Abstract
Inappropriate neutrophil activation contributes to the pathogenesis of acute lung injury (ALI). Apoptosis is essential for removal of neutrophils from inflamed tissues and timely resolution of inflammation. Resolvin E1 (RvE1) is an endogenous lipid mediator derived from the ω-3 polyunsaturated fatty acid eicosapentaenoic acid that displays proresolving actions. Because the balance of prosurvival and proapoptosis signals determines the fate of neutrophils, we investigated the impact of RvE1 on neutrophil apoptosis and the outcome of neutrophil-mediated pulmonary inflammation in mice. Culture of human neutrophils with RvE1 accelerated apoptosis evoked by phagocytosis of opsonized Escherichia coli or yeast. RvE1 through the leukotriene B4 receptor BLT1 enhanced NADPH oxidase-derived reactive oxygen species generation and subsequent activation of caspase-8 and caspase-3. RvE1 also attenuated ERK and Akt-mediated apoptosis-suppressing signals from myeloperoxidase, serum amyloid A, and bacterial DNA, shifting the balance of pro- and anti-survival signals toward apoptosis via induction of mitochondrial dysfunction. In mice, RvE1 treatment enhanced the resolution of established neutrophil-mediated pulmonary injury evoked by intratracheal instillation or i.p. administration of live E. coli or intratracheal instillation of carrageenan plus myeloperoxidase via facilitating neutrophil apoptosis and their removal by macrophages. The actions of RvE1 were prevented by the pan-caspase inhibitor zVAD-fmk. These results identify a mechanism, promotion of phagocytosis-induced neutrophil apoptosis and mitigation of potent anti-apoptosis signals, by which RvE1 could enhance resolution of acute lung inflammation.
Keywords: leukocytes, efferocytosis, innate immunity, resolution of inflammation
Acute inflammation is a tightly regulated self-limited protective response against invading pathogens and tissue injury. Neutrophils, recruited from the circulation, play a prominent role in host defense (1). Neutralization of the offending insult ideally prompts resolution of inflammation and restoration of tissue homeostasis. Effective resolution of inflammation critically depends on inhibition of neutrophil influx and timely removal of infiltrating neutrophils that is governed by active resolution programs (2). Failure of the initial acute inflammatory response to resolve in a timely manner is now considered as a characteristic feature of many common diseases, including acute lung injury or the acute respiratory distress syndrome (3).
Neutrophil apoptosis has emerged as a critical control point in resolving inflammation. Emigrated neutrophils undergo apoptosis before being removed by scavenger macrophages (4, 5). The fate of neutrophils can, however, be profoundly influenced by prosurvival and proapoptosis cues from the inflammatory microenvironment. Recent studies using a variety of gene knockout, transgenic, and pharmacological strategies in diverse models of inflammation, including acute lung injury (ALI) and sepsis showed that modulating neutrophil apoptosis can profoundly affect the outcome of inflammation. For instance, delaying neutrophil apoptosis by myeloperoxidase (MPO) prolonged lung injury (6), whereas cyclin-dependent kinase inhibitor drugs (7, 8) or aspirin-triggered 15-epi-LXA4 (9) augmented neutrophil apoptosis parallel with enhancing resolution. Suppressed neutrophil apoptosis appears to be a component of the pathophysiology in patients with acute respiratory distress syndrome (10) and sepsis (11).
Resolvin E1 (RvE1) is biosynthesized from the ω-3 polyunsaturated fatty acid eicosapentaenoic acid (EPA) during the resolution phase of acute inflammation with leukocyte 5-lipoxygenase playing a pivotal temporal role in the biosynthesis pathway (12, 13). RvE1 binds to ChemR23 and the leukotriene B4 (LTB4) receptor BLT1 (14, 15) and attenuates neutrophil migration (2), reduces production of inflammatory cytokines, enhances generation of the proresolving mediator lipoxin A4 (16) and phagocytosis of apoptotic neutrophils by macrophages (13), thereby attenuating inflammation in several disease models, including peritonitis (13), polymicrobial sepsis (17), bacterial pneumonia (18), and allergic airway inflammation (19, 20). Little is known about whether RvE1 could affect neutrophil apoptosis, a critical control point in resolution of inflammation. Here, we report that RvE1 shortened the life span of human neutrophils by accelerating phagocytosis-induced neutrophil death and by mitigating the antiapoptosis signal from diverse inflammatory mediators. Moreover, RvE1 administered at the peak of inflammation augmented caspase-mediated neutrophil apoptosis and enhanced inflammation resolution in three murine models of acute lung injury.
Results
RvE1 Enhances Phagocytosis-Induced Neutrophil Apoptosis and Mitigates Prosurvival Signals.
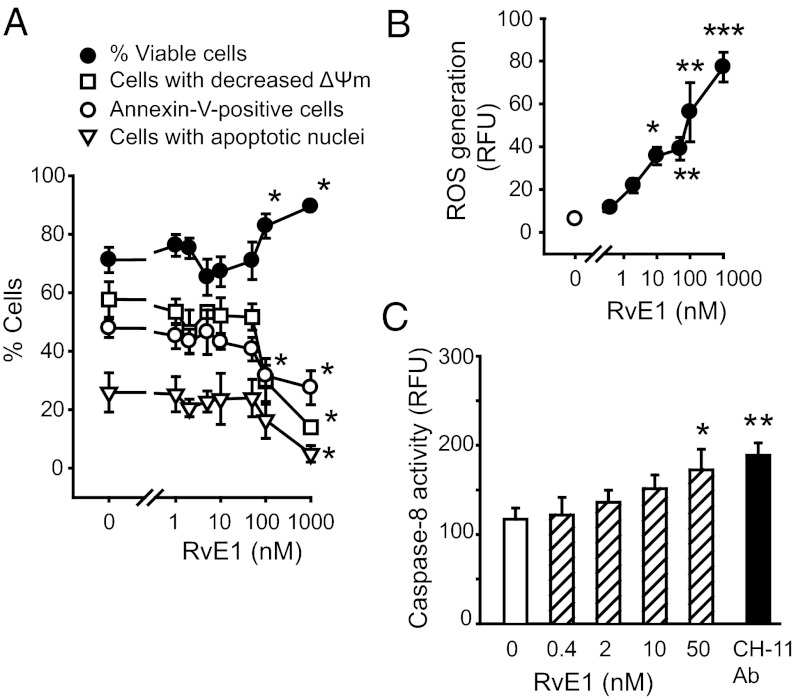
Confirming previous observations (17), RvE1 at lower concentrations (1–50 nM) did not affect apoptosis of naive isolated neutrophils. However, at higher pharmacological concentrations (100–1,000 nM), it prolonged neutrophil longevity by delaying intrinsic apoptosis (Fig. 1A). This was associated with modest elevations in reactive oxygen species (ROS) generation (Fig. 1B and Fig. S1) and caspase-8 activity (Fig. 1C) and robust increases in phosphorylation of ERK (Fig. S1). In subsequent experiments, we used RvE1 at concentrations ≤10 nM.
Fig. 1.
Effects of RvE1 on neutrophil apoptosis. Human neutrophils (5 × 106 cells/mL) were cultured for 24 h with RvE1 and viability, mitochondrial transmembrane potential (ΔΨm) [chloromethyl-X-rosamine (CMXRos) staining] and apoptosis (annexin-V–FITC binding and nuclear DNA content) were assessed. (A) RVE1 at high concentrations suppresses intrinsic apoptosis. Data are means ± SEM (n = 4–11). *P < 0.05 vs. untreated. (B) To monitor ROS production, neutrophils were loaded with H2DCFDA (5 μM) and then left untreated (C, control) or challenged with RvE1 for 15 min. Data are means ± SEM (n = 4–7). *P < 0.05; **P < 0.01; ***P < 0.001 vs. untreated. (C) Caspase-8 activity was assessed at 4 h post-RvE1 with flow cytometry using FITC-labeled z-Ile-Glu(Ome)-Thr-Asp(Ome)-fluoromethylketone (z-IETD-fmk) as a substrate. The effect of the Fas-activating antibody (CH-11 Ab) is shown for comparison. (n = 4–7). *P < 0.05; **P < 0.01 vs. untreated.
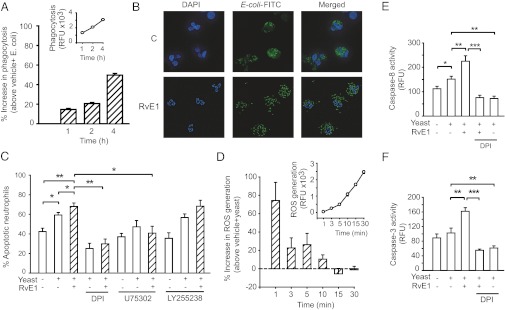
Earlier studies showed that RvE1 enhances phagocytosis of bacteria and apoptotic cells by macrophages (13, 14, 17). We used opsonized E. coli and yeast as target pathogens to evaluate the impact of RvE1 on phagocytosis-induced apoptosis. RvE1-enhanced phagocytosis of FITC-labeled opsonized E. coli by human neutrophils (Fig. 2 A and B). Consistent with published data (21), neutrophils cultured for 24 h with opsonized yeast underwent apoptosis (Fig. 2C). Phagocytosis evoked a rapid, robust ROS production and increased caspase-8 and caspase-3 activity (Fig. 2 D–F). These were further enhanced by RvE1, resulting in augmented neutrophil apoptosis (Fig. 2C). RvE1 augmentation of apoptosis was even more pronounced at 4-h culture (Fig. S2). The RvE1 actions were prevented by the NADPH oxidase inhibitor diphenyleneiodonium (DPI) or the BLT1 antagonist U75302, but not the BLT2 antagonists LY255238 (Fig. 2C). We confirmed that neutrophils express high levels of BLT1, whereas ChemR23 expression was undetectable (Fig. S3).
Fig. 2.
RvE1 enhances phagocytosis-induced neutrophil apoptosis. (A and B) Human neutrophils (5 × 106 cells/mL) were mixed with opsonized FITC-labeled E. coli at a ratio of 1:10 for 1, 2, and 4 h. Extracellular fluorescence was quenched with 0.2% trypan blue and intracellular fluorescence was analyzed with flow cytometry (A) or fluorescence microscopy (B). Inset: representative time course of neutrophil phagocytosis of bacteria. Results are expressed as percentage increase above vehicle plus E. coli (n = 4). (C) Neutrophils were cultured for 24 h with yeast (five yeast particles/neutrophil) with or without RvE1 (10 nM), U75302 (1 μM), LY255238 (1 μM), or DPI (20 μM), stained with acridine orange (10 μg/mL), and apoptosis was assessed by nuclear morphology (condensed or fragmented chromatin) under a fluorescence microscope. Data are means ± SEM (n = 5–6). *P < 0.05; **P < 0.01. (D) ROS generation. The effects of RvE1 are expressed as percentage increase above vehicle plus yeast. Inset: Time course of ROS production by phagocytosing neutrophils. Data are means ± SEM (n = 5). Caspase-8 (E) and caspase-3 (F) activity was assessed at 4 h post-RvE1 with flow cytometry using FITC-labeled z-IETD-fmk and acetyl-Asp-Glu-Val-Asp-fluoromethylketone (Ac-DEVD-fmk), respectively, as substrates. Data are means ± SEM (n = 6). *P < 0.05; **P < 0.01; ***P < 0.001.
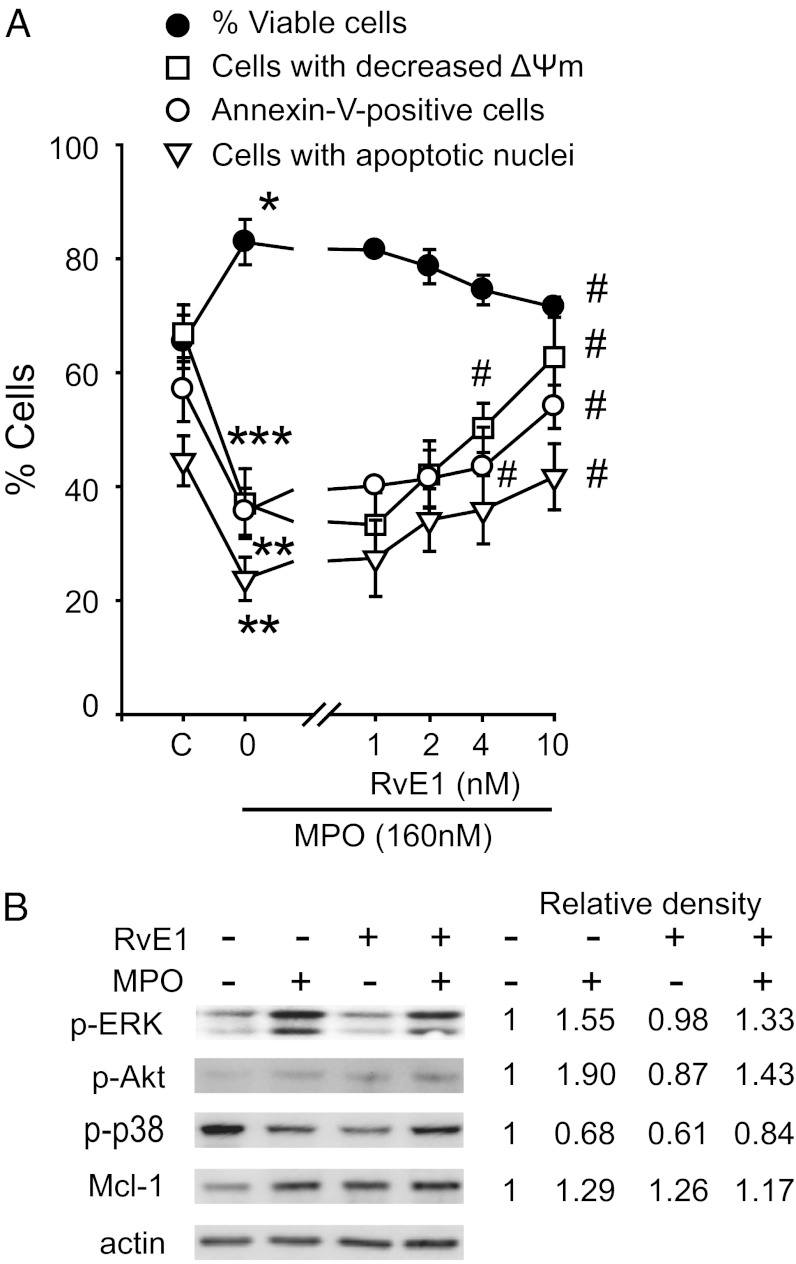
Because RvE1 induces ROS production and ROS may contribute to constitutive neutrophil apoptosis (22), we investigated whether RvE1 could interfere with survival signals from the neutrophil granule enzyme MPO, the acute-phase protein serum amyloid A (SAA) or the bacterial constituent CpG DNA. We have chosen these mediators because of their relevance to acute lung injury (23–25) and their known neutrophil apoptosis-suppressing action through the β2-integrin Mac-1 (6), formyl-peptide receptor 2 (FPR2)/lipoxin A receptor (ALX) (26), and toll-like receptor 9 (TLR9) (27), respectively. As anticipated, MPO (Fig. 3A), SAA, and CpG DNA (Fig. S4) prevented disruption of mitochondrial transmembrane potential (ΔΨm), which precedes development of apoptotic morphology in neutrophils undergoing constitutive apoptosis (26, 28). Exposure of neutrophils to RvE1 (1–10 nM) mitigated the intracellular survival signals produced from MPO (Fig. 3B), SAA, or CpG DNA (Fig. S4), and reduced neutrophil survival by promoting apoptosis. Because MPO, SAA, and CpG DNA evoke rapid phosphorylation of ERK 1/2 and Akt, leading to preservation of Mcl-1 (6, 26, 27), a key regulator of neutrophil apoptosis (29), we further probed the effects of RvE1 on these signaling pathways. RvE1 exerted modest inhibitory actions on ERK and Akt phosphorylation, and Mcl-1 expression evoked by MPO (Fig. 3B), SAA, or CpG DNA (Fig. S4). No significant changes were detected in p38 MAPK phosphorylation (Fig. S4). Furthermore, RvE1 attenuated MPO-stimulated Mac-1 expression on neutrophils in a concentration-dependent fashion (Fig. S5). MPO, SAA, and CpG DNA attenuated activation of caspase-8 that occurs during spontaneous neutrophil apoptosis, and this was almost completely prevented by RvE1 (Fig. S6). Caspase-8 activation is attributed to NADPH oxidase-derived ROS (21, 30). RvE1 reversal of survival signals from MPO, SAA, and CpG DNA was blocked with apocynin and DPI (Fig. S6), suggesting that RvE1 also activated this pathway. RvE1 did not affect Fas-induced neutrophil apoptosis (Fig. S7).
Fig. 3.
RvE1 attenuates MPO suppression of neutrophil apoptosis. (A) Human neutrophils (5 × 106 cells/mL) were cultured for 20 min with RvE1 then with MPO (160 nM) for 24 h. Viability, mitochondrial transmembrane potential (ΔΨm) (CMXRos staining), and apoptosis (annexin-V–FITC binding and nuclear DNA content) were then assessed. Data are means ± SEM (n = 4–7). *P < 0.05; **P < 0.01; ***P < 0.001 vs. untreated (C, control). #P < 0.01 vs. MPO-treated. (B) Impact on MAP kinases and Mcl-1. Neutrophils were lysed after culture with MPO (160 nM) ± RvE1 (10 nM) for 30 min (MAP kinases) or 1 h (for Mcl-1). Proteins were subjected to immunoblotting with antibodies to phosphorylated kinases, Mcl-1, or actin. Numerical values indicate relative density of bands normalized with density of actin bands. Results are representative of three separate experiments.
RvE1 Promotes Neutrophil Apoptosis and Enhances Resolution of Lung Inflammation.
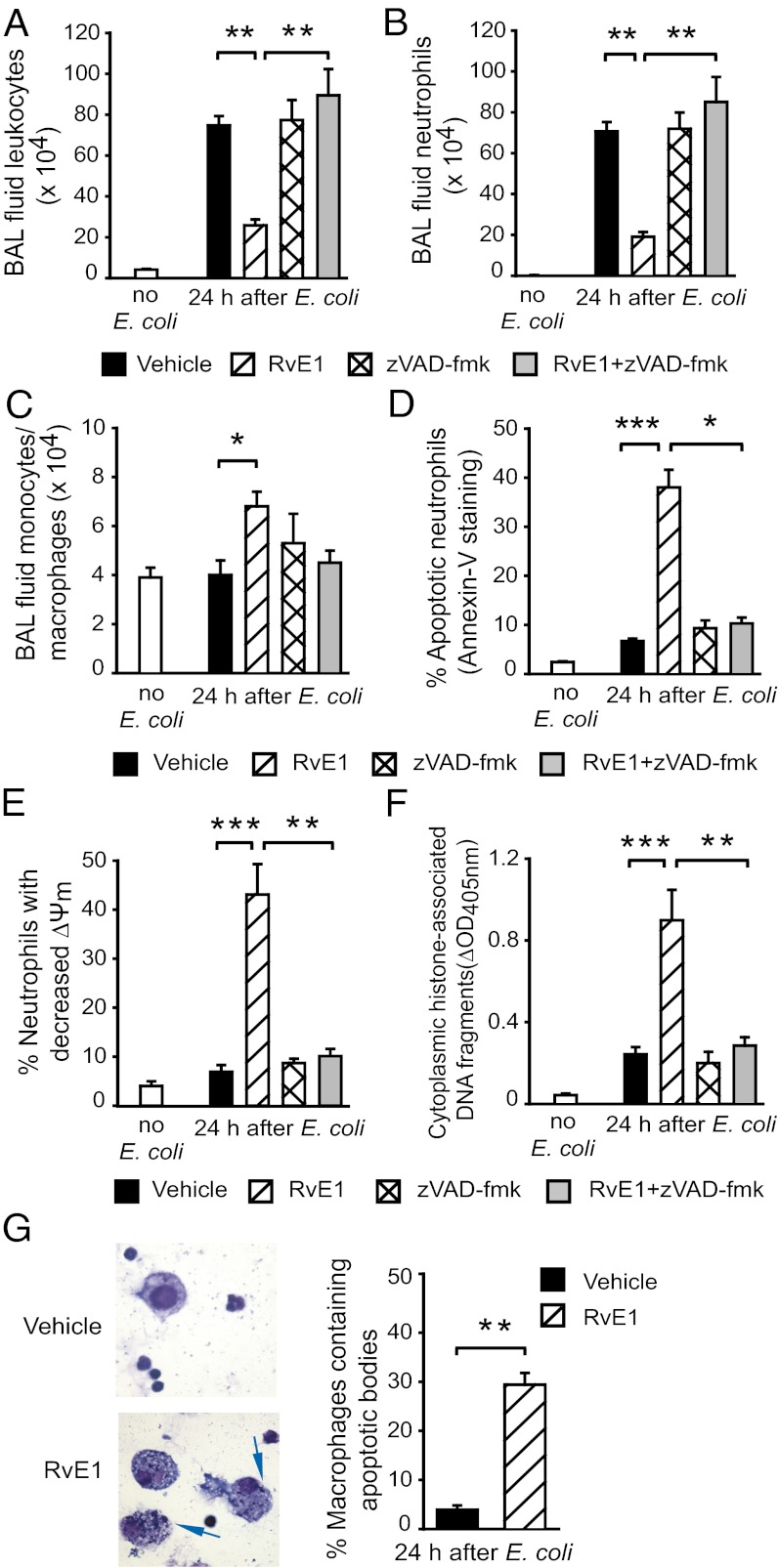
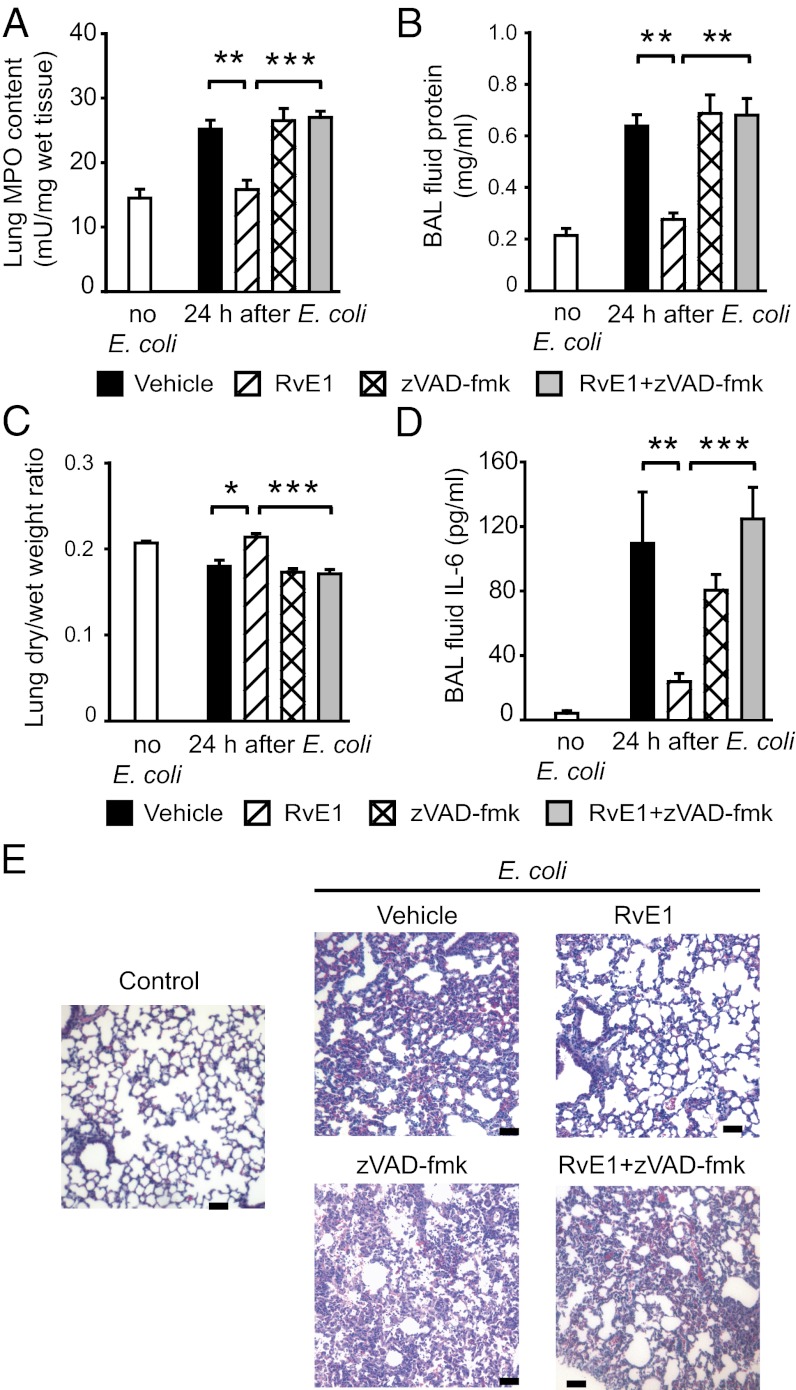
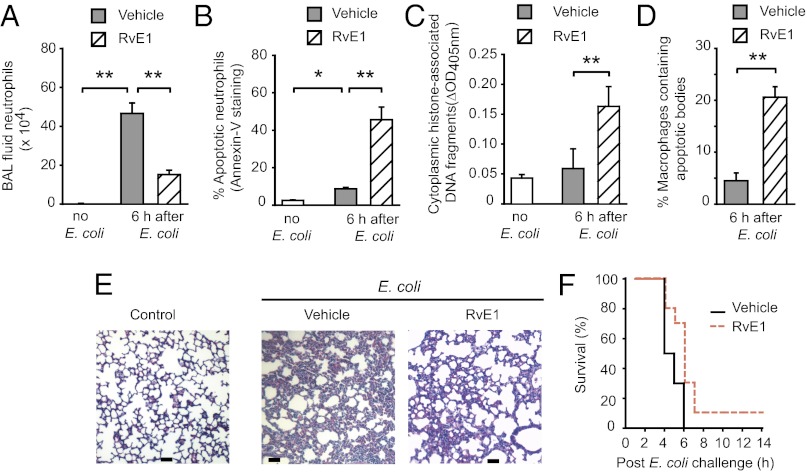
Having shown the ability of RvE1 to promote neutrophil apoptosis in vitro, we investigated the impact of RvE1 treatment on the resolution of inflammation in three mouse models of neutrophil-mediated acute lung injury. In the E. coli pneumonia model, treatment with RvE1 reduced neutrophil accumulation in the airways (Fig. 4 A and B) and increased bronchoalveolar lavage (BAL) fluid monocyte/macrophage numbers (Fig. 4C) concomitant with increases in the number of apoptotic neutrophils (Fig. 4 D–F) and macrophages containing apoptotic bodies (Fig. 4G). Antiinflammatory actions of RvE1 were also evident, as indicated by markedly reduced inflammatory cell infiltrate (Fig. 5A), attenuation of edema formation (Fig. 5 B and C), and BAL fluid IL-6 level (Fig. 5D) and less-severe lung injury (Fig. 5E). The i.p. administration of the pan-caspase inhibitor zVAD-fmk prevented the beneficial actions of RvE1 (Figs. 4 and 5), indicating a critical role of neutrophil apoptosis in these events.
Fig. 4.
RvE1 enhances resolution of E. coli–evoked pneumonia. Six hours after intratracheal instillation of 107 live E. coli, mice were treated with vehicle or RvE1 (25 μg/kg, i.p.) and/or the pan-caspase inhibitor zVAD-fmk (10 μg/kg, i.p. three times at 4-h intervals). Mice were killed 24 h later and BAL fluid total leukocyte (A), neutrophil (B), and monocyte/macrophage counts (C), the percentage of annexin-V–positive (apoptotic) neutrophils (D), the percentage of neutrophils with decreased mitochondrial transmembrane potential (ΔΨm) (E), BAL cell cytoplasmic histone-associated DNA fragments (F), and the number of BAL fluid macrophages containing apoptotic bodies (arrows, Left) (G) were determined. Data are means ± SEM (n = 6–8 mice per group). *P < 0.05, **P < 0.01; ***P < 0.001.
Fig. 5.
RvE1 enhances resolution of E. coli–evoked pneumonia. Six hours after intratracheal instillation of 107 live E. coli, mice were treated with vehicle or RvE1 (25 μg/kg, i.p.) and/or the pan-caspase inhibitor zVAD-fmk (10 μg/kg, i.p. three times at 4-h intervals). Mice were killed 24 h later and lung myeloproxidase content (A), BAL fluid protein (B), lung dry-to-wet weight ratio (C), and BAL fluid IL-6 level (D) were determined. Data are means ± SEM (n = 6–8 mice per group). *P < 0.05, **P < 0.01; ***P < 0.001. (E) Lung tissue sections from naive mice (control), mice with inflammation evoked by E. coli, or mice treated with vehicle, RvE1, and/or zVAD-fmk for 24 h. Hematoxylin and eosin stain (H&E); scale bars: 100 μm.
In the E. coli peritonitis–associated ALI model, treatment with RvE1 decreased BAL fluid neutrophil numbers without affecting monocyte/macrophage numbers (Fig. 6A and Fig. S8) and reduced lung MPO content (Fig. S8) with concomitant increases in the number of apoptotic neutrophils (Fig. 6 B and C). RvE1 also increased the number of macrophages containing apoptotic bodies (Fig. 6D), attenuated pulmonary edema and BAL fluid IL-6 (Fig. S8), and tissue injury (Fig. 6E). E. coli (109) challenge resulted in a mortality rate of 70% within 6 h; whereas, 70% of RvE1-treated mice were alive at 6 h (Fig. 6F).
Fig. 6.
RvE1 attenuates lung inflammation and prolongs survival of mice with E. coli peritonitis. One hour after i.p. injection of 2 × 108 live E. coli, mice were treated with vehicle or RvE1 (25 μg/kg, i.p.). Mice were killed at 6-h post–E. coli and BAL fluid neutrophil counts (A), the percentage of annexin-V–positive (apoptotic) neutrophils (B), BAL fluid cell cytoplasmic histone-associated DNA fragments (C), and the percentage of macrophages containing apoptotic bodies (D) were determined. Data are means ± SEM (n = 6 mice per group). *P < 0.05, **P < 0.01. (E) Lung tissue sections from naive mice (control) and mice challenged with E. coli and treated with RvE1 or vehicle. H&E stain; scale bars: 100 μm. (F) Kaplan-Meier survival plots for mice challenged intraperitoneally with 109 live E. coli and treated with RvE1 or vehicle (n = 10 per group). P = 0.0315 by the log-rank test.
The proresolving action of RvE1 was also investigated in the carrageenan plus MPO instillation-induced lung injury model. In this model, carrageenan evokes a spontaneously self-resolving inflammation that can be prolonged by coadministration of MPO parallel with suppression of neutrophil apoptosis (6). Treatment with RvE1 facilitated resolution of established inflammation when administered at near the peak of inflammation (24 h) (Fig. S9). RvE1 decreased BAL fluid total leukocyte and neutrophil numbers with increases in the number of monocytes/macrophages (Fig. S9). Reduced neutrophil accumulation occurred parallel with increases in the number of apoptotic neutrophils as assessed by positive staining for annexin V, increased caspase-3 activity, the amount of cytoplasmic histone-associated DNA fragments, and collapse of ΔΨm (Fig. S9). RvE1 increased the number of macrophages containing apoptotic bodies, reduced edema formation, BAL fluid IL-6 level, and lung injury (Fig. S9). Coadministration of zVAD-fmk with RvE1 rendered animals resistant to treatment with RvE1 (Fig. S9).
Discussion
Clearance of neutrophils from inflamed tissues is fundamental to resolution of inflammation. Omega-3 polyunsaturated fatty acid–derived lipid mediators, including RvE1, formed during the resolution phase of acute inflammation attenuate proinflammatory mediator responses (13, 16–18) and neutrophil infiltration, and enhance phagocytosis of apoptotic cells (2, 31). The present results indicate that by promoting apoptosis in neutrophils in situ, RvE1 enhances resolution of inflammation in three different mouse models of ALI/acute respiratory distress syndrome (ARDS). RvE1 augments phagocytosis-induced apoptosis and counters intracellular prosurvival signals from diverse proinflammatory mediators in human neutrophils in vitro.
Our results identify RvE1 as a modulator of neutrophil apoptosis, an important control point of the resolution of inflammation (2, 5) and provide several insights into the underlying molecular mechanisms. At concentrations ≤ 10 nM, RvE1 per se did not affect the constitutive death program in neutrophils (17, and the present study), whereas it activated intracellular pathways to enhance phagocytosis-induced apoptosis and to counter prosurvival signals. However, RvE1 at 100–1,000-nM concentration range evoked modest increases in ROS production and caspase-8 activity, but also triggered competing survival signals through sustained ERK and Akt activation, shifting the balance in neutrophils toward survival. These latter actions of RvE1 resemble the actions of GM-CSF that delay neutrophil apoptosis (21).
Phagocytosis of complement-opsonized targets requires Mac-1 and induces apoptosis via NADPH oxidase-derived ROS-mediated activation of caspase-8 (21). This occurs despite ERK activation following Mac-1 ligation (32), indicating that cross-talk between intracellular pro- and anti-apoptotic signals generated in phagocytosing neutrophils places the balance on the apoptotic pathway triggered by phagocytosis. Our findings show that RvE1 enhances phagocytosis of opsonized bacteria and yeast, which, in turn, leads to increased ROS generation by NADPH oxidase and activation of caspase-8 and caspase-3, reinforcing the shift of balance toward apoptosis. This conclusion relies on pharmacological inhibition of NADPH oxidase with DPI. Although DPI also inhibits other flavoprotein-using enzymes, including nitric oxide (NO) synthase, NO is not essential for phagocytosis-induced neutrophil apoptosis (30). Of note, RvE1 in the concentration range of 1–10 nM was reported to enhance phagocytosis of zymosan, live E. coli, and apoptotic neutrophils by human and murine macrophages, leading to a macrophage phenotype switch without evoking apoptosis (13, 14, 17).
Earlier studies showed that MPO signaling through Mac-1 (6), SAA through FPR2/ALX (26) and CpG DNA through TLR-9 (27) generate survival cues for neutrophils through concomitant activation of the ERK and Akt pathways, leading to prevention of Mcl-1 degradation and collapse of mitochondrial function. In human neutrophils, RvE1 produced modest decreases in ERK and Akt phosphorylation by MPO, SAA, or CpG DNA and evoked ROS generation and caspase-8/caspase-3 activation, consistent with enhanced apoptosis. Complete blockade of the RvE1 actions by apocynin and DPI lend additional support for RvE1 activation of the NADPH oxidase-derived ROS-caspase-8 proapoptosis circuit. Of note, RvE1 also decreased MPO-stimulated up-regulation of Mac-1, implicating disruption of an MPO-mediated autocrine and paracrine loop for perpetuation of the inflammatory response (6).
RvE1 can interact with the LTB4 receptor BLT1 and the ChemR23 receptor (15, 31), both of which may mediate cell type–specific actions. In contrast to BLT1, human neutrophils express low levels of ChemR23 mRNA, but the expression and function of the ChemR23 protein is still uncertain (14, 15). In our experiments, ChemR23 protein expression was undetectable with flow cytometry. RvE1 has been reported to act as a partial agonist on BLT1 and to antagonize the proinflammatory actions of LTB4 mainly through inhibition of neutrophil trafficking (15). Our results with selective LTB4-receptor antagonists now identify BLT1 as the predominant receptor mediating the apoptosis promoting action of RvE1 in vitro, indicating that resolution mechanisms may also be activated via BLT1. Thus, RvE1 may exert different proresolution actions via distinct receptors. It promotes neutrophil apoptosis through BLT1 and enhances phagocytosis of apoptotic cells through ChemR23 (14, 33), which suggests that concurrent actions on both receptors may be critical for optimal resolution. It is interesting to note that clearance of apoptotic cells via ChemR23 also occurs at similar low- or subnanomolar RvE1 concentrations (14, 17, 34) as those required to induce neutrophil apoptosis.
Extending observations in mouse models of aspiration pneumonia and asthma (18–20), we also found that RvE1 facilitates resolution of inflammation in clinically relevant mouse models of ALI/ARDS; i.e., intratracheal instillation of E. coli or carrageenan plus MPO and E. coli septicemia–associated acute lung injury. Although the benefits of a diet enriched with EPA have been recognized in reducing disease severity and mortality in patients with ALI or acute respiratory distress syndrome (35, 36) parallel with reduced neutrophil accumulation in the lung (37), the current results raise the possibility that EPA-derived RvE1 mediated some of these clinical effects. MPO, SAA, and CpG DNA have been implicated in mediating E. coli pneumonia and E. coli septicemia–associated lung injury (23–25), whereas carrageenan evokes self-resolving pulmonary inflammation that can be prolonged by coadministration of MPO (6). Our results indicate that enhanced resolution of inflammation by RvE1 was intimately linked to caspase-dependent apoptosis of neutrophils within the airways. Thus, RvE1 promoted apoptosis in large proportions of BAL fluid neutrophils. Furthermore, the pan-caspase inhibitor zVAD-fmk prevented RvE1-induced dramatic drop in the number of neutrophils in the airways and pulmonary tissues, indicating that neutrophil apoptosis is required for their timely clearance, and even aggravated lung injury likely due to persisting presence of neutrophils.
Pretreatment with RvE1 has been reported to reduce neutrophil recruitment in response to inflammatory stimuli (reviewed in 31). We now show that a bolus injection of RvE1 administered at near peak of inflammation was efficient to affect neutrophils within the lung, indicating its therapeutic potential. Thus, RvE1 attenuation of neutrophil trafficking combined with enhanced apoptosis and clearance contribute to its proresolution properties. Furthermore, RvE1 also reduced vascular permeability and release of the proinflammatory cytokine IL-6, consistent with an anti-inflammatory role. In our ALI models, we observed increased number of monocytes/macrophages in the airways in response to RvE1, which is consistent with facilitation of tissue repair (2, 38, 39) and the original properties defining RvE1 actions (12, 40). The high percentage of macrophages with apoptotic bodies indicates enhanced clearance by RvE1 of apoptotic neutrophils and other cells. Phagocytosis of apoptotic cells induces the release of mediators with proresolution and repair properties, such as IL-10 and TGF-β (2, 38), which contributes to the resolution.
Our in vitro and in vivo work shows that RvE1 shifts the balance between competing pro- and anti-apoptotic signals toward apoptotic pathways, thereby affecting the fate of emigrated neutrophils. RvE1 promotion of neutrophil apoptosis represents a clinically relevant mechanism for facilitating removal of emigrated neutrophils as we demonstrated here in three mouse models of ALI. Neutrophils will likely encounter RvE1 during inflammation when they have already received prosurvival cues from the inflammatory microenvironment and being engaged in phagocytosing opsonized pathogens. Thus, RvE1 joins the cyclin-dependent kinase inhibitor drugs (7, 8), 15-epi-LXA4 (9) and TNF-related apoptosis-inducing ligand (41) as neutrophil apoptosis-inducing agent, but exerts its actions through different and unique molecular mechanisms.
Taken together, these data provide evidence for a mechanism, facilitation of neutrophil apoptosis, by which eicosapentaenoic acid-derived RvE1 could promote resolution of ALI/ARDS. We found that RvE1 via the BLT1 receptor promoted phagocytosis-induced neutrophil apoptosis and attenuated anti-apoptosis signals from MPO, SAA, and CpG DNA in vitro. RvE1 administered at a clinically relevant time point, near the peak of inflammation consistently enhanced resolution of lung inflammation in three murine models of ALI by enhancing apoptosis in emigrated neutrophils and facilitating their removal by macrophages. Our findings reinforce the concept of therapeutic targeting of neutrophil apoptosis for enhancing the resolution of ALI/ARDS and other inflammatory pathologies.
Materials and Methods
Neutrophil Culture, Assessment of Apoptosis, and Phagocytosis.
Human neutrophils (purity >96%, apoptotic <3%), resuspended in HBSS containing 10% (vol/vol) autologous serum, were cultured with RvE1 (0.4–1,000 nM; Resolvyx Pharmaceuticals) and then challenged with MPO (160 nM), SAA (10 μg/mL), or E. coli DNA (CpG DNA, 1.6 μg/mL) with or without apocynin (100 μM) or diphenyleneiodonium chloride (20 μM). Apoptosis was assessed by annexin-V staining, nuclear morphology, DNA cleavage, and intracellular caspase-3 and caspase-8 activity (6, 26). For quantitative analysis of phagocytosis, neutrophils were mixed with opsonized FITC-labeled E. coli at a ratio of 1:10 and intracellular fluorescence was analyzed (21). To assess apoptosis, neutrophils were cultured for 24 h with Saccharomyces cerevisae (five yeast particles/neutrophil) with or without RvE1 (10 nM), the LTB4 receptor BLT1 antagonist U75302 (1 μM), the BLT2 receptor antagonist LY255238 (1 μM), or DPI (20 μM), and the percentage of neutrophils with apoptotic nuclei was determined.
Murine Acute Lung Injury.
Female BALB/c mice (aged 8–12 wk; Charles River Laboratories) were injected intraperitoneally 2 × 108 −109 live E. coli (9, 23) or intratracheally with 107 live E. coli or 0.1 mL of 0.25% λ-carrageenan plus 10 μL of 16-μM MPO (6). At the peak of inflammation, mice were treated with RvE1 (25 μg/kg, i.p.) or the pan-caspase inhibitor z-Val-Ala-DL-Asp-fluoromethylketone (zVAD-fmk; 10 μg/kg, three times at 4-h intervals). At the indicated times, the lungs were lavaged or processed for histology without lavage. BAL fluid leukocyte count, protein, IL-6 levels, and neutrophil apoptosis were determined (6). Full details and other methods are described in SI Materials and Methods.
Statistical Analysis.
Values represent mean ± SEM. Statistical comparisons were made by ANOVA using ranks followed by Dunn's multiple contrast hypothesis tests, the Wilcoxon signed rank test or by the Mann-Whitney u test. P <0.05 were considered statistically significant. Kaplan-Meyer survival curves were compared using the log-rank test.
Supplementary Material
Acknowledgments
We thank Dr. Charles N. Serhan for helpful discussions and critical reading of the manuscript. This work was supported by Grant MOP-97742 (to J.G.F.) from the Canadian Institutes of Health Research.
Footnotes
Conflict of interest statement: P.G. is president of Resolvyx Pharmaceuticals and retains founder stock in the company.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206641109/-/DCSupplemental.
References
- 1.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 4.Savill JS, et al. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 6.El Kebir D, József L, Pan W, Filep JG. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ Res. 2008;103:352–359. doi: 10.1161/01.RES.0000326772.76822.7a. [DOI] [PubMed] [Google Scholar]
- 7.Rossi AG, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 8.Koedel U, et al. Apoptosis is essential for neutrophil functional shutdown and determines tissue damage in experimental pneumococcal meningitis. PLoS Pathog. 2009;5:e1000461. doi: 10.1371/journal.ppat.1000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Kebir D, et al. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med. 2009;180:311–319. doi: 10.1164/rccm.200810-1601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matute-Bello G, et al. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;156:1969–1977. doi: 10.1164/ajrccm.156.6.96-12081. [DOI] [PubMed] [Google Scholar]
- 11.Keel M, et al. Interleukin-10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood. 1997;90:3356–3363. [PubMed] [Google Scholar]
- 12.Serhan CN, et al. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from ω-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arita M, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arita M, et al. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 16.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seki H, et al. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol. 2010;184:836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoki H, et al. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Haworth O, Cernadas M, Levy BD. NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation. J Immunol. 2011;186:6129–6135. doi: 10.4049/jimmunol.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang B, Hirahashi J, Cullere X, Mayadas TN. Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis: Cross-talk between caspase 8, reactive oxygen species, and MAPK/ERK activation. J Biol Chem. 2003;278:28443–28454. doi: 10.1074/jbc.M210727200. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Loison F, Luo HR. Neutrophil spontaneous death is mediated by down-regulation of autocrine signaling through GPCR, PI3Kgamma, ROS, and actin. Proc Natl Acad Sci USA. 2010;107:2950–2955. doi: 10.1073/pnas.0912717107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brovkovych V, et al. Augmented iNOS expression and increased NO production reduce sepsis-induced lung injury and mortality in myeloperoxidase-null mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L96–L103. doi: 10.1152/ajplung.00450.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bozinovski S, et al. Serum amyloid A opposes lipoxin A₄ to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc Natl Acad Sci USA. 2012;109:935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz DA, et al. CpG motifs in bacterial DNA cause inflammation in the lower respiratory tract. J Clin Invest. 1997;100:68–73. doi: 10.1172/JCI119523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Kebir D, et al. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: A novel mechanism for resolution of inflammation. J Immunol. 2007;179:616–622. doi: 10.4049/jimmunol.179.1.616. [DOI] [PubMed] [Google Scholar]
- 27.József L, Khreiss T, Filep JG. CpG motifs in bacterial DNA delay apoptosis of neutrophil granulocytes. FASEB J. 2004;18:1776–1778. doi: 10.1096/fj.04-2048fje. [DOI] [PubMed] [Google Scholar]
- 28.Maianski NA, et al. Functional characterization of mitochondria in neutrophils: A role restricted to apoptosis. Cell Death Differ. 2004;11:143–153. doi: 10.1038/sj.cdd.4401320. [DOI] [PubMed] [Google Scholar]
- 29.Moulding DA, Quayle JA, Hart CA, Edwards SW. Mcl-1 expression in human neutrophils: Regulation by cytokines and correlation with cell survival. Blood. 1998;92:2495–2502. [PubMed] [Google Scholar]
- 30.Coxon A, et al. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: A homeostatic mechanism in inflammation. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 31.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLeish KR, Klein JB, Coxon PY, Head KZ, Ward RA. Bacterial phagocytosis activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades in human neutrophils. J Leukoc Biol. 1998;64:835–844. [PubMed] [Google Scholar]
- 33.Cash JL, et al. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med. 2008;205:767–775. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell EL, et al. Resolvin E1 promotes mucosal surface clearance of neutrophils: A new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 35.Gadek JE, et al. Enteral Nutrition in ARDS Study Group Effect of enteral feeding with eicosapentaenoic acid, γ-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Crit Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Pontes-Arruda A, Aragão AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, γ-linoleic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med. 2006;34:2325–2333. doi: 10.1097/01.CCM.0000234033.65657.B6. [DOI] [PubMed] [Google Scholar]
- 37.Pacht ER, et al. Enteral nutrition with eicosapentaenoic acid, γ-linolenic acid, and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Crit Care Med. 2003;31:491–500. doi: 10.1097/01.CCM.0000049952.96496.3E. [DOI] [PubMed] [Google Scholar]
- 38.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: New opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 39.Hasturk H, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 40.Hong S, et al. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 41.McGrath EE, et al. TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. J Leukoc Biol. 2011;90:855–865. doi: 10.1189/jlb.0211062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.