buffer A. The membranes were washed again with PBS-Tween and then incubated for approximately 5 min in pH 7.6, 0.05 M Tris–HCl containing 0.02% H2O2 and 0.2 mM 3.3’ diaminobentidine-tetrahydrochloride (DAB – 4HCl).
Determination of CA-III levels
The CA-III concentrations in several chicken tissues were determined using the previously described competitive ELISA method [13]. Briefly, a flat-bottom micro-ELISA plate (Maxisorp Nunc-Immuno Plate; Nunc. Roskilde, Denmark) was incubated for 16 h at 4°C in 0.1 mL of pH 9.6, 0.1 M NaHCO3 containing 0.03 mg/mL of the antibody to chicken CA-III. The plates were then washed 3 times with 0.3 mL of PBS and blocked at 23°C for 30 min with 0.2 mL of 0.5% BSA in 0.05 M Tris–HCl (pH 8.0). Each well was then washed 3 times with 0.3 mL of PBS-Tween. Duplicate ELISAs were performed using purified CA-III (6–800 ng/mL), biotinylated chicken CA-III and tissue samples were diluted with buffer A, respectively. Tissue extracts (0.1 mL) diluted 50–5,000 with buffer A were subjected to immunoassay in duplicate. The biotinylated CA-III was allowed to compete with the standard CA-III or the tissues samples and incubated for 16 h at 4°C. Each well was then washed 3 times with PBS-Tween, and 0.1 mL/well of avidin and biotinylated horseradish peroxidase complex (ABC reagent, Wako Pure Chemical Industries Ltd, Tokyo, Japan) was added. After 30 min, each well was washed 3 times with PBS-Tween. Peroxidase activity was measured after the addition of 0.1 mL of the ABTS microwell peroxidase substrate (Kirkegaard & Perry Laboratories Inc,). The reaction was terminated after 10 min by the addition 0.1 mL of 1% SDS, and the absorbance at 405 nm was recorded on an automatic ELISA (SH-1000; Corona Electric Co., Ltd., Ibaraki, Japan).
The concentrations of CA-II in the erythrocytes from the chickens with muscular dystrophy were determined by the competitive ELISA method as previously reported [14].
Immunohistochemistry
Endogenous peroxidase activity was blocked in the deparaffinized, rehydrated tissue sections by using 0.3% H2O2 in methanol, followed by immersion in normal goat serum (2% in PBS) for 20 min to block the FC receptors. Monospecific antisera (diluted 1:2,000) against chicken CA-III localized to the respective isozymes in a 1-h–long primary reaction were used. Antibody binding was visualized using the Vectastain Elite avidin-biotin-peroxidase complex kit (ABC-POD reagent kit; Vector, Burlingame, CA., USA) and DAB – 4HCl according to the manufacturer’s protocol. Samples were observed and photographed under a light microscope; the cell count was determined by densitometry. The percentage of fiber type composition in the muscle samples of the normal and DA chickens was calculated from the immunohistochemical localization of CA-III.
Results
Western blotting analysis
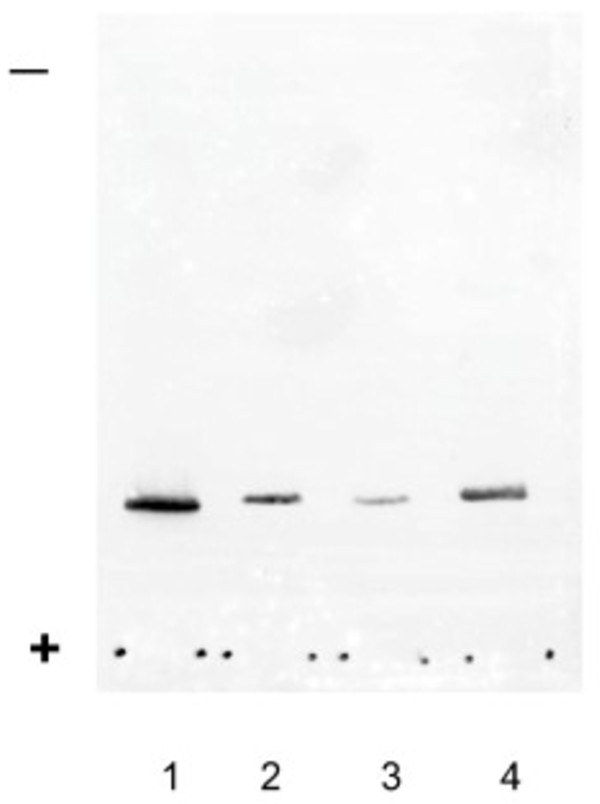
Figure 1 shows a single band on the western blot probed with anti-chicken CA-III serum from the extracts of the superficial pectoral and tensor fasciae latae muscles, and purified chicken CA-III. The molecular weight of CA-III in the extracts from the normal WL-chickens and those with muscular dystrophy were similar to that of the purified chicken CA-III. The western blot of Figure 2 shows that CA-III was present in the hemolysates of DA, female WL, male WL chickens. The molecular weight of these bands was approximately 28,000.
Figure 1.
Western blots of the muscle extracts from normal and dystrophic chickens separated by SDS-PAGE and analyzed using rabbit anti-chicken CA-III antiserum. Lane 1, extract of the superficial pectoral muscle from a dystrophic chicken; lane 2, extract of the superficial pectoral muscle from a normal chicken; lane 3, extract of the tensor fasciae latae muscle from a normal chicken; lane 4, extract of the tensor fasciae latae muscle from a dystrophic chicken; and lane 5, purified chicken CA-III.
Figure 2.
Western blot of the hemolysate from normal and dystrophic chickens separated by SDS-PAGE. Lane 1, purified chicken CA-III; lane 2, hemolysate from a dystrophic chicken; lane 3, hemolysate from a normal male chicken; and lane 4, hemolysate from a normal female chicken.
CA-III and CA-II levels
The concentrations of CA-III in the tissue samples from normal and DA chickens determined by competitive ELISA assays are shown in Table 1. For the normal chickens, the highest content of CA-III was found in the obliquus externus abdominis muscle. The levels of CA-III in the obliquus externus abdominis muscle did not differ between normal and DA chickens. The levels of CA-III in the tensor fasciae latae muscle of the DA chickens was approximately 3-fold higher than that of normal animals and approximately 27-fold higher in the superficial pectoral muscle of the DA chickens. The CA-III levels in kidney and liver and in cardiac muscles were less than in skeletal muscles. The CA-III levels in the liver and lung, and in cardiac muscles of DA chickens were approximately 3–7 fold higher than in normal chickens. The levels of CA-III in the kidneys of the DA chickens were approximately one-third of that seen in the controls.
Table 1.
Concentrations of carbonic anhydrase III in the organ samples from normal male and muscular dystrophic chickens
| Samples |
normal male chicken (n = 5) |
male dystrophic chicken |
dystrophy/normal |
|
|---|---|---|---|---|
| A | B | |||
| |
(μg/g of wet) |
(μg/g of wet) |
(μg/g of wet) |
|
| Obliquus externus abdominis muscle |
99 ± 49 |
81.8 |
62.2 |
0.6 |
| Tensor fasciae latae muscle |
61 ± 45 |
207.7 |
175.9 |
3.1 |
| Superficial pectoral muscle |
8.4 ± 7.8 |
274.9 |
173.8 |
26.6 |
| Kidney |
3.2 ± 3.8 |
1.4 |
1.1 |
0.4 |
| Liver |
0.2 ± 1.1 |
1.3 |
1.6 |
7.2 |
| Lung |
1.0 ± 0.4 |
5.3 |
4.2 |
4.8 |
| Cardiac muscle |
0.2 ± 0.1 |
0.6 |
0.6 |
3 |
| |
(μg/g of Hb) |
(μg/g of Hb) |
(μg/g of Hb) |
|
| Erythrocyte |
1.9 ± 0.8a) |
27.5 |
28.7 |
15 |
| |
(ng/mL) |
(ng/mL) |
(ng/mL) |
|
| Plasma | 105 ± 37a) | 149 | 144 | 1.4 |
The levels of CA-III in the liver and the superficial pectoral muscle of normal female chickens were 0.9 ± 1.0 and 21 ± 5.3 μg/g wet tissues, respectively, which were significantly higher than in normal males (P < 0.05). CA-III levels in other tissues did not differ between the sex of normal chickens.
The concentrations of CA-III in the erythrocytes of the chickens with muscular dystrophy were approximately 15-fold higher than those of the controls. Moreover, the concentrations of CA-III in plasma of DA chickens were approximately 1.4-fold higher than those in normal chickens. The concentrations of CA-II in the erythrocytes of the DA chickens No. A and B were 93.6 and 119.6 mg/g of Hb, respectively, and 113.6 ± 32.3 mg/g of Hb in erythrocytes of normal male chickens (17 weeks old) [14].
Immunohistochemical staining of CA-III
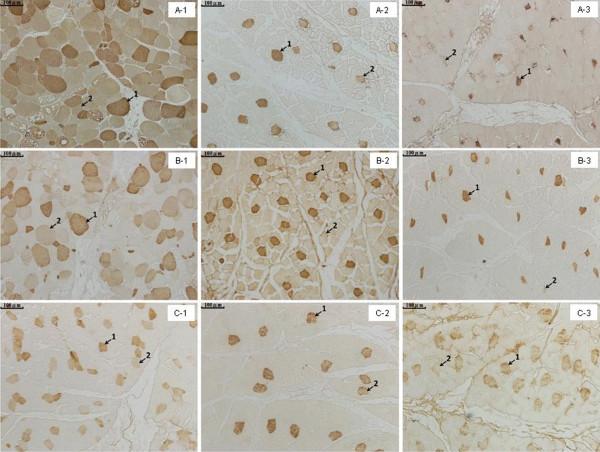
The immunohistochemical reaction of the CA-III antiserum observed in the muscle fibers of the normal and DA chickens are shown in Figure 3. The shape of these fibers in the CA-III-stained normal chicken muscle was a polygon (Figure 3 C-1–3), whereas they were hypertrophied and pathologically rounded in the fibers of the superficial pectoral muscle from DA chickens (Figure 3 A-1, B-1).
Figure 3.
Immunohistochemical localization of CA-III in the muscles from normal and muscular dystrophic chickens.A-1, the superficial pectoral muscle of a dystrophic chicken A; A-2, the tensor fasciae latae muscle of a dystrophic chicken A; A-3, the obliquus externus abdominis muscle of a dystrophic chicken A; B-1, the superficial pectoral muscle of a dystrophic chicken B; B-2, the tensor fasciae latae muscle of a dystrophic chicken B; B-3, the obliquus externus abdominis muscle of a dystrophic chicken B; C-1, the superficial pectoral muscle of a normal chicken; C-2, the tensor fasciae latae muscle of a normal chicken; and C-3, the obliquus externus abdominis muscle of a normal chicken. Arrow 1 indicates strongly stained CA-III. Arrow 2 indicates weakly stained CA-III. Scale bars: 100 μm.
Vacuolar degeneration was also observed in the superficial pectoral muscles of the DA chickens, while such lesions were not observed in the muscle fibers from the tensor fasciae latae muscle and the obliquus externus abdominis muscle.
The muscle fibers were classified into 3 types depending on the immunohistochemical staining intensity of CA-III; strongly, weakly, and not stained. Table 2 shows the proportions of the 3 kinds of staining patterns in the muscles of normal and DA chickens. In the superficial pectoral muscles of the DA animals, the numbers of strongly stained fibers were about 12- and 9-fold greater than found in normal chickens, respectively; while the numbers of weakly stained fibers were about 3- and 2-times greater. In the tensor fasciae latae muscles of the DA chickens, the numbers of strongly stained fibers were about 2- and 4-times higher that of normal chickens, and the numbers of weakly stained fibers were about 2-and 4-times higher. In the obliquus externus abdominis muscle of the DA chickens, the numbers of strongly stained fibers were about 2- and 3-times greater than that of normal chickens. The numbers of weakly stained fibers were about 0.6- and 0.4-times less than that of normal chickens.
Table 2.
The number and classification of the muscle fibers from normal and muscular dystrophic chickens based on the intensity of CA-III immunostaining
| |
|
Stain reactions of CA-III |
|||
|---|---|---|---|---|---|
| Muscle | Chicken | Strong | Weak | Negative | Total cell count |
| Superficial pectoral muscle |
Dystrophy |
|
|
|
|
| |
A |
246 |
253 |
501 |
1,000 |
| |
B |
177 |
170 |
653 |
1,000 |
| |
Normal |
20 |
78 |
902 |
1,000 |
| Tensor fasciae latae muscle |
Dystrophy |
|
|
|
|
| |
A |
52 |
72 |
876 |
1,000 |
| |
B |
104 |
183 |
713 |
1,000 |
| |
Normal |
24 |
48 |
928 |
1,000 |
| Obliquus externus abdominis muscle |
Dystrophy |
|
|
|
|
| |
A |
61 |
62 |
877 |
1,000 |
| |
B |
103 |
35 |
862 |
1,000 |
| Normal | 38 | 94 | 868 | 1,000 | |
a): Nishita et al.[10].
Discussion
CA-III levels in chicken tissue
The levels of CA-III in the liver of DA chickens were approximately 7-fold higher than that of normal chickens (males and females). In the lung and cardiac muscle of DA chickens, the levels of CA-III were 3–5- times higher than in normal chickens, but the kidney level of CA-III was approximately one-third of that present in normal individuals and vastly increased compared to other organs analyzed.
Chown et al.[9] reported that the concentration of CA-III in adult chicken pectoral muscle was 2–4 μg/g wet tissue and 60–500 μg/g wet tissue in the leg (red muscles). Mizuno [7] reported that Mn SOD activities in the superficial pectoral muscles of DA chickens declined with age as in normal chickens, whereas Cu-Zn SOD activities increased until 4 weeks of age. The activity of this enzyme was still twice as high as that of the normal birds at 16 weeks of age. In control chickens, both Cu-Zn SOD and Mn SOD activities declined with age. Increased activities of SOD during the early stages of development suggest an increased turnover of active oxygen species caused by early onset of avian muscular dystrophy. These biochemical changes are considered to represent altered metabolism within muscle cells and not to invasion by phagocytes or other cells.
Mizuno [7,15] also suggested that increased levels of catalase, glutathione peroxidase, and glutathione reductase in DA chickens indicates increased capacity for oxygen-free radical turnover within the muscle cells and that oxygen-free radicals and related activated oxygen species may play a role in inducing cellular damage.
Gailly et al. [16] reported that exposure of human kidney proximal tubule immortalized HK-2 cells, an established model for normal human PT cells, to 1 mM H2O2 induced a significant increase in CA-III mRNA expression. In the present study, the elevation of CA-III in the muscle fibers and other tissues from DA chickens is probably induced by an increase in the reactive oxygen species present. Comparatively, the obliquus externus abdominis muscles of normal and DA chickens, which contain high levels of CA-III, are probably not affected by the increased level of reactive oxygen species. Therefore, we propose that chicken CA-III is useful biomarker of radical-induced damage.
Immunohistochemical staining of CA-III
Barnard et al. [17] showed 5 major fiber types in chicken skeletal muscles namely, I, IIA, IIB, IIIA, and IIIB. Fibers of types I, IIA, and IIB in the pectoral muscle of 50–80 day old normal chickens composed 0%, <1%, and >99% of the tissue. Although the localizations of CA-III into specific fiber types were not made in this study, about 2% of fibers in the superficial pectoral muscles of normal chickens reacted strongly and 8% weakly for CA-III. It is suggested that some fibers of types IIA and IIB contained CA-III. Further, Barnard et al. [17] noted that muscular dystrophy induced fiber composition changes in a characteristic manner. Fibers of types I, IIA, and IIB in the pectoral muscles from 50–80 day old DA chickens composed 0%, <2%–5%, and >90%–95% of the tissue. Pathological changes within the fibers occur selectively in the type IIB fibers. The minority type IIA fibers become slightly hypertrophied and after several weeks become pathologically rounded in DA chickens. The present study showed that the type I fibers show no pathological changes in the DA chickens, at least for several months.
In the present study, CA-III in the superficial pectoral muscle from DA chickens had a strong or weak immunohistochemical staining intensity at an equal frequency (21%). We suggest that some of the type IIB fibers in the DA chickens synthesized CA-III. The number of muscle fibers reacting strongly with anti-CA-III in the superficial pectoral muscles was about 12-times that in normal chickens. The muscle fibers stained with anti-CA-III in the superficial pectoral muscles of DA chickens were hypertrophied and became pathologically rounded with time. We, therefore, suggest that fiber types IIA and IIB contain CA-III in the superficial pectoral muscles of DA chickens.
The number of fibers in the tensor fasciae latae muscles and the obliquus externus abdominis muscles of the DA chickens staining strongly with anti-CA-III were about 3- and 2-times as numerous as in normal chickens, respectively. However, pathological changes within the fibers were not observed.
Levels of CA-III and CA-II in plasma and erythrocytes
CA-III is relatively specific for skeletal muscle and should be useful as a diagnostic marker for various diseases [18,19]. Mokuno et al.[20] determined the serum CA-III levels in 143 patients with 4 types of progressive muscular dystrophy (limb-girdle dystrophy (LG), duchenne muscular dystrophy (DMD), facioscapulohumeral dystrophy (FSH), and congenital dystrophy). The serum CA-III levels in the LG, DMD, FSH, and congenital dystrophy patients were 0.8-, 2.5-, 0.3-, and 1.7-times that of normal individuals, respectively. In the present study, the plasma CA-III levels of 2 male muscular DA chickens were about 1.5-times that of the controls, as previously determined [13].
To our knowledge, no study on the CA-III and CA-II levels in the erythrocytes of human patients and chickens with muscular dystrophy has been published. In the present study, the CA-III levels in the erythrocytes of DA male chickens were about 15-times higher than those in normal male chickens, and 4-times higher than those in normal females [13]. As described above, Mizuno [7] and Park et al. [8] reported that SOD activities in the superficial pectoral muscle were significantly elevated in DA chickens. These findings suggest an increased turnover of reactive oxygen species such as superoxide anion or hydrogen peroxide in cases of muscular dystrophy.
Georgieva et al. [21] suggested that the compensatory elevation of erythrocyte catalase levels in chickens with alimentary muscular dystrophy was due to impaired antioxidant status following the oxidative and ecological stress produced by the disease.
In the present study, elevation of the CA-III level in the erythrocytes of DA chickens was probably induced by an increase in reactive oxygen species. However, to confirm this, determination of CA-III levels in the plasma and erythrocytes of DA chickens will be required. Nevertheless, our results suggest that chicken CA-III is a likely biomarker of radical-induced damage.
The erythrocyte CA-II levels in male DA chickens were similar to those in normal male and females [14]. These data suggest that synthesis of CA-II in chicken erythrocytes is not affected by reactive oxygen species.
Conclusions
The levels of CA-III in the superficial pectoral, tensor fasciae latae muscles, and the plasma and erythrocytes from chickens with muscular dystrophy were higher than those in the normal chickens. The increase of CA-III levels in the muscles was visualized by CA-III immunohistochemistry showing an increase in both strongly and weakly stained fibers. These results demonstrate that CA-III is an effective marker for muscular dystrophy in the chicken; this finding also has potential for applications in other animals and in humans.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TN carried out the study design and performed the data analyses and also made the draft of the manuscript. DY and NI collected samples, processing and carried out the calculation of data. KO performed the data analyses and helped to draft the manuscript. KA contributed to the study design, the analyses of data and the writing of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Toshiho Nishita, Email: nishida@azabu-u.ac.jp.
Daisuke Yorifuji, Email: yorifuji-da806@pref.miyagi.jp.
Kensuke Orito, Email: oritok@azabu-u.ac.jp.
Nobutsune Ichihara, Email: ichihara@azabu-u.ac.jp.
Kazuyoshi Arishima, Email: arishima@azabu-u.ac.jp.
Acknowledgements
We wish to thank Professor, PhD Yoichi Matsuda, Nagoya University Avian Bioscience Research Center for providing the muscular dystrophy affected chickens.
References
- Asmundson VS, Julian LM. Inherited muscle abnormality in the domestic fowl. J Hered. 1956;47:248–252. [Google Scholar]
- Pizzey JA, Barnard EA. Structural change in muscles of the dystrophic chicken. II. Progression of the histopathology in the pectoralis muscle. Neuropathol Appl Neurobiol. 1983;9:149–164. doi: 10.1111/j.1365-2990.1983.tb00332.x. [DOI] [PubMed] [Google Scholar]
- Libelius R, Jirmanová I, Lundquist I, Thesleff S, Barnard EA. T-tubule endocytosis in dystrophic chicken muscle and its relation to muscle fiber degeneration. Acta Neuropathol. 1979;48:31–38. doi: 10.1007/BF00691788. [DOI] [PubMed] [Google Scholar]
- Liu PK, Barnard EA, Barnard PJ. Blood plasma pyruvate kinase as a marker of muscular dystrophy. Properties in dystrophic chickens and hamsters. Exp Neurol. 1980;67:581–600. doi: 10.1016/0014-4886(80)90128-4. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Hudecki MS, Rosenberg PA, Pollina CM, Kunkel LM. Cell and fiber-type distribution of dystrophin. Neuron. 1988;1:411–420. doi: 10.1016/0896-6273(88)90191-2. [DOI] [PubMed] [Google Scholar]
- Mizuno Y. Superoxide dismutase activity in early stages of development in normal and dystrophic chickens. Life Sci. 1984;34:909–914. doi: 10.1016/0024-3205(84)90294-7. [DOI] [PubMed] [Google Scholar]
- Park JH, Hill EJ, Chou TH, LeQuire V, Roelofs R, Park CR. Mechanism of action of penicillamine in the treatment of avian muscular dystrophy. Ann N Y Acad Sci. 1979;317:356–369. doi: 10.1111/j.1749-6632.1979.tb56548.x. [DOI] [PubMed] [Google Scholar]
- Chown P, Moyle S, Jeffery S, Cater ND, Barnard EA, Barnard PJ. Elevations of muscle carbonic anhydrase III and glucose-6-phosphate dehydrogenase during dystrophic chicken development. Neurochem Pathol. 1983;1:279–292. [Google Scholar]
- Räisänen SR, Lehenkari P, Tasanen M, Rahkila P, Härkönen PL, Väänänen HK. Carbonic anhydrase III protects cells from hydrogen peroxide-induced apoptosis. FASEB J. 1999;13:513–522. doi: 10.1096/fasebj.13.3.513. [DOI] [PubMed] [Google Scholar]
- Zimmerman UJ, Wang P, Zhang X, Bogdanovich S, Forster R. Anti-oxidative response of carbonic anhydrase III in skeletal muscle. IUBMB Life. 2004;56:343–347. doi: 10.1080/1521-6540400000850. [DOI] [PubMed] [Google Scholar]
- Nishita T, Matsushita H. Comparative immunochemical studies of carbonic anhydrase III in horses and other mammalian species. Comp Biochem Physiol B. 1988;91:91–96. doi: 10.1016/0305-0491(88)90118-6. [DOI] [PubMed] [Google Scholar]
- Nishita T, Tomita Y, Yorifuji D, Orito K, Ochiai H, Arishima K. Purification of chicken carbonic anhydrase isozyme-III (CA-III) and its measurement in White Leghorn chickens. Acta Vet Scand. 2011;53:63. doi: 10.1186/1751-0147-53-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita T, Tomita Y, Imanari T, Ichihara N, Orito K, Arishima K. Biochemical and developmental characterization of carbonic anhydrase II from chicken erythrocytes. Acta Vet Scand. 2011;53:16. doi: 10.1186/1751-0147-53-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y. Glucose-6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase and glyceraldehyde-3-phosphate dehydrogenase activities in early stages of development in dystrophic chickens. J Neurol Sci. 1985;68:47–60. doi: 10.1016/0022-510X(85)90049-8. [DOI] [PubMed] [Google Scholar]
- Gailly P, Jouret F, Martin D, Debaix H, Parreira KS, Nishita T, Blanchard A, Antignac C, Willnow TE, Courtoy PJ, Scheinman SJ, Christensen EI, Devuyst O. A novel renal carbonic anhydrase type III plays a role in proximal tubule dysfunction. Kidney Int. 2008;74:52–61. doi: 10.1038/sj.ki.5002794. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Lyles JM, Pizzey JA. Fibre types in chicken skeletal muscles and their changes in muscular dystrophy. J Physiol. 1982;331:333–354. doi: 10.1113/jphysiol.1982.sp014375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R, Jeffery S, Carter ND. Radioimmuoassay of human muscle carbonic anhydrase III in dystrophic states. Clin Chim Acta. 1982;119:229–305. doi: 10.1016/0009-8981(82)90343-6. [DOI] [PubMed] [Google Scholar]
- Hibi N, Shima K, Tashiro K, Tsuzuki K, Tsukada Y, Hirai H. Development of a highly sensitive enzyme-immunoassay for serum carbonic anhydrase III. J Neurol Sci. 1984;65:333–340. doi: 10.1016/0022-510X(84)90096-0. [DOI] [PubMed] [Google Scholar]
- Mokuno K, Riku S, Matsuoka Y, Sobue I, Kato K. Serum carbonic anhydrase III in progressive muscular dystrophy. J Neurol Sci. 1985;67:223–228. doi: 10.1016/0022-510X(85)90118-2. [DOI] [PubMed] [Google Scholar]
- Georgieva NV, Stoyanchev K, Bozakova N, Jotova I. Combined effects of muscular dystrophy, ecological stress, and selenium on blood antioxidant status in broiler chickens. Biol Trace Elem Res. 2011;142:532–545. doi: 10.1007/s12011-010-8782-2. [DOI] [PubMed] [Google Scholar]