Abstract
Prostate cancer is the second leading cause of cancer death among United States men. However, disease aggressiveness is varied, with low-grade disease often being indolent and high-grade cancer accounting for the greatest density of deaths. Outcomes are also disparate among men with high-grade prostate cancer, with upwards of 65% having disease recurrence even after primary treatment. Identification of men at risk for recurrence and elucidation of the molecular processes that drive their disease is paramount, as these men are the most likely to benefit from multimodal therapy. We previously showed that androgen-induced expression profiles in prostate development are reactivated in aggressive prostate cancers. Herein, we report the down-regulation of one such gene, Sparcl1, a secreted protein, acidic and rich in cysteine (SPARC) family matricellular protein, during invasive phases of prostate development and regeneration. We further demonstrate a parallel process in prostate cancer, with decreased expression of SPARCL1 in high-grade/metastatic prostate cancer. Mechanistically, we demonstrate that SPARCL1 loss increases the migratory and invasive properties of prostate cancer cells through Ras homolog gene family, member C (RHOC), a known mediator of metastatic progression. By using models incorporating clinicopathologic parameters to predict prostate cancer recurrence after treatment, we show that SPARCL1 loss is a significant, independent prognostic marker of disease progression. Thus, SPARCL1 is a potent regulator of cell migration/invasion and its loss is independently associated with prostate cancer recurrence.
Keywords: Hevin, synaptic cleft 1, urogenital sinus, extracellular matrix
Prostate cancer is the most common noncutaneous malignancy and the second leading cause of cancer death in United States men. Controversy currently exists over the best treatment strategy for men with high-risk disease (clinical stage ≥T2c, Gleason score 8–10 or prostate-specific antigen > 20 ng/mL) because 56–65% of these men recur after definitive local therapy (1–5). This finding highlights the need for a better understanding of the biologic determinants driving disease progression for both prognostic and therapeutic development.
We and others have recently illustrated that pathways essential for prostate organogenesis are reactivated in prostate cancer (6, 7). During organogenesis, androgens induce epithelial-mesenchymal interactions in the urogenital sinus (UGS) and drive its differentiation into a prostate (8). We examined early prostate organogenesis shortly after initial androgen exposure, when urogenital sinus epithelia (UGE) migrate and invade into the surrounding mesenchyme and determined that the genes defining this developmental stage were similarly regulated in the transition between low- and high-grade prostate cancers (6). Among these genes, SPARCL1 (SPARC-like 1/Hevin/SC1), a member of the secreted protein, acidic and rich in cysteine (SPARC) family of matricellular proteins, was down-regulated specifically during embryonic periods of androgen-induced epithelial invasion (6) and in aggressive prostate cancers (6, 9). Sparcl1 has been shown to mitigate adhesion and to inhibit both fibroblast migration and wound healing (10). The mechanisms through which Sparcl1 regulates cellular adhesion and migration are not well understood; however, Sparcl1 has been shown to bind type I collagen, a component of the extracellular matrix that potentiates tumor cell migration and invasion (11–13). Although the C-terminal domain of SPARCL1 is highly homologous to SPARC, an inhibitor of prostate tumorigenesis and progression (14), the relationship of SPARCL1 itself to prostate cancer aggressiveness has not been well characterized.
Herein, we describe specific roles for SPARCL1 that originate during prostate formation and are reprised in prostate cancer progression. We demonstrate that SPARCL1 restricts epithelial invasion both during androgen-induced prostate development and in prostate cancer. Mechanistically, we demonstrate that SPARCL1 blocks the activation of the Ras homolog gene family, member C (RHOC), thereby inhibiting cellular movement. We consistently find that SPARCL1 is not only down-regulated in localized, high-grade prostate cancer lesions, but is also further repressed in prostate cancer metastases, thus implicating SPARCL1 as a biomarker of lesions with metastatic potential. Consistent with this finding, in multivariate analyses we find that the loss of SPARCL1 expression is significantly prognostic of metastatic recurrence after surgery. Our findings suggest that loss of SPARCL1 leads to an increase in the migratory potential of prostatic epithelial cells, resulting in a more aggressive and invasive phenotype and thereby driving disease recurrence. These data support the potential utility of SPARCL1 as an independent prognostic factor for prostate cancer progression.
Results
Sparcl1 Inhibits Embryonic Epithelial Bud Expansion in the Prostate.
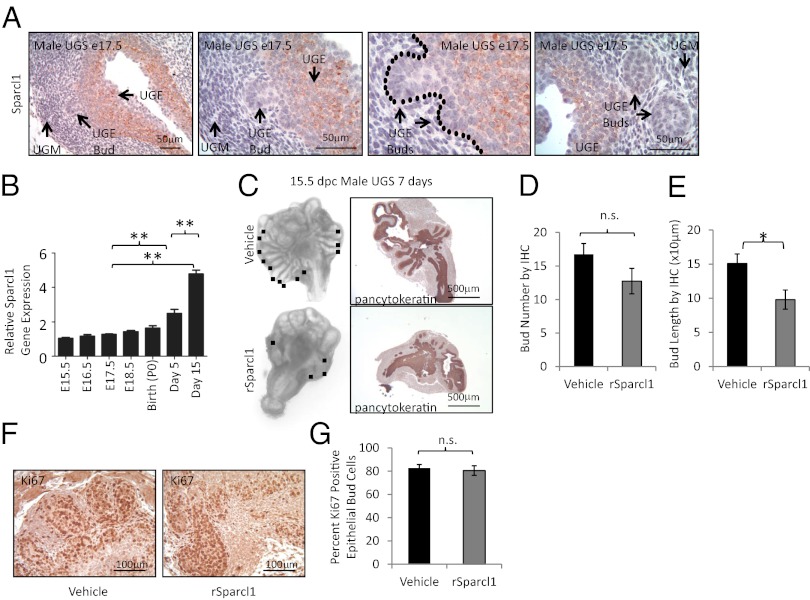
Physiologic prostate growth occurs in an undifferentiated UGS when androgens induce rapid proliferation and invasion of the UGE into the surrounding mesenchyme to form epithelial prostate buds (15, 16). During this phase of development, we previously noted a marked suppression of Sparcl1 gene expression (6). Consistent with this finding, we observed a discrete loss of Sparcl1 protein expression in the invasive epithelial buds compared with the UGE core (Fig. 1A). Following initial epithelial bud elongation, prostate development continues during branching morphogenesis, a stage that begins in utero and is complete by postnatal day 30 (16). During this phase, we noted a significant rise in Sparcl1 gene expression that inversely correlated with physiologic androgen levels and paralleled the percent completion of branching morphogenesis (Fig. 1B) (17). When added to undifferentiated prostate rudiments [embryonic day (E)15.5 male UGS] in organ culture, recombinant Sparcl1 inhibited prostate development. Compared with control-treated UGS, Sparcl1-treated UGS exhibited a significant decrease in the number of prostate epithelial buds observed in whole UGS (Fig. 1C). However, when examined by immunohistochemistry (IHC), we noted that Sparcl1-treated UGS had multiple small buds that were not identifiable in whole-mount preparations (Fig. 1 C, D, and F). Consistent with this finding, bud length was significantly decreased upon exposure to Sparcl1 (Fig. 1E), suggesting that although bud initiation occurs, bud elongation is abrogated. Despite diminished prostate epithelial bud outgrowth, Sparcl1-treated UGS showed epithelial proliferation comparable to control tissue (Fig. 1 F and G). Collectively, these observations suggest that the loss of Sparcl1 expression is necessary for epithelial bud migration and elongation into the surrounding mesenchyme during prostate development.
Fig. 1.
Sparcl1 inhibits androgen-induced fetal prostate bud elongation. (A) Sparcl1 expression in male mouse E17.5 UGS as detected by IHC. (B) Sparcl1 expression examined by quantitative PCR during prostate development. Statistical analysis performed by one-way ANOVA with Newman–Keuls post hoc test (mean ± SEM; n ≥ 3; **P < 0.0001). (C) Male E15.5 UGS cultured in vitro with vehicle or Sparcl1 (10 μg/mL) for 7 d (n ≥ 13) and examined by IHC. The black box indicates bud. (D) Sparcl1 inhibits bud number, but not significantly as measured by IHC (n = 4). (E) Sparcl1 significantly inhibits bud length in UGS cultured in vitro. Bud length determined from photomicrographs for vehicle (n = 43) and Sparcl1 (n = 30) -treated UGS (n = 3 UGS); *P = 0.01. (F and G) Sparcl1 does not inhibit epithelial proliferation as examined by IHC for Ki67 in UGS in vitro cultures. Ki67-positive and -negative cells within the epithelial bud were counted from IHC sections of E15.5 male UGS cultured in vitro with vehicle or Sparcl1 (n = 3 UGS). Statistical analysis for C and G performed by Student t test (mean ± SEM). n.s., not significant.
Sparcl1 Expression Is Suppressed During Adult Prostate Regeneration.
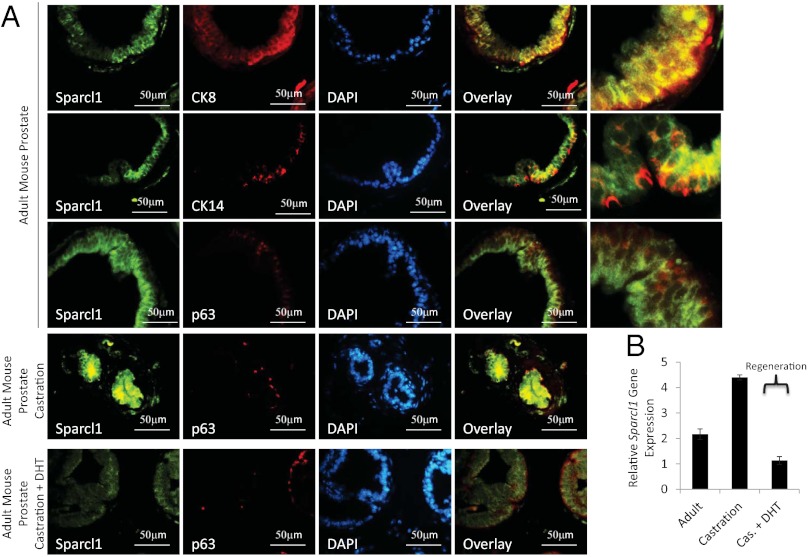
Because Sparcl1 expression is specifically suppressed in migrating epithelial cells during prostate development, we evaluated Sparcl1 expression during androgen-mediated regression and regeneration in the adult prostate. In the mature mouse gland, Sparcl1 is expressed predominantly in luminal (CK8+) epithelial cells; however, a subpopulation of basal cells (p63 and CK14+) coexpress Sparcl1, as indicated by IHC and immunofluorescence (IF) (Fig. 2A, and Fig. S1 A and B). SPARCL1 expression in human prostate epithelial cells is similar to that in the mouse (Fig. S1D). Following androgen withdrawal (castration), both Sparcl1 gene and protein expression were elevated (Fig. 2 and Fig. S1C). Similar to development, Sparcl1 expression was suppressed during androgen-induced prostatic regrowth (Fig. 2). Taken together, these findings indicate that Sparcl1 expression is repressed during phases of androgen-stimulated prostatic epithelial growth and invasion in both the embryo and the adult. Considering Sparcl1’s role in regulating adhesion and migration, our results suggest that Sparcl1 suppresses epithelial expansion and migration in the prostate.
Fig. 2.
Sparcl1 expression is decreased during prostate regeneration in adult mouse. Decreased Sparcl1 protein (A) and gene (B) expression during androgen-induced regrowth determined by IF (A) and quantitative PCR (B) as compared in adult mouse prostate, adult mouse prostate 3 wk following castration, and adult mouse prostrate treated with dihydrotestosterone (DHT) for 3 d following castration (regenerating prostate) (mean ± SD; n = 3).
SPARCL1 Does Not Inhibit Proliferation in the Prostate.
Sparcl1 markedly inhibited prostate epithelial bud elongation; however, comparable expression of proliferation markers in Sparcl1-treated prostate organ cultures suggests that Sparcl1 does not regulate proliferation in the prostate. Because Sparcl1’s role in proliferation is varied, we further defined SPARCL1-mediated regulation of prostatic epithelial cell growth (10, 18, 19). We examined cellular proliferation and death in SPARCL1-treated prostate cells and demonstrated that SPARCL1 did not restrict the growth of multiple prostate cancer cell lines (Fig. S2A). SPARCL1 also did not significantly affect cell cycle progression (Fig. S2B) or cellular proliferation (Fig. S2C). SPARCL1 also did not affect cell death (Fig. S2D). Consistent with prostate organ cultures, these data indicate that SPARCL1 does not regulate cellular proliferation or death in the prostate.
SPARCL1 Inhibits Prostate Cell Adhesion, Migration, and Invasion.
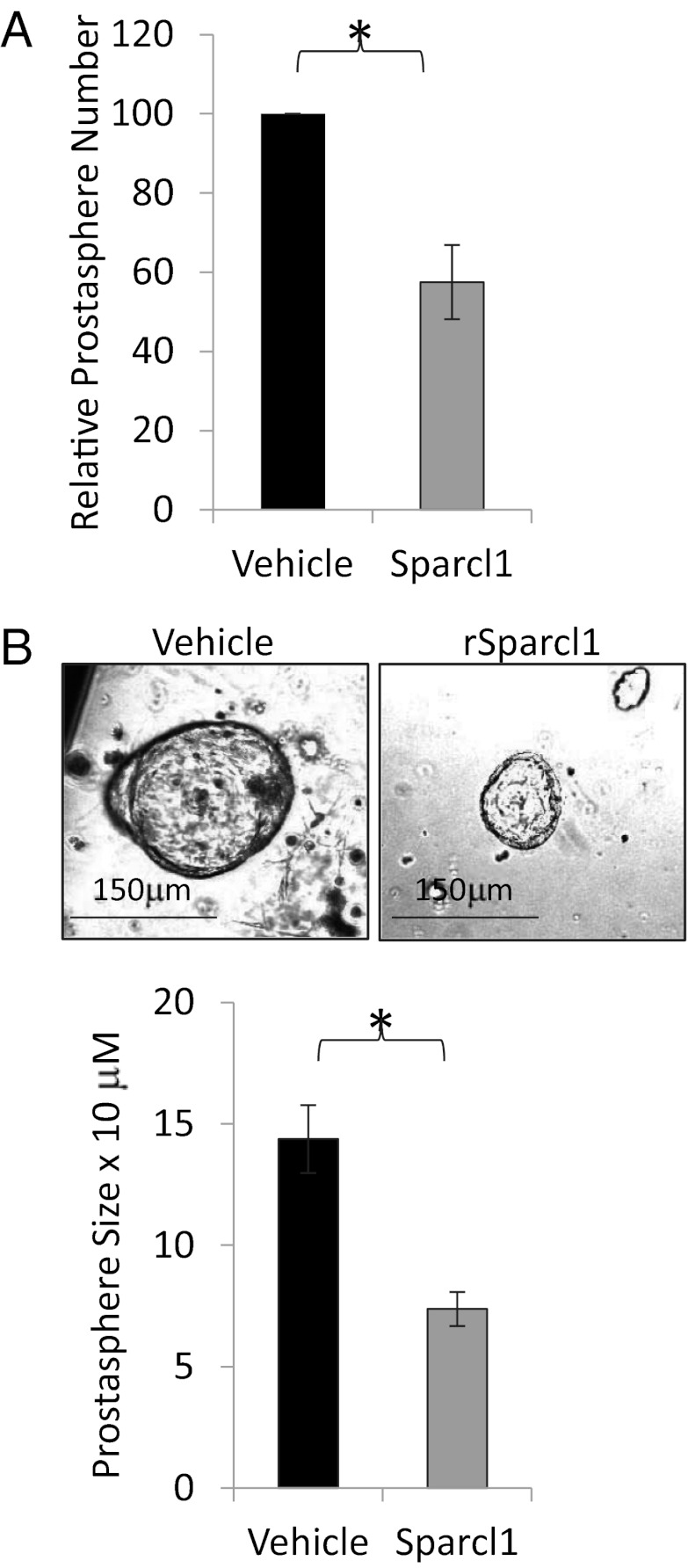
We hypothesized that loss of Sparcl1 expression permits epithelial migration and invasion in prostate organogenesis and regeneration, and conversely that Sparcl1 expression restricts these functions in the adult gland. To examine this theory, we used a 3D invasion assay in which single-cell epithelial isolates from adult murine prostates can be cultured in Matrigel to form “prostaspheres.” This process is dependent on proliferation and 3D migration and invasion into an extracellular matrix. Addition of Sparcl1 to this matrix significantly limited prostasphere number (Fig. 3A) and size (Fig. 3B) without affecting differentiation (CK14, CK8, and p63) or proliferation (Ki67) (Fig. S3A). Augmenting Sparcl1 before or after prostasphere initiation yielded a similar effect (Fig. S3 B and C). Similar to the mouse, SPARCL1 restricts prostasphere formation in benign adult human primary prostate epithelial cells (Fig. S3D).
Fig. 3.
Sparcl1 restricts benign prostate epithelial cell invasion. Sparcl1 inhibits prostasphere number (A) and size (B). Adult mouse prostate epithelial cells disassociated into single cells, cultured in Matrigel and treated with Sparcl1 (10 μg/mL) or vehicle for 14 d to form prostaspheres. Statistical analysis performed by Student t test (mean ± SEM; n = 4; *P ≤ 0.005).
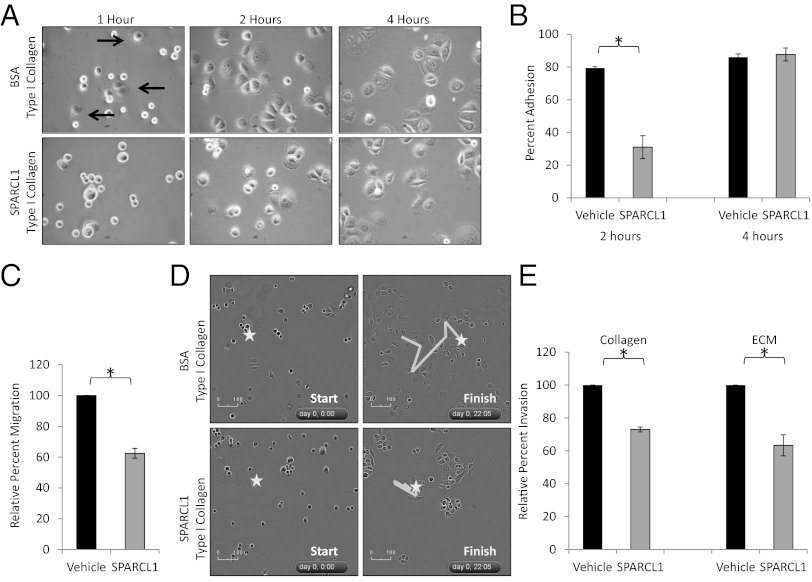
Because prostasphere culture requires attachment to an extracellular matrix, and previous studies have shown that Sparcl1 is antiadhesive (10, 20), we tested the hypothesis that SPARCL1 may prevent prostate cellular adhesion to various extracellular matrices. SPARCL1 delayed or abrogated adhesion of multiple prostate cancer cell lines and primary benign prostate cells to type I collagen, a key element within the extracellular matrix, and one to which SPARCL1 has been shown to bind (Fig. 4 A and B, Fig. S4, and Movies S1 and S2.) (11). Because adhesion is an initiating event leading to a migratory/invasive phenotype, we further examined how SPARCL1 affects cellular migration and extracellular matrix invasion and found that SPARCL1 inhibited prostate cell migration across a membrane (Fig. 4C). To better elucidate this phenotype, we tested the effects of SPARCL1 on type I collagen-mediated movement. Time-lapse microscopy of prostate cancer cells on a type I collagen matrix containing SPARCL1 or vehicle demonstrated that SPARCL1 not only delayed adhesion to type I collagen, but also inhibited migration following adhesion (Fig. 4D, and Movies S1 and S2). Because invasion can be viewed as migration through a matrix, cells that migrate ineffectively should also show defects in invasion. Accordingly, SPARCL1 also inhibited prostate cancer cell invasion as assayed in type I collagen-based extracellular matrices (Fig. 4E). Collectively, these data support a role for SPARCL1 in regulating the migratory and invasive potential of prostate cancer cells by inhibiting their adhesive and migratory properties.
Fig. 4.
SPARCL1 inhibits adhesion, migration, and invasion of prostate cancer cells. (A and B) Adhesion of PC3 cells following incubation on a type I collagen matrix containing BSA (10 μg/mL) or SPARCL1 (10 μg/mL) (n = 3). Arrows indicate adhered cells. Magnification in A, 400×. (C) Migration of PC3 cells incubated with BSA (10 μg/mL) or SPARCL1 (10 μg/mL) across a filter for 20 h (n = 3). (D) Cell adhesion and migration recorded by time-lapse microscopy for 22 h of PC3 cells on a type I collagen matrix containing SPARCL1 (10 μg/mL) or BSA (10 μg/mL). (E) Invasion of PC3 cells incubated with BSA (10 μg/mL) or SPARCL1 (10 μg/mL) of type I collagen- or Matrigel-coated filters for 20 h (n = 3). Statistical analysis performed by Student t test (mean ± SEM; *P ≤ 0.005).
SPARCL1 Inhibits RHOC GTPase-Mediated Prostate Cancer Cell Migration.
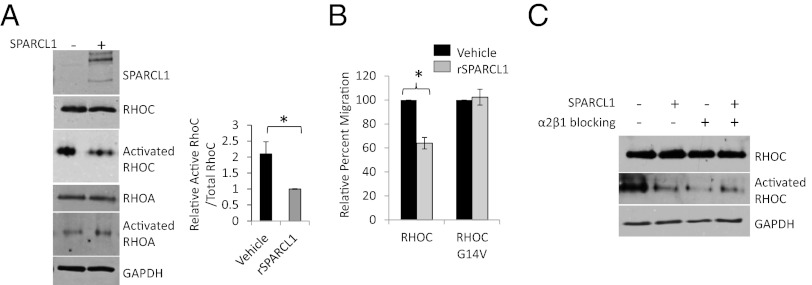
RHOC has established roles in promoting cancer cell adhesion, migration, invasion, and metastatic progression (21, 22). Type I collagen engagement of its cognate receptor (α2β1-integrin) has been shown to promote prostate cancer invasion through RHOC (12). As Sparcl1 has been shown to bind to type I collagen, we hypothesized that SPARCL1 restricts epithelial migration by directly disrupting the function of type I collagen-RHOC–induced migration (11). Following adhesion to a type I collagen/SPARCL1 matrix, prostate cancer cells exhibited cellular dynamics (Movies S1 and S2), consistent with inhibition of RHOC but not RHOA (23). To address the possibility that SPARCL1 inhibits type I collagen-induced RHOC activation, we measured RHOC activation in prostate cancer cells following adhesion to one of two different matrices: a type I collagen matrix containing either BSA (control) or SPARCL1. Specific immunoprecipitation (IP) of its active (GTP-bound) form demonstrated that RHOC activation was significantly suppressed when cells were grown on a type I collagen matrix containing SPARCL1 (Fig. 5A). This effect was specific for RHOC because SPARCL1 did not affect activation of RHOA (Fig. 5A). SPARCL1 appeared to suppress migration largely by inhibiting RHOC activation, as the effect of SPARCL1 was rescued by overexpressing a constitutively active RHOC mutant (RHOC G14V) (Fig. 5B). In addition, we found that inhibition of the type I collagen receptor with a neutralizing antibody resulted in RHOC inhibition that was comparable to that mediated by SPARCL1. In contrast, simultaneous exposure to SPARCL1 and a α2β1-integrin–blocking antibody did not further enhance RHOC inhibition, suggesting that SPARCL1 and α2β1-integrin function through the same pathway (Fig. 5C). Collectively, these data indicate that SPARCL1 inhibits type I collagen-induced RHOC mediated migration.
Fig. 5.
SPARCL1 inhibits type I collagen-induced RHOC-mediated migration. (A) PC3 cells grown on a type I collagen matrix containing SPARCL1 (10 μg/mL) or BSA (10 μg/mL). Specific IP of activated (GTP-bound) RHOA/B/C and immunoblot (IB) for RHOA and RHOC. Pre-IP lysates were examined for total RHOC, RHOA, GAPDH, and SPARCL1 expression. ImageJ quantification of activated RHOC (normalized to total pre-IP RHOC). Statistical analysis performed by Student t test (mean ± SEM; n = 4; *P = 0.02). (B) PC3 cells transiently transfected with RHOC or constitutively active RHOC (G14V), treated with SPARCL1 (10 μg/mL) or vehicle and allowed to migrate across a filter for 20 h. Statistical analysis performed by Student t test (mean ± SEM; n = 3; *P = 0.013). (C) PC3 cells transiently transfected with pcDNA3.1- or hSPARCL1/pcDNA3.1, treated with isotype control or a α2β1-integrin blocking antibody, and then grown on a type I collagen matrix. Specific IP of activated (GTP-bound) RHOA/B/C and IB for RHOC. Pre-IP lysates IB for total RHOC and GAPDH expression.
SPARCL1 Expression Inversely Correlates with Prostate Cancer Aggressiveness.
Because SPARCL1 regulated cell invasion, we postulated that SPARCL1 may correlate with and potentially modulate locally aggressive prostate cancers. To examine this theory, we first evaluated Sparcl1 protein expression in two genetic animal models of prostate cancer. Hi-Myc transgenic mice develop murine prostatic intraepithelial neoplasia (mPIN) and locally invasive adenocarcinoma caused by prostate specific overexpression of c-Myc (24). In Hi-Myc mice, Sparcl1 expression was decreased in invasive prostate adenocarcinoma (Fig. S5A). We further examined Sparcl1 expression in both primary and metastatic lesions isolated from transgenic adenocarcinoma of the mouse prostate (TRAMP) mice, a model with high rates of metastasis (25). Compared with benign adjacent glands and mPIN, Sparcl1 expression was decreased both in invasive prostate adenocarcinoma and in lesions which had metastasized to the liver (Fig. S5 B and C). Taken together, these data indicate Sparcl1 loss predates metastasis and therefore may have prognostic value.
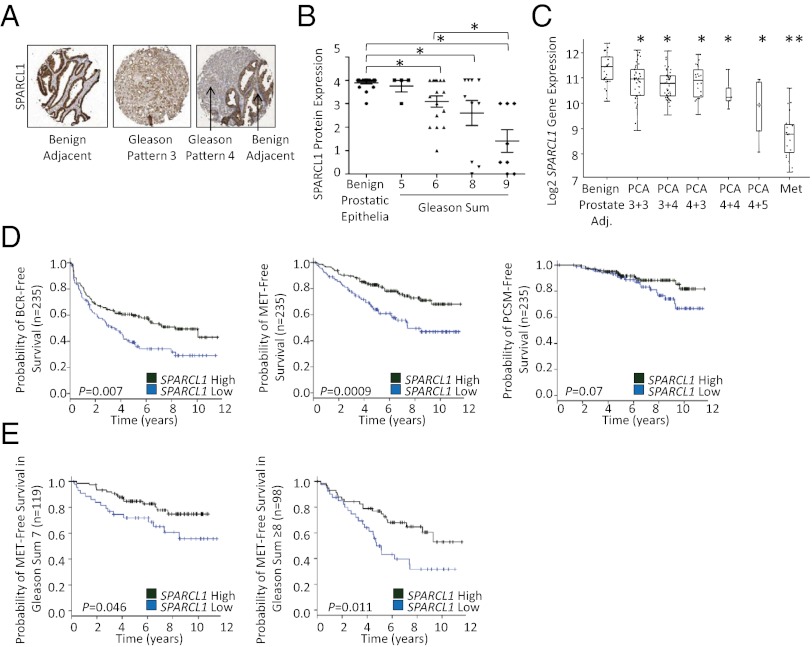
In human prostate cancer, Gleason grade is the strongest single predictor of prostate cancer lethality (1). Low-grade (sum 6 or less) rarely progress, whereas men with high-grade tumors (sum 8–10) frequently progress to metastasis and death, even after radical treatment (1, 26). IHC analysis of SPARCL1 expression on tissue microarrays (TMAs) demonstrated a statistically significant inverse correlation between Gleason grade and SPARCL1 expression (Fig. 6 A and B). Consistent with this finding, analyses of 10 datasets indicated that parallel to protein expression, SPARCL1 gene expression declined continuously as grade increased with the most striking loss seen in metastatic lesions (Fig. 6C and Fig. S6 A–C) (27–36) (Oncomine; www.oncomine.org). In contrast, SPARCL1 gene expression was not significantly lost in benign prostatic hyperplasia or PIN (Fig. S6 D and E) (27, 28, 37) (Oncomine). Interestingly, gene-profiling data from the same cohorts showed that RHOC gene expression did not correlate with prostate cancer grade (Fig. S6 F and G) (27, 28), suggesting that alterations in RHOC activity as opposed to expression levels, mediate RHOC-induced metastatic progression (38).
Fig. 6.
Loss of SPARCL1 expression correlates with Gleason grade and is an independent marker for prostate cancer recurrence. (A and B) SPARCL1 expression is inversely proportional to prostate cancer Gleason grade as determined by IHC in prostate adenocarcinoma Gleason sum 5 (n = 4), 6 (n = 16), 8 (n = 10), and 9 (n = 8), and benign adjacent glands (n = 20) from radical prostatectomies as JHU Gleason grade TMAs. Statistical analysis performed by one-way ANOVA with Bonferroni post hoc test (mean ± SEM; *P ≤ 0.002). (C) SPARCL1 expression is inversely proportional to prostate cancer Gleason grade. Analysis performed on data sets from Taylor et al. for SPARCL1 gene expression (27). Statistical analysis performed by one-way ANOVA. *Prostrate cancer (PCA) vs. benign adjacent and **Met vs. PCA P ≤ 0.01. (D and E) Loss of SPARCL1 expression is prognostic of prostate cancer recurrence. (D) Kaplan–Meier curves for SPARCL1 in a high-risk prostate cancer cohort from the Mayo Clinic for BCR (P = 0.007), MET (P = 0.0009), and PCSM (P = 0.07) endpoints (n = 235). (E) Kaplan–Meier curves for SPARCL1 in a Gleason sum 7 cohort (n = 119, P = 0.046) and a Gleason sum ≥8 cohort (n = 98, P = 0.011) from the Mayo Clinic for MET free survival. Statistical analysis found in SI Materials and Methods.
We additionally investigated SPARCL1 expression in other primary cancers and their metastases and also found it decreased in a variety of cancer types, including bladder (39), breast (40), and lung (41) (Oncomine) (Fig. S6I) with further supression in metastases compared with primary tumors (Fig. S6J) (41–47) (Oncomine). These observations suggest that SPARCL1 suppression is a conserved and critical step in cancer progression to metastasis.
Loss of SPARCL1 Expression Is an Independent Marker of Recurrence After Prostatectomy.
A subset of men with clinically localized prostate cancer experience disease recurrence even after primary treatment. Although current models incorporating Gleason grade, pathologic stage, and other clinical parameters predict recurrence (48), further delineation of risk is needed. We postulated that SPARCL1 loss could add prognostic power to these traditionally used variables. We examined SPARCL1 expression by IHC in a matched nested case-control cohort designed to evaluate prognostic factors for recurrence following prostatectomy (defined as prostate-specific antigen ≥0.2 ng/mL, metastasis, or prostate cancer death) independent of Gleason grade, pathologic stage, age, and other clinical variables [The Johns Hopkins University (JHU) progression array] (32) (Fig. S7A). We found that loss of SPARCL1 expression in prostate adenocarcinoma was independently associated with a 3.48-fold (95% confidence interval 1.02–11.85; P = 0.046) higher risk of prostate cancer recurrence (Fig. S7).
We validated this finding in an independent cohort using Affymetrix exon microarray analysis in a prospectively-designed study of high-risk men who underwent radical prostatectomy at the Mayo Clinic (Fig. S8A). We evaluated the prognostic utility of SPARCL1 using three clinical endpoints: biochemical recurrence (BCR), metastatic disease (MET) as defined by a positive bone scan or CR/MRI evidence of metastatic disease, and prostate cancer-specific mortality (PCSM). Kaplan–Meier analyses show loss of SPARCL1 is a powerful single-gene predictor of aggressive prostate cancer (Fig. 6D). For BCR, loss of SPARCL1 expression was associated with a median time-to-progression of 3.5 y compared with greater than 8 y for high SPARCL1-expressing men. Similarly, for MET-free survival, men with loss of SPARCL1 expression had 5 y MET-free survival of ∼60% vs. ∼80% for men with high SPARCL1 expression. These data suggest that even in a high-risk cohort, where most individuals are expected to experience recurrence at some point after surgery, loss of SPARCL1 expression defines a subgroup where BCR will occur sooner and the risk for developing metastatic disease and prostate cancer death is significantly higher.
Furthermore, multivariable Cox regression analyses of the Mayo Clinic cohort confirmed the JHU observation that loss of SPARCL1 expression is independently prognostic of prostate cancer aggressiveness with significant hazard ratios (HR) for predicting BCR, MET, and PCSM [HR 1.40, P = 0.0045; HR 1.62, P = 0.0007; HR 1.77, P = 0.0028, respectively] (Fig. S8B). In groups of men stratified by Gleason score (sum 7 and sum ≥8), SPARCL1 suppression significantly identified men at increased risk of developing metastatic disease (Gleason sum 7, HR 1.55, P = 0.03 and Gleason sum ≥8 HR 1.86 P = 0.03) (Fig. 6E). In fact, in these Gleason subgroups, multivariable Cox regression analyses of SPARCL1 and standard prognostic factors including stage demonstrated that loss of SPARCL1 expression was the only statistically significant predictor of recurrence (Fig. S8C).
Discussion
Enrichment of embryonic gene-expression signatures has been demonstrated in multiple solid malignancies, substantiating the paradigm of embryonic reawakening in cancer and the utility of embryonic systems to model cancer progression (6, 7, 49). With this approach, we show that the developmental regulation of Sparcl1 expression is paralleled in prostate cancer. Similar to periods of physiologic growth, we illustrate an inverse correlation between SPARCL1 expression and high-grade localized prostate cancer as well as metastatic lesions. Consistent with its role in physiologic epithelial invasion during development, we demonstrate that the loss of SPARCL1 expression increases the migratory and invasive properties of prostate epithelial cells through a RHOC-mediated process. We further demonstrate that loss of SPARCL1 expression is not only associated with aggressive disease, but is also independently associated with disease recurrence following treatment, indicating that loss of SPARCL1 expression in the primary tumor may drive metastasis rather than solely being a marker of metastatic lesions. Taken together, these data suggest that by suppressing RHOC mediated migration, SPARCL1 plays a key role in modulating the metastatic potential of cancer and further defines loss of SPARCL1 as an early marker of aggressive prostate cancer.
Recent studies show type I collagen stimulation of the α2β1-integrin promotes prostate cancer cell migration through RHOC activation (12, 50). We demonstrate here that SPARCL1, a type I collagen-binding protein, attenuates type I collagen-induced RHOC activation and this corresponds to decreased RHOC-mediated migration in the prostate (11). RHOC has been shown to affect the localization of active Rac1, a distinct member of the RHO family (23). Consistent with that study and our finding that SPARCL1 negatively regulates RHOC activity, a separate report using a small molecule inhibitor against Rac1 suggests that Sparcl1 inhibits Rac1-dependent migration in fibroblasts (10). Furthermore, although RHOC expression is elevated in multiple cancers, including breast (51), bladder (52), and nonsmall-cell lung carcinoma (53), its expression levels do not correlate with prostate cancer aggressiveness. This finding suggests that unlike other tumors, which overexpress RHOC, prostate cancers may regulate RHOC-mediated migration via modulation of SPARCL1 expression. Together, these studies suggest a role for SPARCL1 as a master regulator of RHOC-RAC1 mediated cellular migration and invasion.
We demonstrate that SPARCL1 may have clinical utility as a prognostic marker that is independently associated with prostate cancer recurrence. Thus, SPARCL1 expression may identify patients who are in greatest need of additional therapies. In addition, we outline a key biologic role for SPARCL1 in prostate cancer. Thus, it is possible that treatments targeting this pathway could attenuate the metastatic potential of localized cancers and we believe that further understanding of the factors modulating SPARCL1 will have important clinical implications for both prognostic and therapeutic development.
Materials and Methods
Cellular and molecular biology assays including RNA isolation, RT-PCR, in vitro organ culture, antibodies, immunoblotting, IF, 3D prostate invasion, cell culture, cell growth, cell cycle, apoptosis, adhesion, migration, invasion, and activated RHO assays are described in SI Materials and Methods. Animal studies including prostate regeneration, Hi-Myc mice, and TRAMP mice are described in SI Materials and Methods. Cohort studies including the JHU Gleason grade TMAs, the JHU progression analyses, and the Mayo Clinic progression analyses are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank John Isaacs for cell lines, and Angelo DeMarzo, Rolf Brekken, Berry Nelkin, Michael Haffner, Srinivasan Yegnasubramanian, and Bruce Trock for helpful discussions. We thank The Johns Hopkins University Flow Cytometry and Cell Imaging Core Facilities and the Prostate Cancer Biorepository Network (PCBN), supported by the Department of Defense Prostate Cancer Research Program, DOD Award No W81XWH-10-2-0056 and W81XWH-10-2-0046. This work was supported by a Flight Attendant Medical Research Institute Young Clinical Scientist Award (to P.J.H.); The Patrick C. Walsh Prostate Cancer Fund (P.J.H. and E.M.S.); a Howard Hughes Medical Institute Early Careers Physician Scientist Award (to E.M.S.); the American Urological Association/Astellas Rising Star Award (to E.M.S.); and National Institutes of Health Grant P30CA006973 (to L.M.).
Footnotes
Conflict of interest statement: N.E., I.A.V., M.G., and E.D. are employees of and have stock in GenomeDx Biosciences, Inc.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203525109/-/DCSupplemental.
References
- 1.Eggener SE, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185:869–875. doi: 10.1016/j.juro.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loeb S, et al. What are the outcomes of radical prostatectomy for high-risk prostate cancer? Urology. 2010;76:710–714. doi: 10.1016/j.urology.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierorazio PM, et al. Long-term survival after radical prostatectomy for men with high Gleason sum in pathologic specimen. Urology. 2010;76:715–721. doi: 10.1016/j.urology.2009.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Amico AV, et al. Analyzing outcome-based staging for clinically localized adenocarcinoma of the prostate. Cancer. 1998;83:2172–2180. doi: 10.1002/(sici)1097-0142(19981115)83:10<2172::aid-cncr16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Pierorazio PM, et al. Preoperative characteristics of high-Gleason disease predictive of favourable pathological and clinical outcomes at radical prostatectomy. BJU Int. 2012 doi: 10.1111/j.1464-410X.2012.10986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaeffer EM, et al. Androgen-induced programs for prostate epithelial growth and invasion arise in embryogenesis and are reactivated in cancer. Oncogene. 2008;27:7180–7191. doi: 10.1038/onc.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pritchard C, et al. Conserved gene expression programs integrate mammalian prostate development and tumorigenesis. Cancer Res. 2009;69:1739–1747. doi: 10.1158/0008-5472.CAN-07-6817. [DOI] [PubMed] [Google Scholar]
- 8.Cunha GR. Mesenchymal-epithelial interactions: Past, present, and future. Differentiation. 2008;76:578–586. doi: 10.1111/j.1432-0436.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- 9.Nelson PS, et al. Hevin, an antiadhesive extracellular matrix protein, is down-regulated in metastatic prostate adenocarcinoma. Cancer Res. 1998;58:232–236. [PubMed] [Google Scholar]
- 10.Sullivan MM, Puolakkainen PA, Barker TH, Funk SE, Sage EH. Altered tissue repair in hevin-null mice: inhibition of fibroblast migration by a matricellular SPARC homolog. Wound Repair Regen. 2008;16:310–319. doi: 10.1111/j.1524-475X.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- 11.Hambrock HO, et al. SC1/hevin. An extracellular calcium-modulated protein that binds collagen I. J Biol Chem. 2003;278:11351–11358. doi: 10.1074/jbc.M212291200. [DOI] [PubMed] [Google Scholar]
- 12.Hall CL, et al. Type I collagen receptor (alpha2beta1) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia. 2008;10:797–803. doi: 10.1593/neo.08380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong T, et al. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:7427–7437. doi: 10.1158/1078-0432.CCR-03-0825. [DOI] [PubMed] [Google Scholar]
- 14.Said N, et al. The role of SPARC in the TRAMP model of prostate carcinogenesis and progression. Oncogene. 2009;28:3487–3498. doi: 10.1038/onc.2009.205. [DOI] [PubMed] [Google Scholar]
- 15.Meeks JJ, Schaeffer EM. Genetic regulation of prostate development. J Androl. 2011;32:210–217. doi: 10.2164/jandrol.110.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;76:641–659. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pritchard CC, Nelson PS. Gene expression profiling in the developing prostate. Differentiation. 2008;76:624–640. doi: 10.1111/j.1432-0436.2008.00274.x. [DOI] [PubMed] [Google Scholar]
- 18.Claeskens A, et al. Hevin is down-regulated in many cancers and is a negative regulator of cell growth and proliferation. Br J Cancer. 2000;82:1123–1130. doi: 10.1054/bjoc.1999.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esposito I, et al. Tumor-suppressor function of SPARC-like protein 1/Hevin in pancreatic cancer. Neoplasia. 2007;9:8–17. doi: 10.1593/neo.06646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girard JP, Springer TA. Modulation of endothelial cell adhesion by hevin, an acidic protein associated with high endothelial venules. J Biol Chem. 1996;271:4511–4517. doi: 10.1074/jbc.271.8.4511. [DOI] [PubMed] [Google Scholar]
- 21.Wu M, Wu ZF, Rosenthal DT, Rhee EM, Merajver SD. Characterization of the roles of RHOC and RHOA GTPases in invasion, motility, and matrix adhesion in inflammatory and aggressive breast cancers. Cancer. 2010;116(11, Suppl):2768–2782. doi: 10.1002/cncr.25181. [DOI] [PubMed] [Google Scholar]
- 22.Hakem A, et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 2005;19:1974–1979. doi: 10.1101/gad.1310805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vega FM, Fruhwirth G, Ng T, Ridley AJ. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J Cell Biol. 2011;193:655–665. doi: 10.1083/jcb.201011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellwood-Yen K, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 25.Gingrich JR, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
- 26.Pound CR, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 27.Taylor BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross AE, et al. Gene expression pathways of high grade localized prostate cancer. Prostate. 2011;71:1568–1577. doi: 10.1002/pros.21373. [DOI] [PubMed] [Google Scholar]
- 29.Chandran UR, et al. Differences in gene expression in prostate cancer, normal appearing prostate tissue adjacent to cancer and prostate tissue from cancer free organ donors. BMC Cancer. 2005;5:45. doi: 10.1186/1471-2407-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu YP, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22:2790–2799. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 31.Holzbeierlein J, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaTulippe E, et al. Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res. 2002;62:4499–4506. [PubMed] [Google Scholar]
- 33.Lapointe J, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varambally S, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8:393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Ramaswamy S, et al. Multiclass cancer diagnosis using tumor gene expression signatures. Proc Natl Acad Sci USA. 2001;98:15149–15154. doi: 10.1073/pnas.211566398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 37.Tomlins SA, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 38.Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;1796:91–98. doi: 10.1016/j.bbcan.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006;24:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 40.Richardson AL, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Bhattacharjee A, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci USA. 2001;98:13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radvanyi L, et al. The gene associated with trichorhinophalangeal syndrome in humans is overexpressed in breast cancer. Proc Natl Acad Sci USA. 2005;102:11005–11010. doi: 10.1073/pnas.0500904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segal NH, et al. Classification and subtype prediction of adult soft tissue sarcoma by functional genomics. Am J Pathol. 2003;163:691–700. doi: 10.1016/S0002-9440(10)63696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segal NH, et al. Classification of clear-cell sarcoma as a subtype of melanoma by genomic profiling. J Clin Oncol. 2003;21:1775–1781. doi: 10.1200/JCO.2003.10.108. [DOI] [PubMed] [Google Scholar]
- 45.Liao YL, et al. Identification of SOX4 target genes using phylogenetic footprinting-based prediction from expression microarrays suggests that overexpression of SOX4 potentiates metastasis in hepatocellular carcinoma. Oncogene. 2008;27:5578–5589. doi: 10.1038/onc.2008.168. [DOI] [PubMed] [Google Scholar]
- 46.Xu L, et al. Gene expression changes in an animal melanoma model correlate with aggressiveness of human melanoma metastases. Mol Cancer Res. 2008;6:760–769. doi: 10.1158/1541-7786.MCR-07-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ki DH, et al. Whole genome analysis for liver metastasis gene signatures in colorectal cancer. Int J Cancer. 2007;121:2005–2012. doi: 10.1002/ijc.22975. [DOI] [PubMed] [Google Scholar]
- 48.Han M, et al. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517–523. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 49.Ben-Porath I, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iiizumi M, et al. RhoC promotes metastasis via activation of the Pyk2 pathway in prostate cancer. Cancer Res. 2008;68:7613–7620. doi: 10.1158/0008-5472.CAN-07-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Golen KL, et al. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin Cancer Res. 1999;5:2511–2519. [PubMed] [Google Scholar]
- 52.Kamai T, et al. Significant association of Rho/ROCK pathway with invasion and metastasis of bladder cancer. Clin Cancer Res. 2003;9:2632–2641. [PubMed] [Google Scholar]
- 53.Shikada Y, et al. Higher expression of RhoC is related to invasiveness in non-small cell lung carcinoma. Clin Cancer Res. 2003;9:5282–5286. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.