Abstract
Components of modular cellulases, type-A cellulose-binding modules (CBMs) bind to crystalline cellulose and enhance enzyme effectiveness, but structural details of the interaction are uncertain. We analyzed cellulose binding by EXLX1, a bacterial expansin with ability to loosen plant cell walls and whose domain D2 has type-A CBM characteristics. EXLX1 strongly binds to crystalline cellulose via D2, whereas its affinity for soluble cellooligosaccharides is weak. Calorimetry indicated cellulose binding was largely entropically driven. We solved the crystal structures of EXLX1 complexed with cellulose-like oligosaccharides to find that EXLX1 binds the ligands through hydrophobic interactions of three linearly arranged aromatic residues in D2. The crystal structures revealed a unique form of ligand-mediated dimerization, with the oligosaccharide sandwiched between two D2 domains in opposite polarity. This report clarifies the molecular target of expansin and the specific molecular interactions of a type-A CBM with cellulose.
Keywords: plant cell wall, X-ray crystallography
Gigatons of cellulose are synthesized annually by plants, which use this glucose-based polymer as an inert reinforcing material in their cell walls. As a combustible fuel and in the form of textiles, paper, lumber, and other materials, cellulose has vast economic value, prompting great interest in modifying it by enzymes or other means, particularly for conversion to liquid transportation fuels (1, 2). Cellulose is synthesized as micrometer-long microfibrils with ∼24 β- (1, 4)-d-glucans tightly packed into a cablelike array ∼3 nm wide, containing both crystalline and less-ordered regions (3). The crystalline regions have four faces in which either the hydroxyl-rich hydrophilic edges of the glucans or the hydrophobic pyranose rings populate surfaces of distinctive character. Cellulolytic enzymes typically consist of a catalytic domain linked to a cellulose-binding module (CBM) that potentiates enzyme activity by proximity and targeting effects (4–6). Type-A CBMs use a flat surface populated with aromatic residues to bind to crystalline cellulose whereas type-B CBMs use a deep groove to bind individual twisted glucan chains found in disordered cellulose (4, 7).
Although there are numerous published structures of type-B CBMs complexed with cellooligosaccharides (8–12), analogous structures for type-A CBMs have proved difficult to produce, and so the current concept of type-A CBM interaction with cellulose is based on indirect evidence. The current hypothesis, that type-A CBMs bind to the hydrophobic face of crystalline cellulose, stems from the observation that they have an open surface with a planar strip of aromatic residues suitably positioned to bind the planar surface of the aligned pyranose rings in crystalline cellulose (4, 13). Site-directed mutagenesis showed these aromatic residues to be essential for binding crystalline cellulose (14–17), and calorimetry indicated binding was mainly driven by increases in entropy, consistent with freeing of constrained water at the hydrophobic surfaces of the protein and cellulose (18). Supporting this idea, electron microscopy indicated that type-A CBMs bind to the hydrophobic face of crystalline cellulose (19). However, structural details of the interaction of type-A CBMs with cellulose are lacking because suitable protein:ligand complexes have not been crystallized to date. Such structures could help elucidate how type-A CBMs may modify cellulose (20, 21) and potentiate cellulase action (4–6, 22), with consequences for the engineering and technology of cellulose use as a material and as a feedstock for biofuel production.
Here we report energetic and structural details of cellulose binding by a type-A CBM that is a tightly integrated part of EXLX1, a bacterial expansin from Bacillus subtilis (23, 24). Expansins were first discovered as nonenzymatic plant proteins that loosen plant cell walls (25), with key roles in plant cell growth and developmental processes that involve cell-wall modification, such as leaf abscission, fruit softening, and pollen tube penetration of the stigma (26, 27). Bacterial expansins were recently recognized by virtue of their structural similarity to plant expansins (23, 28). They facilitate microbial colonization of plants, with consequences for pathogen virulence and biocontrol (23, 29).
We focused on EXLX1 because it is readily expressed in active form in heterologous expression systems, whereas plant expansins have proved recalcitrant to this approach. As is true for other expansins, domain D1 of EXLX1 shows distant structural similarity to family-45 glycoside hydrolases, but has no detectable lytic activity against the polysaccharide components of plant cell walls (23). Domain D2 forms an open, nearly flat surface resembling that of type-A CBMs (23, 24). Detailed analysis of EXLX1 indicated that both domains are essential for plant cell-wall loosening activity and domain D2 almost exclusively mediates binding to cellulose (24).
In this study, we analyzed EXLX1 binding to cellulose and assessed whether it competes for binding sites with type-A or type-B CBMs. Additionally, we obtained calorimetric and crystallographic data to reveal the thermodynamics and physical nature of EXLX1 binding to cellulose. Our results provide insight into the specific target of EXLX1 and, by extension, of other expansins and type-A CBMs.
Results
EXLX1 Binding to Cellulose.
The predominant forms of cellulose used to characterize CBM binding are, in decreasing degree of crystallinity, bacterial microcrystalline cellulose (BMCC), Avicel, and phosphoric-acid–swollen cellulose (PASC) (30–32). BMCC and Avicel have higher binding capacity for type-A CBMs compared with type-B and have been used to distinguish between the two groups of CBMs, whereas PASC has comparable binding capacities for the two types (14, 32–35).
We compared EXLX1 binding to cellulose with that of two type-A CBMs (CBM3 and CBM10) and two type-B CBMs (CBM17 and CBM28). The purity of all proteins was checked by SDS/PAGE (Fig. S1). Protein binding to Avicel and BMCC was fitted to a Langmuir-type model as described before (14, 18, 36). Binding data approximate a one-site Langmuir isotherm up to protein concentrations of 20–25 μM (Fig. S2). At higher concentrations, there was evidence of a second binding site of much lower affinity, which was observed previously for type-A and type-B CBMs (18, 34). For simplicity, we ignored this site because its relative contribution to binding was negligible at the protein concentrations used here. Binding to BMCC resembled that of type-A CBMs, although with fewer binding sites, and for Avicel its binding properties were intermediate between the two types of CBMs (Table 1), with a dissociation constant Kd ∼2 μM.
Table 1.
Characterization of the binding of EXLX1 (including Alexa-labeled EXLX1) and various CBMs to Avicel and BMCC
| Substrate | Protein | Bmax (μmol/g of substrate ± SEM) | Kd (μM ± SEM) |
| BMCC | CBM3 (type-A) | 9.9 ± 0.6 | 0.5 ± 0.2 |
| CBM10 (type-A) | 6.4 ± 0.2 | 4.2 ± 0.3 | |
| EXLX1 | 1.0 ± 0.1 | 1.9 ± 0.4 | |
| CBM17 (type-B) | Not detected | Not detected | |
| CBM28 (type-B) | Not detected | Not detected | |
| Avicel | CBM10 (type-A) | 2.7 ± 0.2 | 6.7 ± 0.8 |
| CBM3 (type-A) | 0.6 ± 0.1 | 0.7 ± 0.2 | |
| EXLX1 | 0.3 ± 0.0 | 2.3 ± 0.2 | |
| CBM17 (type-B) | 0.3 ± 0.0 | 2.1 ± 0.4 | |
| CBM28 (type-B) | 0.1 ± 0.0 | 3.7 ± 1.0 | |
| Alexa-EXLX1 | 0.3 ± 0.0 | 2.7 ± 0.5 | |
| PASC | EXLX1 | 3.5 ± 0.1 | 1.0 ± 0.1 |
| CBM17 (type-B) | 9.7 ± 1.0 | 1.2 ± 0.5 |
Hepes (50 mM) pH 7.5, 5 mM CaCl2, 25 mM NaCl was used as a buffer.
Although cellulose binding for most CBMs is reversible, there are examples of CBMs that bind to crystalline cellulose irreversibly (14, 19, 37), which can complicate the analysis. We found that EXLX1 binds Avicel and BMCC reversibly (assayed at low concentrations, [EXLX1]free ≤ Kd; Fig. S3).
Binding Competition Between EXLX1 and CBMs.
To assess overlap in binding sites, we measured EXLX1 binding to Avicel ± a fixed amount of a type-A or type-B CBM. We used Avicel because, unlike BMCC, it contained binding sites for both types of CBMs. The CBM amount was enough to occupy 80–85% of the corresponding binding sites. Type-A, but not type-B, CBMs substantially reduced EXLX1 binding (Fig. 1). BSA (a negative control) did not interfere with EXLX1 binding. These results show that the cellulose surfaces recognized by EXLX1 overlap, at least partially, with those of type-A CBMs.
Fig. 1.
Lineweaver-Burk plots of Alexa-labeled EXLX1 binding to Avicel in the presence of nonlabeled EXLX1, type-A CBMs (17 μM CBM3, 40 μM CBM10) and type-B CBMs (20 μM CBM17, 20 μM CBM28). BSA was used as a negative control. The values represent the apparent affinity of EXLX1 for Avicel (μM ± SEM) in the presence of the competitor in parenthesis. † P < 0.01 compared with binding of EXLX1 in the absence of a competitor (n = 5, F test). No comp.: No competitor.
Specificity and Thermodynamics of EXLX1 Binding.
We tested EXLX1 binding to various soluble plant cell-wall components by isothermal titration calorimetry (ITC), which can be used to calculate the thermodynamic parameters (ΔH, ΔS, and ΔG), Kd, and stoichiometry of binding. Most glycans that we tested (cellopentaose, xyloglucan hepta-oligosaccharide, xylohexaose, mannohexaose, arabinan, galactan, arabinoxylan, and xyloglucan) resulted in no heat change upon mixing with EXLX1 in solution, other than the heat of dilution. Cellohexaose and mixed-linkage β- (1, 3) (1, 4)-d-glucan (MLG) gave small heat releases, with a gradual reduction as more glycan was added, indicating weak binding by EXLX1 (Kd > 1 mM) (Fig. S4). Alanine substitutions of W125, W126, and Y157 in the triple EXLX1 variant TTT eliminated this heat release, demonstrating their importance in binding of these ligands (Fig. S4). These aromatic residues form a planar surface in D2 (similar to type-A CBMs) and have been implicated in cellulose binding and cell-wall loosening activity (24). The tight binding of EXLX1 to crystalline cellulose (Kd ∼2.0 μM) and weak affinity for soluble cellooligosaccharides (Kd > 1.0 mM) are also features of type-A CBMs (4, 15). In contrast, type-B CBMs, such as CBM17, show high affinity for soluble cellooligosaccharides (33), which was not seen with EXLX1 (Fig. S4).
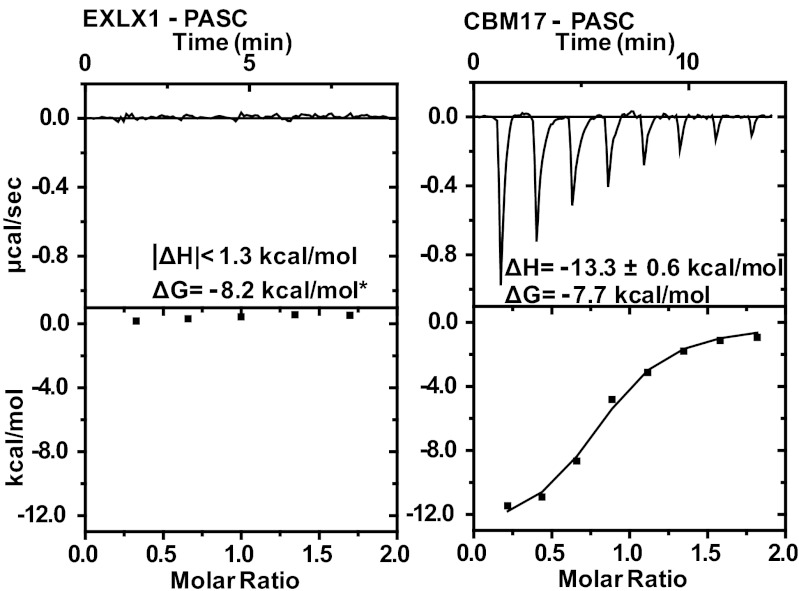
Our results indicate that insoluble cellulose is the primary binding target of the flat aromatic surface of EXLX1. Our attempts to measure the thermodynamic parameters of EXLX1 binding to Avicel and BMCC with ITC were unsuccessful because the low-binding capacity of these materials for EXLX1 required the use of excessively high amounts of cellulose, resulting in unstable baselines and undetectable ΔH signal upon EXLX1 injection. In comparison, PASC showed higher EXLX1-binding capacity (Bmax = 3.5 μmol/g versus 0.3 μmol/g for Avicel) (Table 1, Fig. S2), in part because of its greater exposed surface area. Still, ΔH for EXLX1 binding to PASC was below the ITC detection limit (Fig. 2). In contrast, PASC binding by type-B CBM17 yielded a large ΔH (−13.3 ± 0.6 kcal mol−1) and entropy decreased (T*ΔS = −5.6 kcal mol−1). Based on these results, we conclude that ΔH of EXLX1 binding to PASC is at least tenfold lower in absolute value than the ΔH for CBM17 binding to PASC (that is, |ΔH|<1.3 kcal mol−1). Therefore, the change in free energy associated with EXLX1 binding to PASC, ΔG = −RTlnKa = −8.2 kcal mol−1 (Fig. 2), is mostly the result of increased entropy (ΔG = ΔH-T*ΔS, T: temperature in Kelvin).
Fig. 2.
Binding interaction between EXLX1 and PASC as investigated by isothermal titration calorimetry. The binding parameters are indicated inside each graph. The interaction of CBM17 with PASC was used as positive control. *ΔG was calculated from the Kd value in Table 1. The experiment was repeated three times with similar results.
Crystal Structure of the EXLX1–Cellohexaose Complex.
We produced crystals of EXLX1 complexed with cellohexaose (the hexameric cellooligosaccharide) to gain insight into the interaction of EXLX1 with cellulose. The structure was solved and refined to 2.1-Å resolution with a crystallographic R factor of 0.172 and an R free of 0.201 (Table S1).
Analysis of the structure revealed a unique protein:ligand arrangement. One molecule of cellohexaose is packed between the aromatic surfaces of domain D2 of two EXLX1 molecules, designated (A) and (B), with the reducing end of cellohexaose pointing toward the D1 domain of EXLX1(B) (Fig. 3A). Dynamic light scattering of EXLX1 in solution showed that EXLX1 is a monomer (size estimated as 19 kDa) both in the absence and presence of cellohexaose, indicating dimerization by cellohexaose does not occur in solution, at least under the conditions tested.
Fig. 3.
Structure of EXLX1 with cellohexaose. (A) Sandwich structure consisting of two molecules of EXLX1 (A and B) and one molecule of cellohexaose (green). (B) Direct hydrogen bonds and CH–π interactions between each EXLX1 molecule and cellohexaose. Glucose numbering starts from the reducing end of cellohexaose.
The structures of EXLX1(A) and (B) are essentially identical to each other and to the existing crystal structure of EXLX1 (PDB#: 3D30), which was obtained without bound ligands [rmsd = 0.443 and 0.491 Å for superposition of 3D30 with EXLX1(A) and EXLX1(B) respectively] (Fig. S5). There is no change in the position of the side chains of amino acid residues that are crucial for cellulose and cell-wall–modifying activity, including D71, Y73, D82, K119, W125, W126, and Y157 (24). The only minor change is that W125 is slightly tilted in both EXLX1 monomers compared with 3D30 (Fig. S5).
Binding of CBMs to cellulose is partly mediated by hydrophobic interactions between aromatic amino acids and pyranose rings (4). Consistent with such a mechanism, the aromatic rings of W125, W126, and Y157 in both EXLX1(A) and EXLX1(B) are nearly parallel to the planes of the pyranose rings of cellohexaose, which adopt the undistorted 4C1 chair conformation. The distance between the aromatic and the pyranose rings is 3.0–4.0 Å, allowing CH–π interactions (38, 39). In EXLX1(A), W126 makes CH–π interactions with glucose (G) residue G4 (numbering of pyranose rings starts from the reducing end of cellohexaose), and W125 interacts with G2 and to a lesser extent with G1 (Fig. 3B). The aromatic ring of Y157 is located between G5 and G6 (5.1 and 5.3 Å from ring centers, respectively), likely interacting with both. In EXLX1(B), W125 is stacked against G5 and W126 makes CH–π interactions with G3 and to a lesser extent with G2 (Fig. 3B). Similarly, Y157 in EXLX1(B) is located between two pyranose rings, G1 and G2, 5.0 and 5.9 Å from the ring centers (Fig. 3B).
The distances between W125, W126, and Y157 do not match the 5.5-Å interval between pyranose rings of cellohexaose and so it is not possible for all aromatic amino acids to overlap precisely with pyranose rings at the same time (Fig. 4). In both EXLX1(A) and EXLX1(B), W125 and W126 overlap better with pyranose rings than does Y157 (Figs. 3B and 4), suggesting that these residues are more important for cellulose binding than Y157. Supporting this idea, the single amino acid variants W125A and W126A bound cellulose less effectively than wild-type EXLX1, whereas the Y157A variant was less affected (24).
Fig. 4.
Alignment and registration of aromatic residues of EXLX1 with the pyranose rings of cellohexaose in the protein:ligand complex (Left) and comparison with the spacing of analogous surface aromatic residues in four other type-A CBMs. PDB numbers of the structures are indicated in parentheses.
There are few direct H bonds between cellohexaose and EXLX1, formed only by K119 (Fig. 3B). In EXLX(A), K119 forms H bonds with the C2 and C3 hydroxyl groups of G5; whereas, in EXLX(B) it is hydrogen bonded with the C3 hydroxyl of G1 and the C6 hydroxyl of G2 as well as with the G1:G2 glycosidic oxygen. The direct contact of K119 with cellohexaose is in agreement with mutagenesis data implicating K119 in cellulose binding and cell-wall loosening (24). Indirect H bonding via water can be seen but contribute little to CBM binding (40).
Structures of EXLX1 with Cellotetraose, Hemithiocellodextrin, and MLG.
In the EXLX1:cellohexaose structure, domain D1 did not contact cellohexaose, possibly because of its weak affinity for cellulose (24). In attempts to obtain complexes where the glucans interact directly with residues in D1, we successfully produced crystals of EXLX1 with cellotetraose, hemithiocellodextrin (HTC; DP = 10) and oligosaccharides from MLG, with the last two ligands being longer than cellohexaose. HTC is a water-soluble analog of cellodecaose (water insoluble), except that every other glycosidic linkage is replaced with a thio-glycosidic linkage (41). We hoped that use of long oligosaccharides such as HTC and MLG would result in EXLX1 structures where the ligands spanned both EXLX1 domains.
In all three of these structures, the ligands bind to EXLX1 in a fashion similar to that found for cellohexaose (Fig. S6). The interactions of D2 with all three ligands are dominated by CH–π interactions mediated by aromatic amino acids W125, W126, and Y157 with minimal contribution by direct H bonding. Sandwich structures formed in all these cases, with the ligand bound between the aromatic surfaces of D2 domains of two EXLX1 in opposite polarity. Thus, this sandwich structure seems to be a thermodynamically favorable configuration with various cellulose-like oligosaccharides.
These structures also show that domain D1 does not contribute to ligand binding in these complexes. This suggests that D1 binding to glucans is extremely weak, consistent with binding results (24), and thus domain D1 may depend on D2 binding to cellulose for performing its loosening action on cellulose and cell-wall networks.
Discussion
EXLX1 domain D2 has characteristics of a type-A CBM. Our structures of EXLX1 with cellohexaose and other oligosaccharides provide the first structures of a type-A CBM in complex with a cellulose-like oligosaccharide and reveal the structural details of type-A CBM binding to cellulose. Although cellohexaose is more flexible in solution than are the glucan chains in crystalline cellulose, it is the closest water-soluble equivalent to crystalline cellulose. Therefore, lessons learned from the structure of EXLX1 complexed with cellohexaose can provide information about the binding of EXLX1 (and potentially other type-A CBMs and expansins) to cellulose.
Our results show that EXLX1 binding to cellohexaose and related oligosaccharides is dominated by CH–π interactions of W125, W126, and Y157 with the glucan's pyranose rings, in a configuration similar to that hypothesized for type-A CBMs. The registration of these aromatic residues with the pyranose rings is shown in Fig. 4, which also illustrates the spacing of analogous aromatic residues from the binding surfaces of four other type-A CBMs. The number and general arrangement of the aromatic residues are similar among the protein surfaces, but the spacing shows substantial variability in the specific geometry of the binding interactions.
ITC showed that ΔH for EXLX1 binding to cellulose is a small component of ΔG of binding (< 16%), indicating that an increase in entropy drives binding, mostly likely due to release of water constrained to unfavorable configurations at the hydrophobic surfaces of cellulose and protein. Similarly, the binding of type-A CBMs to cellulose is believed to be driven by positive entropic changes due to the release of bound water (18). The binding affinities of D2 and other type-A CBMs to soluble cellooligosaccharides are ∼1,000-fold lower than to cellulose, possibly because of the loss in entropy when the cellooligosaccharides lose conformational freedom and the increase in enthalpy when they are forced into an energetically unfavorable conformation, whereas these thermodynamic penalties are not incurred when the protein binds to an already constrained glucan on the cellulose surface. A comparison among cellooligosaccharide conformations shows that cellohexaose bound to EXLX1 is intermediate between the flat chains in cellulose and the twisted chains in type-B CBM complexes (Fig. S7). These observations are consistent with the competition results of Fig. 1 and indicate that EXLX1 may bind to the more ordered regions of cellulose rather than to the disordered regions targeted by type-B CBMs.
One particularly intriguing observation in our study is that two molecules of EXLX1 form a sandwich structure with one oligosaccharide between the aromatic surfaces to two D2 domains. This configuration appears to be unique, yet it occurred with all four oligosaccharides that formed an EXLX1 crystal complex. A second unique observation is that the two D2 surfaces bind the oligosaccharides in opposite polarities with respect to the reducing end of the glucan chain. This likewise seems remarkable. The closest comparison may be found in the ligand-mediated dimer of the E78R mutant of the type-B CMB, PeCBM29-2 (42), but in this structure the two proteins bind the oligosaccharide in the same polarity and the oligosaccharide is found in the twisted configuration characteristic of type-B CBMs. Furthermore, the two PeCBM29-2 proteins make numerous direct H bonds with each other that stabilize the dimer, whereas such interactions are not seen in the EXLX1:oligosaccharide structures, where the two proteins are connected only via the oligosaccharide.
The unusual binding of EXLX1 to cellulose-like oligosaccharides in opposing polarities may be possible because similar and relatively nonspecific CH–π interactions are made by both proteins on open surfaces. In addition, binding in opposite polarities could be enabled by the scarcity of direct H bonds, which require the -OH groups to be in specific positions and orientations. In nature, most -OH groups in crystalline cellulose would be H bonded to adjacent glucose residues in the same plane, thus limiting their availability for binding with surface proteins. This aspect of EXLX1 binding may influence the dynamics of its movement on cellulose surfaces and may be common in type-A CBMs, one of which has been shown to diffuse along the surface of cellulose, although it is not clear whether it displays movement anisotropy along a single microfibril (43) as has been observed for cellobiohydrolase I (Cel7A) (44). Our results suggest that EXLX1 could move bidirectionally on a microfibril.
The exact target of expansin has been a subject of interest and speculation in an effort to understand the site and mechanism of its wall-loosening action (26, 45). Our results indicate that EXLX1 shares binding sites with type-A CBM3, reported to be located on the hydrophobic face of cellulose microfibrils (19). These results, along with the arrangement of aromatic residues on the D2 surface and the hydrophobic nature of D2 binding to cellooligosaccharides, collectively indicate that EXLX1 binds to the hydrophobic face of the cellulose microfibril. However, it is unlikely that EXLX1 binds to the whole hydrophobic face because the binding capacity of the highly crystalline BMCC for EXLX1 is five- to tenfold lower than for CBM3 and CBM10. In addition, there is a slight right-handed twist among aromatic residues W125, W126, and Y157 (Fig. 3 and Fig. S5). This slight twist, which is mirrored in the EXLX1-bound cellohexaose (Fig. S7), may preferentially direct binding to cellulose surfaces with a similar twist. These sites might be junctions between crystalline and disordered cellulose, perhaps similar to the proposed targets of endoglucanase CtCel124, which has distinct binding surfaces for crystalline and noncrystalline cellulose (46). One speculative scenario is that EXLX1 binds to the slightly twisted region of a glucan chain that bridges two cellulose microfibrils. Such stray or disordered glucans are important in strengthening paper, a synthetic network of cellulose fibers that is weakened by EXLX1 (24). In this scenario, D2 binding might assist in unzipping the glucan from the cellulose surface (with energy provided by turgor-generated wall stresses) and would also lead domain D1 to interact with the disordered part of the same glucan chain.
Because the binding of expansin domain D1 to cellulose is extremely weak, we infer that the function of domain D1 is highly dependent on the binding of D2 to cellulose. The weak binding is supported by previous work (24) as well as our current ITC results and the lack of stable interactions between D1 and oligosaccharides in our four EXLX1:ligand structures. Nonetheless, domain D1 is clearly needed for loosening activity by EXLX1 (24). The D1 surface formed by residues D82, D71, and Y73 extends the cellulose-binding surface of D2 and has been shown by site-directed mutagenesis to be critical for weakening filter paper and for loosening plant cell walls (24). Although expansins lack wall lytic activity, domain D1 shows distant structure similarity to GH45 endoglucanases (23). Cellooligosaccharides do stably bind to GH45 endoglucanases (23, 47) where the catalytic site is located in a deep cleft containing several polar residues that help bind the cellooligosaccharide (47). In contrast, EXLX1 domain D1 forms an open surface with a small shoulder near residues D71, D82, and Y73; the surface is much flatter than the one in GH45 endoglucanases, missing residues that stabilize the interaction with cellooligosaccharides, as well as missing key parts of the catalytic machinery (23, 24, 47). Thus, the specific molecular activity of D1 that leads to its wall-loosening activity differs from GH45 action and may not require a stable interaction with the glucan.
In summary, our study revealed both predicted and unexpected features of EXLX1 binding to cellulose, with conclusions that may also hold true for other expansins and type-A CBMs in modular cellulases. The structure of D2 with cellohexaose can be a starting point for molecular dynamics simulations to better understand how the binding of expansin to cellulose may facilitate release of glucans from cellulose microfibrils. Such studies offer the potential for deeper understanding of the biophysical actions of expansins on cell-wall networks and type-A CBMs on cellulose surfaces, with potential applications in many fields.
Materials and Methods
Oligosaccharides and Polysaccharides.
Avicel PH-101 was purchased from FMC Biopolymer. BMCC was prepared from Gluconacetobacter xylinus pellicles as described by Gilkes et al. (36). PASC was prepared from Avicel PH-101 as described by Wood (48). Cellohexaose, cellopentaose, xylohexaose, mannohexaose, xyloglucan heptaoligosaccharide (Cat: O-X3G4), sugar beet arabinan (Cat: P-ARAB), potato galactan (Cat: P-GAPT), barley mixed-linkage glucan (low viscosity, Cat: P-BGBL), wheat arabinoxylan (Cat: P-WAXYL), and tamarind xyloglucan (Cat: P-XYGLN) were purchased from Megazyme. Cellotetraose was purchased from Seikagaku Corp.
Protein Expression, Purification, and Labeling.
EXLX1 and the TTT variant was expressed, purified, and labeled with Alexa Fluor 488 C5-maleimide (Life Technologies) as described by Georgelis et al. (24). The cDNA sequence of Pseudomonas fluorescens CBM10 (PDB#: 1E8R) (35, 49) was synthesized by Life Technologies and the protein was expressed and purified as described by Bolam et al. (5). Clostridium cellulovorans CBM17 (PDB#: 1J83) (10, 33, 34) and Bacillus sp.1139 CBM28 (PDB#: 1UWW) (34, 50) were provided by Dr. Alisdair Boraston (University of Victoria, Victoria, Canada) in lyophilized form. C. thermocellum CBM3 (PDB#: 1NBC) (13, 19) was purchased from Prozomix Limited. All proteins were extensively exchanged into 50 mM Hepes (pH 7.5), 5 mM CaCl2, 25 mM NaCl2 by use of polyethersulfone (PES) concentrating columns (3kD cutoff) (Sartorius Stedium Biotech). The purity of the proteins was visualized by 15% (wt/vol) SDS/PAGE stained with Coomassie Brilliant Blue R-250. SeeBlue Plus 2 was used as a standard (Life Technologies).
Protein Crystallization, Data Collection, Structure Determination, and Refinement.
EXLX1 was concentrated to 30 mg/mL in the presence of cellohexaose or hemithiocellodextrin or MLG or cellotetraose, crystallized by hanging drops, and data were collected at an MM007 rotating anode X-ray generator with CuKα radiation, operating at 1.2 kW of power and Saturn944+ CCD detector (Rigaku Americas). For details see SI Material and Methods.
Isothermal Titration Calorimetry.
The thermodynamic parameters of EXLX1 binding to soluble oligosaccharides, polysaccharides, and PASC were assessed at 25 °C with an ITC-200 microcalorimeter (MicroCal). EXLX1, CBM17, and PASC were dialyzed in 50 mM Hepes (pH 7.5), 5 mM CaCl2, 25 mM NaCl, 0.01% NaN3 for 24 h at room temperature and saccharides were dissolved in 50 mM Hepes (pH 7.5), 5 mM CaCl2, 25 mM NaCl, 0.01% NaN3. EXLX1 was placed into the ITC-200 cell (202.8 μL working volume) at a final concentration of 500 μM. The concentration of CBM17 (positive control) (10, 51) in the cell was 200 μM. Bindings were analyzed by 4 μL injections of ligand concentrated to 10 mg/mL. Cellohexaose was concentrated to 3 mg/mL for injections to a cell containing CBM17. For ITC analysis of the EXLX1 and CBM17 (51) binding to PASC, 10.5 mg/mL PASC (or 36.5 μM of EXLX1 binding sites), and 3.5 mg/mL PASC (or 34 μM of CBM17 binding sites) respectively, were placed in the cell. Binding was analyzed by 4-μL injections of EXLX1 or CBM17 concentrated to 500 μM. Data were processed with Origin 7 (MicroCal).
Cellulose-Binding Assays.
Avicel, BMCC, and PASC binding studies of all proteins were done as described by Georgelis et al. (24). The buffer used in all binding assays was 50 mM Hepes (pH 7.5), 5 mM CaCl2, 25 mM NaCl2 . The binding parameters (dissociation constant and binding capacity) were calculated by fitting the data to a single-site Langmuir isotherm with Graphpad Prism version 5.04 (GraphPad Software).
Binding Competition Assays.
EXLX1 binding to Avicel was studied as described above in the presence of a fixed amount of CBM3 (17 μM), CBM10 (40 μM), CBM17 (20 μM), or CBM28 (20 μM). EXLX1 used in these experiments contained a proportion of one molecule of Alexa-labeled EXLX1 to 24 nonlabeled EXLX1molecules (24). Fixed amounts of BSA (20 μM) and nonlabeled EXLX1 (20 μM) were used as negative and positive control for binding competition, respectively. Binding of EXLX1 to Avicel was assessed by fluorescence of EXLX1-attached Alexa Fluor 488 with a NanoDrop 3300 fluorospectrometer (Thermo Fisher Scientific). The statistics of the binding of Alexa-labeled EXLX1 to Avicel in the presence of competing proteins were calculated with Graphpad Prism 5.04.
Protein Models.
Protein structures were visualized with Chimera software (University of California, San Francisco) (52). Potential H bonds were placed on the structures by use of the FindHbond function of Chimera and relaxing H-bond constraints by 0.4 Å and 20°. The structures were deposited in RCSB Protein Data Bank as #4FER, 4FG2, 4FFT, and 4FG4.
Additional Details.
Supplementary Material
Acknowledgments
We are grateful to Lisa Wilson, Daniel Durachko, and Edward Wagner for technical assistance, Hemant Yennawar (Department of Chemistry, Pennsylvania State University, University Park, PA) for help with X-ray crystallography, Alisdair Boraston (Department of Biochemistry and Microbiology, University of Victoria, Victoria, Canada) for providing CBM17 and CBM28, and Linghao Zhong (Department of Chemistry, Pennsylvania State University, University Park, PA) for helpful discussion. This work was supported by United States Department of Energy Grant DE-FG02-84ER13179 from the Office of Basic Energy Sciences.
Footnotes
The authors declare no conflict of interest.
Data deposition: The crystallography, atomic coordinates, and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 4FER, 4FG2, 4FFT, and 4Fg4).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213200109/-/DCSupplemental.
References
- 1.Himmel ME, et al. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science. 2007;315:804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 2.Rubin EM. Genomics of cellulosic biofuels. Nature. 2008;454:841–845. doi: 10.1038/nature07190. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes AN, et al. Nanostructure of cellulose microfibrils in spruce wood. Proc Natl Acad Sci USA. 2011;108:E1195–E1203. doi: 10.1073/pnas.1108942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boraston AB, Bolam DN, Gilbert HJ, Davies GJ. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochem J. 2004;382:769–781. doi: 10.1042/BJ20040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolam DN, et al. Pseudomonas cellulose-binding domains mediate their effects by increasing enzyme substrate proximity. Biochem J. 1998;331:775–781. doi: 10.1042/bj3310775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hervé C, et al. Carbohydrate-binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects. Proc Natl Acad Sci USA. 2010;107:15293–15298. doi: 10.1073/pnas.1005732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boraston AB, Lammerts van Bureren A, Ficko-Blean E, Abbott DW. Carbohydrate-protein interactions: Carbohydrate-binding modules. In: Kamerling JP, editor. Comprehensive Glycoscience. Amsterdam: Elsevier; 2007. pp. 661–696. [Google Scholar]
- 8.Tsukimoto K, et al. Recognition of cellooligosaccharides by a family 28 carbohydrate-binding module. FEBS Lett. 2010;584:1205–1211. doi: 10.1016/j.febslet.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Bae B, et al. Molecular basis for the selectivity and specificity of ligand recognition by the family 16 carbohydrate-binding modules from Thermoanaerobacterium polysaccharolyticum ManA. J Biol Chem. 2008;283:12415–12425. doi: 10.1074/jbc.M706513200. [DOI] [PubMed] [Google Scholar]
- 10.Notenboom V, et al. Recognition of cello-oligosaccharides by a family 17 carbohydrate-binding module: An X-ray crystallographic, thermodynamic and mutagenic study. J Mol Biol. 2001;314:797–806. doi: 10.1006/jmbi.2001.5153. [DOI] [PubMed] [Google Scholar]
- 11.Boraston AB, et al. Differential oligosaccharide recognition by evolutionarily-related beta-1,4 and beta-1,3 glucan-binding modules. J Mol Biol. 2002;319:1143–1156. doi: 10.1016/S0022-2836(02)00374-1. [DOI] [PubMed] [Google Scholar]
- 12.Charnock SJ, et al. Promiscuity in ligand-binding: The three-dimensional structure of a Piromyces carbohydrate-binding module, CBM29-2, in complex with cello- and mannohexaose. Proc Natl Acad Sci USA. 2002;99:14077–14082. doi: 10.1073/pnas.212516199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tormo J, et al. Crystal structure of a bacterial family-III cellulose-binding domain: A general mechanism for attachment to cellulose. EMBO J. 1996;15:5739–5751. [PMC free article] [PubMed] [Google Scholar]
- 14.McLean BW, et al. Analysis of binding of the family 2a carbohydrate-binding module from Cellulomonas fimi xylanase 10A to cellulose: Specificity and identification of functionally important amino acid residues. Protein Eng. 2000;13:801–809. doi: 10.1093/protein/13.11.801. [DOI] [PubMed] [Google Scholar]
- 15.Nagy T, et al. All three surface tryptophans in Type IIa cellulose binding domains play a pivotal role in binding both soluble and insoluble ligands. FEBS Lett. 1998;429:312–316. doi: 10.1016/s0014-5793(98)00625-5. [DOI] [PubMed] [Google Scholar]
- 16.Linder M, et al. Identification of functionally important amino acids in the cellulose-binding domain of Trichoderma reesei cellobiohydrolase I. Protein Sci. 1995;4:1056–1064. doi: 10.1002/pro.5560040604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinikainen T, Teleman O, Teeri TT. Effects of pH and high ionic strength on the adsorption and activity of native and mutated cellobiohydrolase I from Trichoderma reesei. Proteins. 1995;22:392–403. doi: 10.1002/prot.340220409. [DOI] [PubMed] [Google Scholar]
- 18.Creagh AL, Ong E, Jervis E, Kilburn DG, Haynes CA. Binding of the cellulose-binding domain of exoglucanase Cex from Cellulomonas fimi to insoluble microcrystalline cellulose is entropically driven. Proc Natl Acad Sci USA. 1996;93:12229–12234. doi: 10.1073/pnas.93.22.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehtiö J, et al. The binding specificity and affinity determinants of family 1 and family 3 cellulose binding modules. Proc Natl Acad Sci USA. 2003;100:484–489. doi: 10.1073/pnas.212651999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Din N, et al. C1-Cx revisited: Intramolecular synergism in a cellulase. Proc Natl Acad Sci USA. 1994;91:11383–11387. doi: 10.1073/pnas.91.24.11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Zhang Y, Gao P. A novel function for the cellulose binding module of cellobiohydrolase I. Sci China C Life Sci. 2008;51:620–629. doi: 10.1007/s11427-008-0088-3. [DOI] [PubMed] [Google Scholar]
- 22.Percival Zhang YH, Himmel ME, Mielenz JR. Outlook for cellulase improvement: Screening and selection strategies. Biotechnol Adv. 2006;24:452–481. doi: 10.1016/j.biotechadv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Kerff F, et al. Crystal structure and activity of Bacillus subtilis YoaJ (EXLX1), a bacterial expansin that promotes root colonization. Proc Natl Acad Sci USA. 2008;105:16876–16881. doi: 10.1073/pnas.0809382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Georgelis N, Tabuchi A, Nikolaidis N, Cosgrove DJ. Structure-function analysis of the bacterial expansin EXLX1. J Biol Chem. 2011;286:16814–16823. doi: 10.1074/jbc.M111.225037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McQueen-Mason S, Durachko DM, Cosgrove DJ. Two endogenous proteins that induce cell wall extension in plants. Plant Cell. 1992;4:1425–1433. doi: 10.1105/tpc.4.11.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol Cell Biol. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- 27.Tabuchi A, Li LC, Cosgrove DJ. Matrix solubilization and cell wall weakening by β-expansin (group-1 allergen) from maize pollen. Plant J. 2011;68:546–559. doi: 10.1111/j.1365-313X.2011.04705.x. [DOI] [PubMed] [Google Scholar]
- 28.Yennawar NH, Li LC, Dudzinski DM, Tabuchi A, Cosgrove DJ. Crystal structure and activities of EXPB1 (Zea m 1), a beta-expansin and group-1 pollen allergen from maize. Proc Natl Acad Sci USA. 2006;103:14664–14671. doi: 10.1073/pnas.0605979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahr H, Dreier J, Meletzus D, Bahro R, Eichenlaub R. The endo-β-1,4-glucanase CelA of Clavibacter michiganensis subsp. michiganensis is a pathogenicity determinant required for induction of bacterial wilt of tomato. Mol Plant Microbe Interact. 2000;13:703–714. doi: 10.1094/MPMI.2000.13.7.703. [DOI] [PubMed] [Google Scholar]
- 30.Park S, Johnson DK, Ishizawa CI, Parilla PA, Davis MF. Measuring the crystallinity index of cellulose by solid state (13)C nuclear magnetic resonance. Cellulose. 2009;16:641–647. [Google Scholar]
- 31.Hall M, Bansal P, Lee JH, Realff MJ, Bommarius AS. Cellulose crystallinity—A key predictor of the enzymatic hydrolysis rate. FEBS J. 2010;277:1571–1582. doi: 10.1111/j.1742-4658.2010.07585.x. [DOI] [PubMed] [Google Scholar]
- 32.McLean BW, et al. Carbohydrate-binding modules recognize fine substructures of cellulose. J Biol Chem. 2002;277:50245–50254. doi: 10.1074/jbc.M204433200. [DOI] [PubMed] [Google Scholar]
- 33.Boraston AB, Chiu P, Warren RA, Kilburn DG. Specificity and affinity of substrate binding by a family 17 carbohydrate-binding module from Clostridium cellulovorans cellulase 5A. Biochemistry. 2000;39:11129–11136. doi: 10.1021/bi0007728. [DOI] [PubMed] [Google Scholar]
- 34.Boraston AB, Kwan E, Chiu P, Warren RA, Kilburn DG. Recognition and hydrolysis of noncrystalline cellulose. J Biol Chem. 2003;278:6120–6127. doi: 10.1074/jbc.M209554200. [DOI] [PubMed] [Google Scholar]
- 35.Ponyi T, et al. Trp22, Trp24, and Tyr8 play a pivotal role in the binding of the family 10 cellulose-binding module from Pseudomonas xylanase A to insoluble ligands. Biochemistry. 2000;39:985–991. doi: 10.1021/bi9921642. [DOI] [PubMed] [Google Scholar]
- 36.Gilkes NR, et al. The adsorption of a bacterial cellulase and its two isolated domains to crystalline cellulose. J Biol Chem. 1992;267:6743–6749. [PubMed] [Google Scholar]
- 37.Linder M, Teeri TT. The cellulose-binding domain of the major cellobiohydrolase of Trichoderma reesei exhibits true reversibility and a high exchange rate on crystalline cellulose. Proc Natl Acad Sci USA. 1996;93:12251–12255. doi: 10.1073/pnas.93.22.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohamed MN, Watts HD, Guo J, Catchmark JM, Kubicki JD. MP2, density functional theory, and molecular mechanical calculations of C-H...pi and hydrogen bond interactions in a cellulose-binding module-cellulose model system. Carbohydr Res. 2010;345:1741–1751. doi: 10.1016/j.carres.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Spiwok V, et al. Modelling of carbohydrate-aromatic interactions: ab initio energetics and force field performance. J Comput Aided Mol Des. 2005;19:887–901. doi: 10.1007/s10822-005-9033-z. [DOI] [PubMed] [Google Scholar]
- 40.Pell G, et al. Importance of hydrophobic and polar residues in ligand binding in the family 15 carbohydrate-binding module from Cellvibrio japonicus Xyn10C. Biochemistry. 2003;42:9316–9323. doi: 10.1021/bi0347510. [DOI] [PubMed] [Google Scholar]
- 41.Parsiegla G, Reverbel C, Tardif C, Driguez H, Haser R. Structures of mutants of cellulase Cel48F of Clostridium cellulolyticum in complex with long hemithiocellooligosaccharides give rise to a new view of the substrate pathway during processive action. J Mol Biol. 2008;375:499–510. doi: 10.1016/j.jmb.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 42.Flint J, et al. Ligand-mediated dimerization of a carbohydrate-binding molecule reveals a novel mechanism for protein-carbohydrate recognition. J Mol Biol. 2004;337:417–426. doi: 10.1016/j.jmb.2003.12.081. [DOI] [PubMed] [Google Scholar]
- 43.Jervis EJ, Haynes CA, Kilburn DG. Surface diffusion of cellulases and their isolated binding domains on cellulose. J Biol Chem. 1997;272:24016–24023. doi: 10.1074/jbc.272.38.24016. [DOI] [PubMed] [Google Scholar]
- 44.Igarashi K, et al. High speed atomic force microscopy visualizes processive movement of Trichoderma reesei cellobiohydrolase I on crystalline cellulose. J Biol Chem. 2009;284:36186–36190. doi: 10.1074/jbc.M109.034611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cosgrove DJ. Enzymes and other agents that enhance cell wall extensibility. Ann Rev Plant Phys Plant Mol Biol. 1999;50:391–417. doi: 10.1146/annurev.arplant.50.1.391. [DOI] [PubMed] [Google Scholar]
- 46.Brás JL, et al. Structural insights into a unique cellulase fold and mechanism of cellulose hydrolysis. Proc Natl Acad Sci USA. 2011;108:5237–5242. doi: 10.1073/pnas.1015006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies GJ, Tolley SP, Henrissat B, Hjort C, Schülein M. Structures of oligosaccharide-bound forms of the endoglucanase V from Humicola insolens at 1.9 A resolution. Biochemistry. 1995;34:16210–16220. doi: 10.1021/bi00049a037. [DOI] [PubMed] [Google Scholar]
- 48.Wood TM. Preparation of crystalline, amorphous, and dyed cellulase substrates. Methods Enzymol. 1988;160:19–25. [Google Scholar]
- 49.Raghothama S, et al. Solution structure of the CBM10 cellulose binding module from Pseudomonas xylanase A. Biochemistry. 2000;39:978–984. doi: 10.1021/bi992163+. [DOI] [PubMed] [Google Scholar]
- 50.Jamal S, Nurizzo D, Boraston AB, Davies GJ. X-ray crystal structure of a non-crystalline cellulose-specific carbohydrate-binding module: CBM28. J Mol Biol. 2004;339:253–258. doi: 10.1016/j.jmb.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 51.Boraston AB. The interaction of carbohydrate-binding modules with insoluble non-crystalline cellulose is enthalpically driven. Biochem J. 2005;385:479–484. doi: 10.1042/BJ20041473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pettersen EF, et al. UCSF Chimera—A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.