Abstract
Recent findings suggest that neurons can efficiently repair oxidatively damaged DNA, and that both DNA damage and repair are enhanced by activation of excitatory glutamate receptors. However, in pathological conditions such as ischemic stroke, excessive DNA damage can trigger the death of neurons. Oxidative DNA damage is mainly repaired by base excision repair (BER), a process initiated by DNA glycosylases that recognize and remove damaged DNA bases. Endonuclease VIII-like 1 (NEIL1) is a DNA glycosylase that recognizes a broad range of oxidative lesions. Here, we show that mice lacking NEIL1 exhibit impaired memory retention in a water maze test, but no abnormalities in tests of motor performance, anxiety, or fear conditioning. NEIL1 deficiency results in increased brain damage and a defective functional outcome in a focal ischemia/reperfusion model of stroke. The incision capacity on a 5-hydroxyuracil–containing bubble substrate was lower in the ipsilateral side of ischemic brains and in the mitochondrial lysates of unstressed old NEIL1-deficient mice. These results indicate that NEIL1 plays an important role in learning and memory and in protection of neurons against ischemic injury.
Increased oxidative stress and decreased DNA repair occur during normal brain aging and in disorders in which neurons degenerate, including stroke (1) and Alzheimer’s disease (2, 3). Neuronal cells encounter relatively more oxidative DNA damage due to their high metabolic rate, which is necessary for maintaining their electrochemical signaling functions. Reactive oxygen species generated as a byproduct of metabolic activities can create oxidative DNA lesions in addition to damage to other cellular constituents. Unrepaired oxidative DNA lesions can cause detrimental effects to the cell, including dysregulation of gene expression and cell death. The integrity of DNA repair systems is therefore presumed to be an important prerequisite for proper brain function and oxidative stress resistance.
Oxidative DNA damage is repaired mainly by the base excision repair (BER) system (4). BER is initiated by a DNA glycosylase that recognizes and removes the damaged base. This activity is followed by an endonuclease to process the abasic site, and then a DNA polymerase completes gap filling. The reaction is completed by the activity of a DNA ligase, which seals the nick. Although there are 11 known DNA glycosylases in higher vertebrates, oxidative base lesions are mainly removed by 8-oxoguanine DNA glycosylase 1 (OGG1), endonuclease III like-1 (NTH1), and endonuclease VIII-like (NEIL) family members (NEIL1, -2, and -3) (5).
NEIL1 is a versatile DNA repair enzyme as it has a broad range and overlapping substrate specificity with other DNA glycosylases. NEIL1 can efficiently recognize and remove 5-hydroxyuracil, 5-hydroxycytosine, thymine glycol, and formamidopyrimidine (FAPY) lesions (5). NEIL1 also participates in interstrand cross-link repair and nucleotide excision repair in addition to BER (6, 7). NEIL1 activity is stimulated by physical and functional interactions with several DNA repair and maintenance proteins. These include X-ray repair cross-complementing protein 1 (XRCC1) in complex with polymerase β and ligase IIIα, flap structure-specific endonuclease 1 (FEN1), Werner syndrome, RecQ helicase-like (WRN), proliferating cell nuclear antigen (PCNA), Rad9, Hus1, Rad1 (9-1-1) complex, and Cockayne syndrome group B (CSB) proteins (8). NEIL1 has been shown to localize to mitochondria and nuclei (9). In one study it was shown that neil1−/− mice develop metabolic syndrome and mitochondrial damage, although others have not found a phenotype in NEIL1-deficient mice (8, 10). Further, neil1−/− mice exhibit elevated levels of FAPY lesions in their brains, kidneys, and liver (11). In situ hybridization studies in mouse brain showed that mNEIL1 is widely expressed throughout the brain and that its expression increases with age (12). However, roles of NEIL1 in normal brain function and neurological disorders have not been established.
Stroke is a neurological disease that causes brain damage and injury due to acute oxidative stress. Stroke is the third leading cause of death in the United States (140,000 deaths per year) (13). The role of BER in antagonizing acute oxidative stress may be more significant in stroke survivors. Therefore, understanding the role of BER and its components in animals is a valuable tool. We previously showed that OGG1 prevents the death of neurons in a mouse model of focal ischemic stroke (14). In that study, we also showed that FAPY lesions accumulate even more than 8-oxoG lesions after ischemic stroke in wild-type mice (14). The FAPY lesions are mainly removed by NEIL1 and therefore NEIL1 may play a crucial role in stroke tolerance. Further, neil1−/− mice accumulate more FAPY lesions in their brains without external stress (11), suggesting a potential for compromise of normal brain function in neil1−/− mice.
In this study, we investigated the effect of NEIL1 deficiency on brain function and oxidative stress resistance under normal and induced ischemic stroke conditions. Unstressed animals were subjected to a battery of behavioral tests to study different brain functions. The results indicate that NEIL1 is important in normal brain function and neuronal stress resistance.
Results
Evidence That NEIL1 Is Critical for Memory Retention in Mice.
We first evaluated levels of NEIL1 protein and incision activity in brain tissue samples from 30- to 33-mo-old (n = 6 each) wild-type (WT) and NEIL-1-deficient (neil1−/−) mice. As expected, NEIL1 protein was present in brain tissue samples from WT mice and was not detected in brain tissue samples from neil1−/− mice (Fig. S1A, Left). Levels of thymine glycol incision capacity were significantly lower (∼25%) in nuclear lysates and 5-hydroxyuracil incision capacity was significantly lower (∼33%) in mitochondrial lysates in brain tissue samples from neil1−/− mice compared with WT mice (Fig. S1 A and B, Right and Table 1). A two-tailed Student t test was conducted for significance of the data and the obtained P value is <0.05.
Table 1.
Summary of incision assays conducted
| Sample | Stress | Substrate | Incision |
| Brain nuclear lysates | Unstressed | 5-Hydroxyuracil containing bubble DNA | No |
| Thymine glycol containing DNA | Yes* | ||
| Brain mitochondrial lysates | Unstressed | 5-Hydroxyuracil | Yes† |
| Brain whole cell lysates (Ipsilateral) | Ischemic stroke | 5-Hydroxyuracil | Yes‡ |
| Brain whole cell lysates (Contralateral) | Ischemic stroke | 5-Hydroxyuracil | No |
Significant difference between WT and neil1−/− mice where *P = 0.013, †P = 0.026, and ‡P < 0.05.
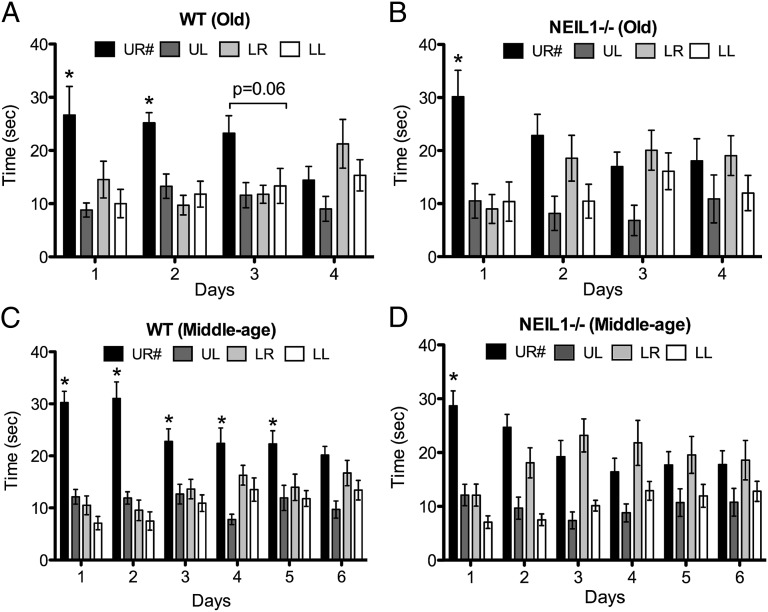
To examine the potential effects of NEIL1 deficiency on brain function, and in the context of aging, we performed a battery of behavioral tests in middle-aged (9- to 13-mo-old mice; n = 14 WT and 17 neil1−/−) and old (30- to 33-mo-old mice; n = 8 WT and 10 neil1−/−) cohorts. The behavioral tests included the Morris water maze (Fig. 1), rotarod, novel object recognition, fear conditioning, and open field tests (Fig. 2). Repeated measures using two-way ANOVA followed by a Bonferroni correction was used to evaluate the significance of the data. We observed a significant difference between the WT and neil1−/− groups in the water maze probe trial phase. All WT and neil1−/− were able to learn the location of the hidden platform as indicated by similar reductions in goal latencies across the 8 d of training (Fig. S2). Old WT mice were able to retain memory of the platform location for up to 2 d, [although not significant, there is a strong trend (P = 0.06) at the day 3 time point] after the last training session, as indicated by spending significantly more time in the target quadrant (Fig. 1A). In contrast, old neil1−/− mice were only capable of remembering the target location during the first 24 h posttraining and failed to recall the platform location when tested 2, 3, and 4 d after training (Fig. 1B). Middle-aged WT mice retained the memory of the platform location for 5 d after training, whereas middle-aged neil1−/− mice lost their recollection of the platform after the first posttraining day (Fig. 1 C and D). Collectively, the results of the water maze testing indicate that the neil1−/− mice are capable of cue-based learning about the hidden platform position as proficiently as WT mice, but exhibited a deficit in their ability to retain the memory about the platform location.
Fig. 1.
Loss of short-term spatial memory in neil1−/− mice during water maze probe trials. Data indicate average time spent in each quadrant ± SE. This test was conducted on age- and sex-matched neil1−/− and WT mice from two different age groups. (A) Old WT, n = 8. (B) Old neil1−/−, n = 10. (C) Middle-aged WT, n = 14. (D) Middle-aged neil1−/−, n = 17. X axis indicates the number of days for which the probe trials were conducted. *P < 0.005. UR#, Upper Right quadrant where the mice learnt to find a platform during the hidden platform training phase. The remaining quadrants are Upper Left (UL), Lower Right (LR), and Lower Left (LL). Repeated measures two-way ANOVA followed by Bonferroni’s test were used for analysis of variance.
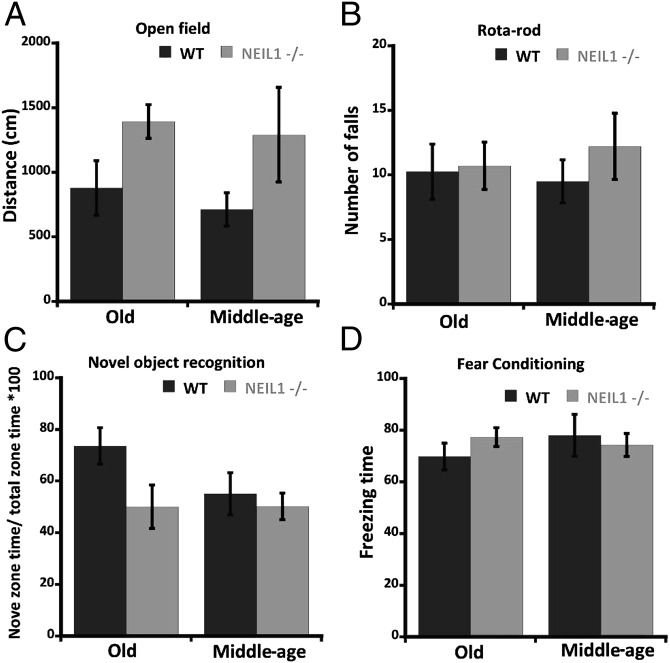
Fig. 2.
Behavioral tests conducted on WT and neil1−/− mice. (A) Open field test indicating the average distance covered by the mice in each group ± SE. (B) Rotarod test indicating the average number of falls within 5 min ± SE. (C) Novel object recognition test indicating the average percentage of time spent in the novel zone ± SE. (D) Fear conditioning test indicating average cued freezing time ± SE conducted on middle-aged and old cohorts. Repeated measures two-way ANOVA followed by Bonferroni’s test were used for analysis of variance.
In each of the other four behavioral tests that were performed, we observed no significant difference between WT and neil1−/− middle-aged or old mice (Fig. 2). These findings suggest that WT and neil1−/− mice have similar levels of anxiety (open field test), motor capabilities (rotarod test), curiosity and short-term memory (novel object recognition test), and short-term fear-based learning ability (fear conditioning test).
neil1−/− Mice Exhibit Greater Brain Damage, Increased Mortality, and a Poorer Functional Outcome in a Model of Focal Ischemic Stroke.
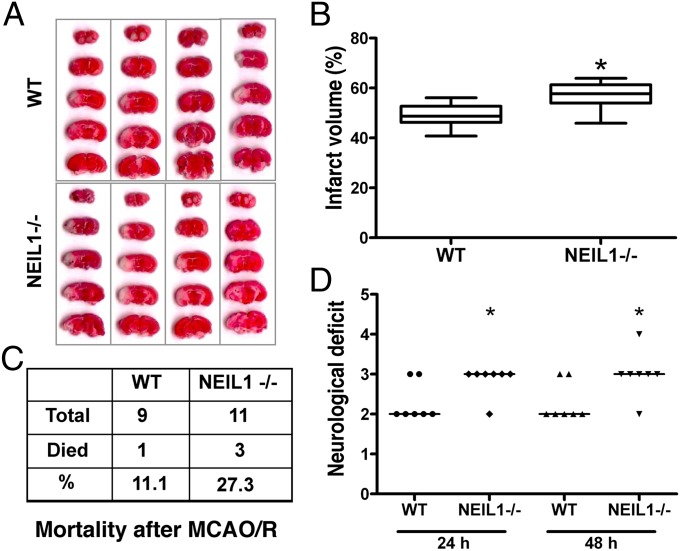
To study the role of NEIL1 and the BER pathway in brain cells subjected to severe stress, we used the middle cerebral artery occlusion/reperfusion (MCAO/R) focal ischemic stroke model using 9 WT and 11 neil1−/− mice at 9–13 mo of age. Cerebral blood flow was measured before, during, and after the surgery in each animal (averages WT: 100, 20, and 80% and neil1−/−: 100, 20, 65%, respectively). During surgery, WT and neil1−/− mice retained 20% of normal blood flow. There was a slight delay in return of blood flow in neil1−/− mice compared with wild type. Poststroke mortality rate, infarct volume, and neurological deficits were measured in each case. The infarct volume was measured in mouse brains that were alive at 48 h after stroke with 2,3,5-triphenyltetrazolium chloride (TTC) staining and neurological deficits are represented for these mice (n = 7 each). The infarct volume was significantly greater in neil1−/− mice compared with WT mice (P = 0.01) as compared using the Mann–Whitney test (Fig. 3 A and B). The observed mortality rate was about 2.5-fold higher in neil1−/− mice (27.3%) compared with WT mice (11.1%) after ischemic stroke (Fig. 3C). The neurological deficit score was also increased in neil1−/− mice compared with WT mice at both 24 and 48 h poststroke (Fig. 3D). The data were analyzed by Kruskal–Wallis one-way ANOVA and found to be significant (P < 0.05).
Fig. 3.
Infarct volume, mortality rate, and neurological deficit after middle cerebral artery occlusion/ reperfusion (MCAO/R) in WT and neil1−/− mice. (A) Mouse brain sections stained with 2,3,5-triphenyltetrazolium chloride (TTC) after 48 h of MCAO/R. (B) Quantification of the infarct volume shown in A. Data represent average percentage of infarct volume ± SE. Mann–Whitney test was performed to analyze these data. (C) Percentage of death in mice within 48 h of the MCAO/R procedure. (D) Scatterplot of the assigned neurological deficit score at 24 and 48 h after the MCAO/R procedure. Median of each data series is represented by a horizontal line. Data were analyzed using a Kruskal–Wallis one-way ANOVA test. *P value less than 0.05.
Loss of Motor Function in neil1−/− Mice After Ischemic Stroke.
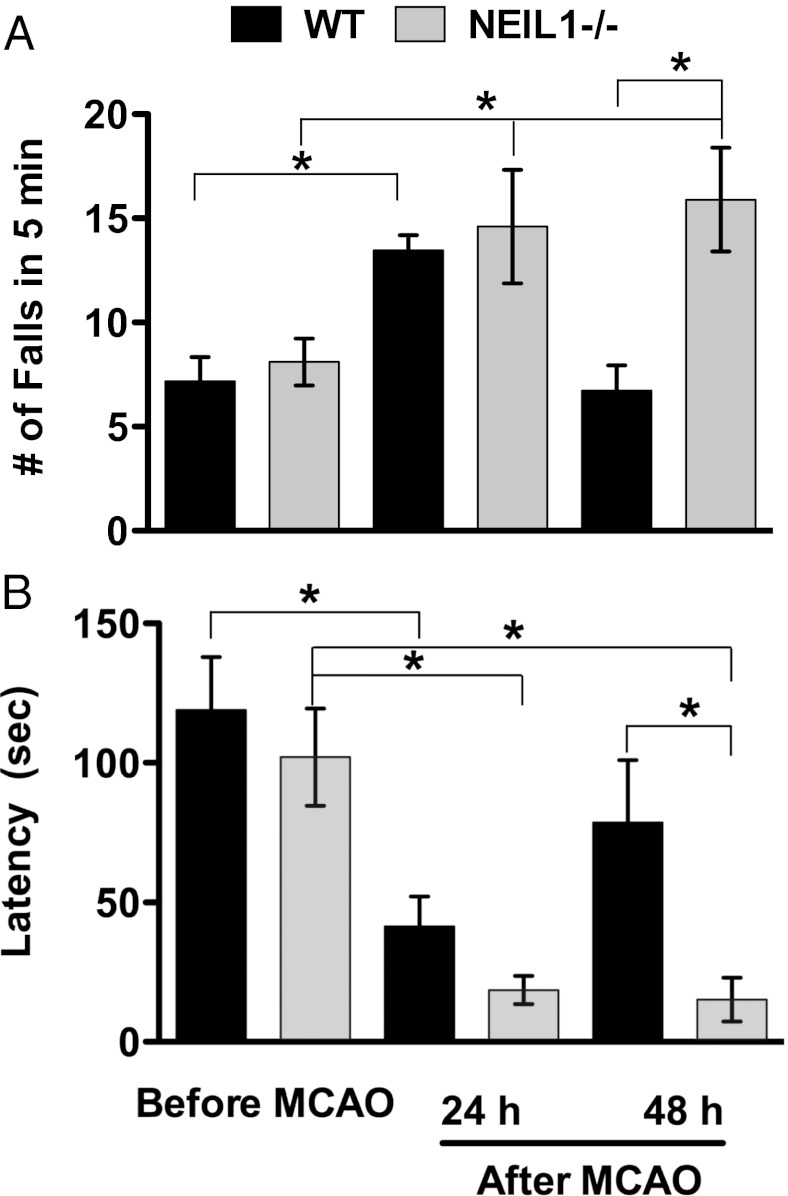
Stroke patients are prone to physical disability or complete loss of motor function (paralysis) due to the degeneration of neurons that occurs by a mechanism involving oxidative stress, DNA damage, and apoptosis (15, 16). To study the effect of loss of NEIL1 on motor function after ischemic stroke, a rotarod test was conducted at 24 and 48 h after stroke in WT (n = 11) and neil1−/− (n = 10) mice. Repeated measures two-way ANOVA with factors for time and animal tag number were analyzed. This was followed by a Bonferroni’s correction as well as least significant differences (LSDs) equivalent to pairwise t test were performed to compare mean prestroke performance with poststroke performance and analyze the significance of the data. Before stroke, NEIL1 mice did not show any difference on the rotarod test relative to WT mice (Fig. 2B). However, at 48 h after stroke the number of falls in 5 min on the rotarod was significantly greater in neil1−/− mice compared with WT mice (Fig. 4A). In other words, WT mice recovered from stroke effect more than neil1−/− mice. The latency to the first fall, an indictor of motor coordination and grip strength was significantly reduced in neil1−/− mice at both 24 and 48 h after MCAO/R (Fig. 4B). This suggests that the loss of NEIL1 in mice exacerbates stroke-induced dysfunction of the motor system, consistent with increased damage to the striatum and cortex in neil1−/− mice (Fig. 3A).
Fig. 4.
Motor dysfunction in neil1−/− and WT mice before and after middle cerebral artery occlusion/ reperfusion (MCAO/R). (A) Average number of falls and (B) average latency of first fall measured during rotarod test. Data indicate average ± SE, where the sample size is WT, n = 11 and neil1−/−, n = 10. A two-way ANOVA followed by Bonferroni’s correction as well as LSD was used to analyze the data. *P value less than 0.05.
NEIL1 Deficiency Results in Reduced DNA Repair Capacity and Enhanced Apoptosis in Brain Cells of Mice Subjected to Stroke.
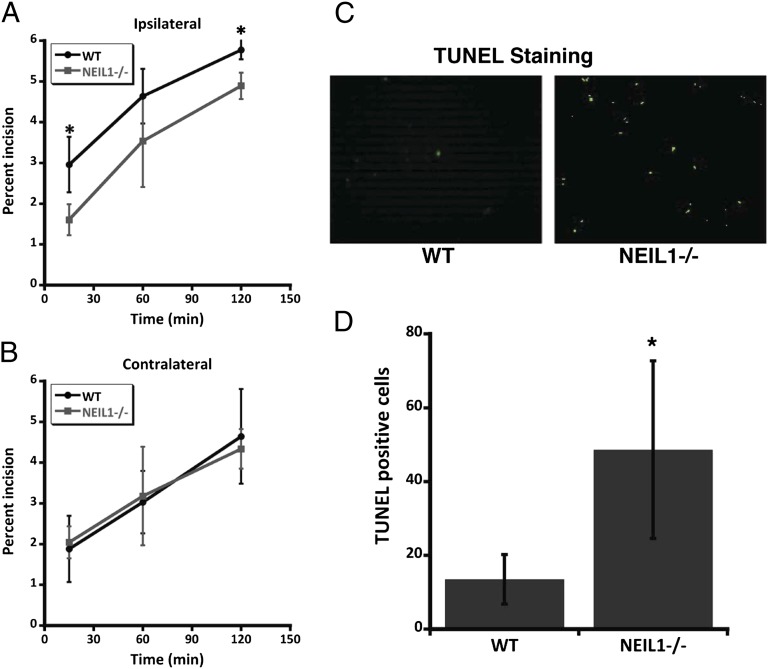
Previous data show that neil1−/− mouse brains accumulate more FAPY-adenine lesions and mitochondrial DNA damage (10, 11). In the present study, we demonstrated that NEIL1 deficiency resulted in greater brain damage and worsened functional outcome in a mouse model of focal ischemic stroke. Because NEIL1 deficiency would be predicted to increase DNA damage, and DNA damage can trigger apoptosis (17), we evaluated DNA repair capacity and apoptosis in the brains of six each of WT and neil1−/− mice that had been subjected to focal ischemic stroke. Data were analyzed by a two-tailed Student t test. The incision capacity in whole cell lysates of cerebral cortex tissue in the ischemic penumbra of neil1−/− mice was significantly reduced compared with WT mice (Fig. 5A). In contrast, levels of DNA incision capacity were similar in tissue from the nonischemic (contralateral) cerebral hemispheres of WT and neil1−/− mice (Fig. 5B).
Fig. 5.
Incision capacity and apoptosis in neil1−/− mouse brains after middle cerebral artery occlusion/ reperfusion (MCAO/R). (A and B) Percentage of incision of 5-hydroxyuracil containing bubble DNA substrate in whole brain lysates of ipsilateral and contralateral areas of the stroked brains. Data indicate average ± SE (*P < 0.05 using a two-tailed Student t test with a sample size of n = 6). (C) Representative picture of a mouse brain section after TUNEL staining with FITC-labeled dUTP and terminal deoxynucleotidyl transferase enzyme in WT and neil1−/− mouse brains after MCAO/R. (D) Quantification of TUNEL+ nuclei in WT and neil1−/− mice. An average of total TUNEL+ cells counted from multiple brain section areas (n = 6 each of WT and neil1−/−) is shown. Data are mean ± SD. *P < 0.05. Data were analyzed by two-tailed Student t test.
Many of the neurons that die after an ischemic stroke do so by a process of programmed cell death called apoptosis, a hallmark of which is nuclear DNA condensation and fragmentation (16). We therefore determined the impact of the reduced DNA repair capacity in neil1−/− mice on the vulnerability of neurons in the ischemic penumbra to apoptosis. A terminal deoxynucleotidyl transferase (TdT) dUTP nick end labeling (TUNEL) assay (single- and double-strand breaks were end labeled with fluorescein-attached dUTP by TdT enzyme) was performed on brain sections from neil1−/− and WT mice at 48 h after focal ischemia/reperfusion. We found that there were significantly more TUNEL+ cells in the ischemic penumbra of neil1−/− mice compared with WT mice (Fig. 5 C and D), indicating that NEIL1 deficiency renders neurons vulnerable to apoptosis following a stroke.
Discussion
Our findings reveal previously unknown roles for NEIL1 in long-term memory retention and in protecting the brain against damage caused by an ischemic stroke. Previous studies have provided evidence that BER is a major pathway for the repair of oxidatively modified DNA bases in neurons (17, 18). Oxidative DNA damage is elevated in neurons during the normal aging process, which may result in impaired expression of genes encoding proteins that play critical roles in synaptic plasticity, learning, and memory (19). We found that middle-aged and old NEIL1-deficient mice are able to learn to find the hidden platform in the water maze, with a rate of acquisition (i.e., learning curve) similar to that of WT mice. However, these mice exhibited a highly significant deficit in their ability to retain the memory of the platform location. Indeed, whereas middle-aged WT mice were able to recall the location of the platform for up to 5 d after training, the NEIL1-deficient mice were only able to recall the platform location for 1 d. Interestingly, our findings suggest that although NEIL1 is required for normal hippocampus-dependent long-term memory retention, this DNA repair enzyme is dispensable for short-term memory and for normal control of locomotion, anxiety, and fear behaviors. Our findings suggest that NEIL1 may be particularly important for the repair of genes encoding proteins critical for long-term memory, a possibility that merits investigation in future studies. No cognitive deficits were reported in previous studies of mice with reduced levels of XRCC1 and mice lacking OGG1 (20, 21), suggesting that NEIL1 may be particularly important in preserving cognitive function.
Whereas ours is unique evidence that NEIL1 plays an important role in protecting neurons against ischemic stroke, previous findings suggest important roles for other BER enzymes in neuroprotection. Mahabir et al. showed that DNA repair gene polymorphisms alter the risk of stroke (22). Luo Y et al. showed that BER activity is significantly reduced after ischemic stroke, implicating an altered posttranslational modification pathway (23). The effect of loss of BER-specific genes in ischemic stroke resistance has only recently been investigated. Cerebral cortical neurons in OGG1-deficient mice exhibit increased vulnerability to ischemia caused by permanent occlusion of the middle cerebral artery compared with WT mice (14). However, more FAPY guanine lesions rather than 8-oxoguanine accumulate after ischemic stroke (14). One of the major DNA glycosylases that repair FAPY lesions is NEIL1, and the present findings demonstrate increased neuronal death and defective functional outcome after focal ischemic stroke in neil1−/− mice compared with age-matched WT mice. We found that at 48 h after the stroke, mice lacking NEIL1 exhibited significantly poorer performance in the rotarod test of motor function compared with wild-type mice. However, because we did not evaluate motor function at later poststroke time points, we cannot rule out the possibility that lack of NEIL1 results in a delay in recovery of motor function rather than a long-lasting/permanent motor deficit. However, this indicates that NEIL1 is important in resistance to adverse effects caused by ischemic stroke. To corroborate these findings with loss of DNA repair capacity, we examined the incision capacity in the whole brain lysates and cellular fractions including nuclear and mitochondrial lysates from poststroke and old neil1−/− and WT mice, respectively (Table 1). We observed a significant reduction of 5-hydroxyuracil incision capacity in a bubble DNA substrate in the ipsilateral part of stroked brain tissues (Fig. 5A) but not in the contralateral part of NEIL1-deficient mice compared with WT mice. Further, the nuclear fractions from unstressed old mice were deficient in incising thymine glycol containing DNA substrate (Fig. 1A). However, the magnitude of the reduction of DNA incision capacity was relatively small, most likely because of overlapping substrate specificity of the NEIL1 enzyme with NEIL2 and NTH1 and because of other backup glycosylase activities. The incision assay may not be sensitive enough to show the level of accumulation of damage in the brain. Previous literature has comprehensively studied and reported that neil1−/− mouse brains accumulate more FAPY lesions in brain tissues using mass-spectrometry and gas-chromatography techniques, which are much more sensitive in measuring the number and type of accumulated oxidative lesions (11). Normal brain function depends on proper mitochondrial function due to the energy demands. DNA repair in mitochondria is necessary for mitochondrial function. NEIL1 localizes to nuclear and mitochondrial compartments (9). A previous study showed accumulation of mitochondrial DNA damage in neil1−/− mouse liver mitochondria (10). We therefore studied the levels of 5-hydroxyuracil incision capacity in the brain mitochondrial fractions from unstressed old mice. We observed a significantly lower incision capacity of 5-hydroxyuracil in a bubble DNA substrate in mitochondrial lysates of old neil1−/− mouse brains in a single end point incision assay. This suggests that neil1−/− mice do have a loss of DNA repair capacity, which could be one of the reasons for the brain dysfunction and vulnerability to oxidative stress.
NEIL1 was initially described as a backup glycosylase to the major DNA glycosylases OGG1 and NTH1, but recent developments in the field suggest that NEIL1 is a versatile DNA repair enzyme that participates in more than one repair pathways (7). Loss of NEIL1 may affect the functions of organs such as brain, liver, pancreas, and kidney as it is highly expressed in these areas (11). The data presented here confirm that NEIL1 is important in cells and not just a backup system for OGG1. Together our data suggest that the initial steps in the BER pathway play a significant role in protecting cells from stroke-induced adverse effects.
Materials and Methods
Mice.
neil1−/− mice were a generous gift from Steven Lloyd (Oregon Health and Science University, Portland, OR). The mice were maintained on a mixed background (C57 and 129), and littermate wild-type and neil1−/− mice were used for all experiments. Mice were maintained in a constant-temperature facility with a 12 h light/12 h dark cycle, and free access to food and water. All procedures were approved by the Animal Care and Use Committee of the National Institute on Aging Intramural Research Program.
Behavioral Tests.
The methods for rotarod, open field, novel object recognition, fear conditioning, and water maze were performed as described previously (24) and are detailed in SI Materials and Methods.
Middle Cerebral Artery Occlusion and Reperfusion.
The focal ischemia/reperfusion model was performed using the intraluminal suture technique described previously (25). The middle cerebral artery was occluded for 1 h. Body temperature was maintained around 37 °C using heating pads and a heating ramp throughout the surgical procedure and afterward until the mouse recovered from anesthesia. In a separate set of experiments anesthetized animals from all groups (three mice per group) underwent cerebral blood flow (CBF) measurements using a laser Doppler perfusion monitor. All CBF measurements were conducted with the mouse fixed in a plastic frame with the probe placed in the region of cerebral cortex perfused by the middle cerebral artery (MCA).
Evaluation of Neurological Deficits and Infarct Volume.
Neurological impairment was assessed by using a five-point neurological deficit score (0, no neurological deficit; 1, failure to extend left paw; 2, circling to the left; 3, falling to the left; and 4, unable to walk spontaneously) (26) and were assessed in a blinded fashion. Median differences were analyzed by a nonparametric Kruskal–Wallis one-way ANOVA test at 5% significance level. At 48 h after the experimental stroke, the mice were euthanized and infarct area was evaluated by TTC staining as described previously (25). For each brain section, the infarct area was determined by subtracting the area of the noninfarcted ipsilateral hemisphere from that of the intact contralateral hemisphere. The percentage of infarct volume was calculated by dividing the sum of the area from all sections of infarction by the total of that of contralateral hemisphere to avoid the influence of tissue edema (27). A nonparametric statistical test (Mann–Whitney test) and also a two-sample t test were conducted to analyze the significance of the data.
TUNEL Staining, Microscopy, and Quantification.
TUNEL was performed on brain sections using a Deadend kit (Promega). Multiple sections from individual brain samples were layered on a glass slide before the TUNEL assay; brain sections were fixed in 4% (vol/vol) formaldehyde and permeablized with 0.2% Triton X-100 and proteinase K as per manufacturer’s instructions. After staining, the sections were mounted in hardset vectashield medium containing DAPI. Additional staining was done with hematoxylin–eosin to locate the boundary of ischemic penumbra (28). The sections were photographed using a fluorescence microscope equipped with AxioVision software. Six WT and six neil1−/− brains were analyzed. To avoid bias, a minimum of four microscopic fields within the ischemic penumbra of each section were photographed. TUNEL+ cells were counted in a double-blinded manner in each of these areas. An average of all of the TUNEL+ cells from these areas counted were taken from each brain section (29). Data were analyzed by Student’s two-tailed t test and the significance level of 5% was used.
Incision Assays.
Brain tissue lysates were prepared by homogenizing with a dounce homogenizer in buffer A (20 mM Hepes, pH 7.5, 50 mM KCl, 2 mM EGTA and complete protease inhibitor (Roche). Lysates were centrifuged at 800 × g for 10 min to remove large cell debris. The resulting lysates were resuspended (2 mg/mL) in 20 mM Hepes (pH 7.0), 150 mM KCl, 2 mM EGTA, 1% (wt/vol) CHAPSO (Sigma-Aldrich), and protease inhibitor mixture and incubated at 4 °C for 1 h with end-over-end rotation. The lysates were centrifuged at 100,000 × g for 1 h, and the supernatants were collected. The samples were flash frozen in liquid nitrogen and stored at −80 °C until use. Protein concentration was determined using the BCA assay kit (Pierce). Mitochondrial fractions were isolated using the differential centrifugation method and lysate was prepared as described previously (30). The samples were flash frozen in liquid nitrogen and stored at −80 °C until use. Protein concentration was determined using the BCA assay kit (Pierce). A two-tailed Student t test was conducted and the differences in the means were considered significant when P < 0.05.
Statistics.
A number of approaches to the analysis of the data were used as the circumstances warranted. When comparing the means (or medians) of two independent samples, a two-sample Student t test was used (or the nonparametric Mann–Whitney test or a Wilcoxon rank sum test). When comparing the means (or medians) among a number of independent samples, a one-way analysis of variance (ANOVA) was used (or the nonparametric Kruskal–Wallis test). In the experiment where each animal was measured on three occasions (prestroke and at 24 and 48 h after stroke), a randomized complete block design was applied to account for the repeated measures on the animals (this is equivalent to a two-sample ANOVA with no interaction term). In the experiment where each animal was probed for a parameter time spent in specific quadrants of water maze repeatedly measured for several days, we used repeated measures two-way ANOVA to compare significant interaction terms. Appropriate post hoc approaches, as mentioned in the results section were conducted to control the false positive error rate. A 5% significance level is used throughout. Graphpad Prism version 5 and Statistical Aanalysis System (SAS) programming were used to calculate statistics in various experiments.
Supplementary Material
Acknowledgments
We thank Dr. Steven Lloyd (Oregon Health and Science University) for the generous gift of NEIL1 knockout mice, Drs. V. Popuri and L. K. Hoh for critically reviewing this manuscript, and Chris Morrell for statistical assistance. This research was supported entirely by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204156109/-/DCSupplemental.
References
- 1.Li P, et al. Mechanistic insight into DNA damage and repair in ischemic stroke: Exploiting the base excision repair pathway as a model of neuroprotection. Antioxid Redox Signal. 2011;14:1905–1918. doi: 10.1089/ars.2010.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 3.Yang JL, Weissman L, Bohr VA, Mattson MP. Mitochondrial DNA damage and repair in neurodegenerative disorders. DNA Repair (Amst) 2008;7:1110–1120. doi: 10.1016/j.dnarep.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson DM, 3rd, Bohr VA. The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair (Amst) 2007;6:544–559. doi: 10.1016/j.dnarep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Ide H, Kotera M. Human DNA glycosylases involved in the repair of oxidatively damaged DNA. Biol Pharm Bull. 2004;27:480–485. doi: 10.1248/bpb.27.480. [DOI] [PubMed] [Google Scholar]
- 6.Couvé-Privat S, Macé G, Rosselli F, Saparbaev MK. Psoralen-induced DNA adducts are substrates for the base excision repair pathway in human cells. Nucleic Acids Res. 2007;35:5672–5682. doi: 10.1093/nar/gkm592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muniandy PA, Liu J, Majumdar A, Liu ST, Seidman MM. DNA interstrand crosslink repair in mammalian cells: step by step. Crit Rev Biochem Mol Biol. 2010;45:23–49. doi: 10.3109/10409230903501819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grin IR, Zharkov DO. Eukaryotic endonuclease VIII-like proteins: New components of the base excision DNA repair system. Biochemistry (Mosc) 2011;76:80–93. doi: 10.1134/s000629791101010x. [DOI] [PubMed] [Google Scholar]
- 9.Hu J, et al. Repair of formamidopyrimidines in DNA involves different glycosylases: Role of the OGG1, NTH1, and NEIL1 enzymes. J Biol Chem. 2005;280:40544–40551. doi: 10.1074/jbc.M508772200. [DOI] [PubMed] [Google Scholar]
- 10.Vartanian V, et al. The metabolic syndrome resulting from a knockout of the NEIL1 DNA glycosylase. Proc Natl Acad Sci USA. 2006;103:1864–1869. doi: 10.1073/pnas.0507444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan MK, et al. Targeted deletion of the genes encoding NTH1 and NEIL1 DNA N-glycosylases reveals the existence of novel carcinogenic oxidative damage to DNA. DNA Repair (Amst) 2009;8:786–794. doi: 10.1016/j.dnarep.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rolseth V, et al. Widespread distribution of DNA glycosylases removing oxidative DNA lesions in human and rodent brains. DNA Repair (Amst) 2008;7:1578–1588. doi: 10.1016/j.dnarep.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu D, et al. Evidence that OGG1 glycosylase protects neurons against oxidative DNA damage and cell death under ischemic conditions. J Cereb Blood Flow Metab. 2011;31:680–692. doi: 10.1038/jcbfm.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niizuma K, Endo H, Chan PH. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem. 2009;109(Suppl 1):133–138. doi: 10.1111/j.1471-4159.2009.05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 17.Harrison JF, et al. Oxidative stress-induced apoptosis in neurons correlates with mitochondrial DNA base excision repair pathway imbalance. Nucleic Acids Res. 2005;33:4660–4671. doi: 10.1093/nar/gki759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karahalil B, Hogue BA, de Souza-Pinto NC, Bohr VA. Base excision repair capacity in mitochondria and nuclei: Tissue-specific variations. FASEB J. 2002;16:1895–1902. doi: 10.1096/fj.02-0463com. [DOI] [PubMed] [Google Scholar]
- 19.Lu T, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 20.Klungland A, et al. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci USA. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeill DR, et al. XRCC1 haploinsufficiency in mice has little effect on aging, but adversely modifies exposure-dependent susceptibility. Nucleic Acids Res. 2011;39:7992–8004. doi: 10.1093/nar/gkr280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahabir S, et al. A prospective study of polymorphisms of DNA repair genes XRCC1, XPD23 and APE/ref-1 and risk of stroke in Linxian, China. J Epidemiol Community Health. 2007;61:737–741. doi: 10.1136/jech.2006.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo Y, et al. Impaired DNA repair via the base-excision repair pathway after focal ischemic brain injury: A protein phosphorylation-dependent mechanism reversed by hypothermic neuroprotection. Front Biosci. 2007;12:1852–1862. doi: 10.2741/2193. [DOI] [PubMed] [Google Scholar]
- 24.Okun E, et al. Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. Proc Natl Acad Sci USA. 2010;107:15625–15630. doi: 10.1073/pnas.1005807107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arumugam TV, et al. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bederson JB, et al. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 27.Swanson RA, Sharp FR. Infarct measurement methodology. J Cereb Blood Flow Metab. 1994;14:697–698. doi: 10.1038/jcbfm.1994.88. [DOI] [PubMed] [Google Scholar]
- 28.Borlongan CV, et al. Glial cell survival is enhanced during melatonin-induced neuroprotection against cerebral ischemia. FASEB J. 2000;14(10):1307–1317. doi: 10.1096/fj.14.10.1307. [DOI] [PubMed] [Google Scholar]
- 29.Khan M, Im YB, et al. 2009. Administration of S-nitrosoglutathione after traumatic brain injury protects the neurovascular unit and reduces secondary injury in a rat model of controlled cortical impact. J Neuroinflammation 6:32.
- 30.Maynard S, de Souza-Pinto NC, Scheibye-Knudsen M, Bohr VA. Mitochondrial base excision repair assays. Methods. 2010;51:416–425. doi: 10.1016/j.ymeth.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.