Like baseball, immunity is a team effort. Various innate/adaptive humoral/cellular components work in unison to clear infections. Determining the importance of each baseball player/immune element to winning/clearing is more difficult than meets the eye. Just as mathematical modeling of individual contributions (“sabermetrics”) revolutionized baseball, “immunometrics” will change our concept of immunity. Measles virus (MV) is a highly transmissible negative-stranded RNA virus that remains a major cause of childhood morbidity and mortality (1). Mortality results from MV infection itself or secondary bacterial infections facilitated by MV-mediated immunosuppression. Following the introduction of effective vaccines during the 1960s, worldwide annual MV mortality decreased from more than 2.8 million to less than 150,000. Vaccine efficacy, in conjunction with the apparent absence of an animal reservoir, potentially makes MV the second human pathogen (after smallpox) to be eradicated by medical intervention (2). MV infection elicits neutralizing antibodies (Abs) that correlate with lifelong measles protection (3). However, the clearance of MV from infected individuals was thought to be caused principally by CD8+ T-cell activity. Infectious MV is detected in the blood by 7 d after exposure and is cleared within 2 wk, coincident with the appearance of T-cell responses (Fig. 1) (4–6). In both humans and monkeys, MV RNA can persist in the blood for months (7, 8), which may be related to MV-mediated immunosuppression, and, ironically, immunological memory as well. In PNAS, modeling MV immunity enables Lin et al. to propose a surprising role for Abs in mediating MV clearance (9).
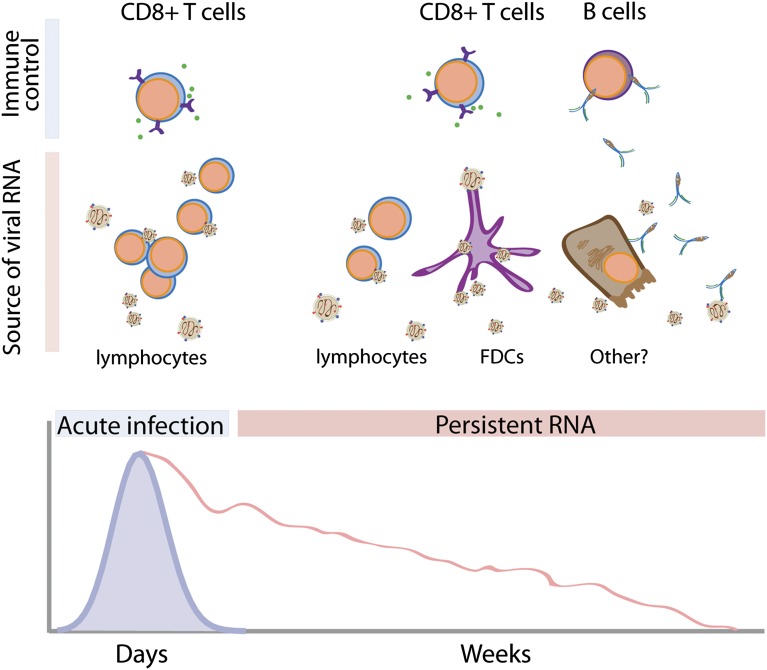
Fig. 1.
The dynamics of MV clearance. MV infection results in transient viremia (purple line, Lower) that is cleared within ∼2 wk, coincident with CD8+ T-cell responses. MV RNA (red line, Lower) persists, however, for as long as months in blood. In the acute phase of infection, most MV is likely produced by infected lymphocytes. The source of persistent MV RNA is unknown, but could come from infected lymphocytes, follicular dendritic cells, or possibly other cell types, including epithelial cells in lung, liver, or kidney. Lin et al.’s modeling studies (9) point to an important role for Abs in clearing persistently infected cells that are the source of MV RNA.
Lin et al. infect rhesus macaques with MV via the respiratory tract, the normal route of viral transmission, and measure infectious MV and virion RNA (i.e., MV RNA) in blood. Infectious virus could be recovered only during the first ∼14 d of infection. In contrast, MV RNA persisted for ∼50 d, as described previously (7, 8). How does this correspond with the immune response? Three immune parameters are included in mathematical models predicting MV RNA dynamics. Neutralizing Ab titers are used as proxy for the humoral response. T-cell responses are inputted as the frequency of IFN-γ–secreting cells in peripheral blood mononuclear cells. Finally, the immunosuppressive influence of regulatory T cells (Tregs) is included as FoxP3 mRNA measured in peripheral blood mononuclear cells (FoxP3 is a transcription factor expressed at high levels by Tregs). The total lymphocyte count is used as a measure of the number of MV susceptible cells in the animal.
By using relatively simple linear differential equations, the authors show that a model based solely on the contribution of effector T cells accounts for clearance of infectious virus, but not the persistence of MV RNA. Instead, and somewhat unexpectedly, accurately modeling MV RNA dynamics requires a major contribution from Abs (and, to a lesser extent, Tregs). These findings elegantly illustrate the sequential coordination of cellular and humoral responses to fully clear MV from infected individuals.
CD8+ T-cell–mediated clearance of infectious virus is consistent with these cells direct killing of infected cells and delivery of cytokines to induce innate antiviral immunity. However, how do Abs clear MV RNA? Answering this question requires knowledge of the nature and source of MV RNA. RNA degrades rapidly when unprotected by virions or other membrane-bound structures. MV RNA may therefore be released by persistently infected cells in the form of exosomes or virions that are not detected as infectious units as a result of their interaction with neutralizing Abs or insensitive culture methods. In this scenario, neutralizing Abs could contribute to clearance of persistent MV RNA by limiting cell-to-cell transmission of low levels of residual infectious virus. At the same time, Abs could play a direct role in eradicating infected cells via Ab-dependent cellular cytotoxicity or complement fixation (10). Less well defined, but still potentially relevant, is that Abs might induce antiviral activity in infected cells by direct interaction with viral proteins on infected cell surfaces (11).
The study of Lin et al. (9) highlights the importance of identifying the source of persistent MV RNA in infected individuals. Studies in primates with fluorescent protein-expressing MV demonstrate that the initial targets of MV infection are macrophages and dendritic cells of the upper respiratory tract (12). MV is amplified in regional lymph nodes and then disseminated hematogenously to multiple organs including lung, liver, and skin (where the telltale rash marks the T-cell response). Infected respiratory epithelial cells shed virus into the airway to enable transmission (13). Any or all of these cells could be the source of MV RNA, as MV RNA persists not just in blood, but also urine, lymphoid tissue, and lung secretions. MV can persistently infect neurons, spreading between cells directly without budding (4). If MV is capable of similar tricks outside the CNS, this would certainly complicate Ab-mediated clearance.
On the contrary, if the prolonged persistence of MV RNA is accompanied by
Modeling MV immunity enables Lin et al. to propose a surprising role for Abs in mediating MV clearance.
viral gene expression, this could contribute to the magnitude of the immune responses and, in particular, the remarkable duration of immunity. Is MV special in this respect? Recent findings in mice infected with “acute” viruses demonstrate a similar surprising duration of viral RNA, which can last for years (14) and be accompanied by viral immunogens capable of activating CD8+ T cells (15, 16). It would certainly make sense for the immune system to retain the genetic information of previous viral infections to maintain memory by occasionally translating retained viral mRNA, although it is clear that antigen persistence is not an absolute prerequisite for maintaining memory T cells (17).
Immunometrics is a powerful approach for deconvoluting the contribution of distinct components of the immune response to infection. As exemplified by Lin et al. (9), immunometrics tests assumptions and raises important questions for further study. At the same time, much remains to be learned on the road to accurate modeling. Above all, we should be ever-vigilant of the danger of immune correlates masking true effector functions. A model that matches the data is not equivalent to a model that explains the data. This is an intellectual trap. Indeed, as Lin et al. show (9), the safer scenario is when models fail, as this paves the path to new testable hypotheses.
Footnotes
The authors declare no conflict of interest.
See companion article on page 14989.
References
- 1.WHO Global reductions in measles mortality 2000-2008 and the risk of measles resurgence. Wkly Epidemiol Rec. 2009;84:509–516. [PubMed] [Google Scholar]
- 2.Moss WJ, Griffin DE. Measles. Lancet. 2012;379:153–164. doi: 10.1016/S0140-6736(10)62352-5. [DOI] [PubMed] [Google Scholar]
- 3.Chen RT, et al. Measles antibody: Reevaluation of protective titers. J Infect Dis. 1990;162:1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 4.Griffin DE, Lin WH, Pan CH. Measles virus, immune control, and persistence. FEMS Microbiol Rev. 2012;36:649–662. doi: 10.1111/j.1574-6976.2012.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Permar SR, et al. Limited contribution of humoral immunity to the clearance of measles viremia in rhesus monkeys. J Infect Dis. 2004;190:998–1005. doi: 10.1086/422846. [DOI] [PubMed] [Google Scholar]
- 6.Permar SR, et al. Role of CD8(+) lymphocytes in control and clearance of measles virus infection of rhesus monkeys. J Virol. 2003;77:4396–4400. doi: 10.1128/JVI.77.7.4396-4400.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riddell MA, Moss WJ, Hauer D, Monze M, Griffin DE. Slow clearance of measles virus RNA after acute infection. J Clin Virol. 2007;39:312–317. doi: 10.1016/j.jcv.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Pan CH, et al. Modulation of disease, T cell responses, and measles virus clearance in monkeys vaccinated with H-encoding alphavirus replicon particles. Proc Natl Acad Sci USA. 2005;102:11581–11588. doi: 10.1073/pnas.0504592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin W-HW, Kouyos RD, Adams RJ, Grenfell BT, Griffin DE. Prolonged persistence of measles virus RNA is characteristic of primary infection dynamics. Proc Natl Acad Sci USA. 2012;109:14989–14994. doi: 10.1073/pnas.1211138109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimmerjahn F, Ravetch JV. Fcgamma receptors: Old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Levine B, et al. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 12.Lemon K, et al. Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog. 2011;7:e1001263. doi: 10.1371/journal.ppat.1001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Vries RD, Mesman AW, Geijtenbeek TB, Duprex WP, de Swart RL. The pathogenesis of measles. Curr Opin Virol. 2012;2:248–255. doi: 10.1016/j.coviro.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Simon ID, van Rooijen N, Rose JK. Vesicular stomatitis virus genomic RNA persists in vivo in the absence of viral replication. J Virol. 2010;84:3280–3286. doi: 10.1128/JVI.02052-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zammit DJ, Turner DL, Klonowski KD, Lefrançois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner DL, Cauley LS, Khanna KM, Lefrançois L. Persistent antigen presentation after acute vesicular stomatitis virus infection. J Virol. 2007;81:2039–2046. doi: 10.1128/JVI.02167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murali-Krishna K, et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]