Abstract
Periostin (Postn) is a matricellular protein preferentially expressed by osteocytes and periosteal osteoblasts in response to mechanical stimulation and parathyroid hormone (PTH). Whether and how periostin expression influences bone anabolism, however, remains unknown. We investigated the skeletal response of adult Postn−/− and Postn+/+ mice to intermittent PTH. Compared with Postn+/+, Postn−/− mice had a lower bone mass, cortical bone volume, and strength response to PTH. PTH-stimulated bone-forming indices were all significantly lower in Postn−/− mice, particularly at the periosteum. Furthermore, in vivo stimulation of Wnt-β-catenin signaling by PTH, as evaluated in TOPGAL reporter mice, was inhibited in the absence of periostin (TOPGAL;Postn−/− mice). PTH stimulated periostin and inhibited MEF2C and sclerostin (Sost) expression in bone and osteoblasts in vitro. Recombinant periostin also suppressed Sost expression, which was mediated through the integrin αVβ3 receptor, whereas periostin-blocking antibody prevented inhibition of MEF2C and Sost by PTH. In turn, administration of a Sost-blocking antiboby partially restored the PTH-mediated increase in bone mass in Postn−/− mice. In addition, primary osteoblasts from Postn−/− mice showed a lower proliferation, mineralization, and migration, both spontaneously and in response to PTH. Osteoblastic gene expression levels confirmed a defect of Postn−/− osteoblast differentiation with and without PTH, as well as an increased osteoblast apoptosis in the absence of periostin. These data elucidate the complex role of periostin on bone anabolism, through the regulation of Sost, Wnt-β-catenin signaling, and osteoblast differentiation.
Intermittent administration of parathyroid hormone (PTH) is currently the only bone anabolic treatment for patients with osteoporosis (1). Intermittent PTH (iPTH) exerts biphasic effects on bone: first, PTH triggers the differentiation of mesenchymal stem cells into the osteoblast lineage (2) and activates bone-lining cells to produce and mineralize a collagen matrix (3); then it stimulates the development and activation of osteoclasts through the regulation of receptor activator of NFκB (RANKL) and osteoprotegerin expression by osteoblasts (4). In turn, bone resorption releases local factors such as IGF1, FGFs, TGF-β, and bone morphogenetic proteins that support osteoblast activity (5). Bone biopsy data in patients treated with teriparatide (PTH 1–34) indicate that bone formation on modeling surfaces accounts for 5–30% of PTH anabolic effects, whereas more than 70% of PTH anabolism occurs through remodeling surfaces (6). Hence, the net effects of iPTH on bone mass and structure depend on the extent of remodeling surfaces (7). Consequently, PTH exerts greater effects on endosteal surfaces than on the periosteum, where remodeling is virtually absent, at least in adults. In turn, RANKL inhibitors may restrict PTH anabolic effects on endosteal surfaces, without affecting bone formation at the periosteum (7–9). PTH has also been shown to inhibit sclerostin (Sost) expression (10), and this effect is mediated by MEF2C (11, 12). Thus, PTH-induced bone mass gain is inhibited at the endosteal and periosteum surfaces in mice overexpressing Sost, as well as in Sost-deficient mice (10, 12). Among all genes regulated by PTH, periostin (Postn) has been shown to be a highly responsive gene (13–16). Postn is a 90-kd secreted extracellular matrix protein that binds integrins αvβ3 (17). Postn-deficient mice develop periodontitis and osteoporosis (18). We recently reported that Postn mRNA and protein levels are rapidly up-regulated by mechanical stimuli and could contribute to Sost inhibition (19). Indeed, the cortical bone response to mechanical loading was absent in Postn−/− mice but was rescued by Sost-blocking antibodies. The preferential expression of Postn in the periosteum, the outer cortex, and in osteocytes raised the intriguing possibility that Postn could be involved in the regulation of Sost expression and bone anabolism in response to PTH.
To test this hypothesis, we characterized the skeletal response to iPTH in Postn−/− mice and investigated the role of Postn in osteoblast signaling and gene expression. Here we show that, consistent with its pattern of expression, Postn is required for PTH anabolic effects on cortical but not on trabecular bone. Moreover, Postn inhibited Sost expression, and this effect was mediated by the integrin αVβ3 receptor. Using TOPGAL reporter mice, we further demonstrated that Postn is involved in the activation of Wnt-β-catenin in response to PTH. Eventually, Postn played an important role in osteoblast differentiation. These data elucidate the complex mechanisms by which Postn regulates bone anabolism.
Results
Postn−/− Mice Are Resistant to PTH Anabolic Effects on Cortical Bone.
At baseline the weight was 22.15 ± 0.67 g in Postn+/+ and 17.8 ± 0.21 g in Postn−/− mice (P < 0.001). During the experiment, the animals gain weight normally without significant differences between genotype and treatment (Fig. 1). Bone mineral density (BMD) was significantly lower at baseline in Postn−/− vs. Postn+/+ mice at the femur (0.073 ± 0.001 vs. 0.078 ± 0.002 g/cm2, respectively, P < 0.01) and the spine (0.073 ± 0.002 vs. 0.079 ± 0.002 g/cm2, respectively, P < 0.05). iPTH stimulated femoral BMD gain over 5 wk in both Postn+/+ and Postn−/− mice; however, BMD gain was significantly less in Postn−/− mice compared with Postn+/+ mice (Fig. 1). iPTH significantly increased femoral cortical bone volume (CtBV), Ct thickness (CtTh), as well as vertebral CtTh in Postn+/+ but not in Postn−/− mice (Fig. 1 and Tables S1 and S2). Cortical bone-forming indices significantly increased in iPTH-treated Postn+/+ but not in Postn−/− mice, and most strikingly differed at the periosteum. iPTH also significantly increased alveolar bone volume in the jaw of Postn+/+ (+18.6% vs. vehicle, P < 0.01) but not in Postn−/− mice (Fig. S1). In contrast, iPTH increased trabecular bone volume to total bone bolume (BV/TV), trabecular thickness (TbTh), and trabecular number (TbN) at the femur and spine similarly in Postn−/− and Postn+/+ mice (Fig. 1 and Tables S1 and S2). Indeed, PTH stimulated bone-forming indices at trabecular surfaces similarly in Postn−/− and Postn+/+ mice (Table S2).
Fig. 1.
Effects of iPTH on bone in Postn−/− and Postn+/+ mice. (A) Femur bone BMD gain. (B) Trabecular BV/TV of the distal femur. (C) CtBV and CtTh at midshaft femur. (D) Fluorescent calcein labels on periosteal and endosteal surfaces. (E) Biomechanical properties of the cortical tibia. (F) Body weight gain. *P < 0.05, **P < 0.01, ***P < 0.001 significant difference vs. vehicle. Bars show mean (± SEM). Closed bars, iPTH; open bars, vehicle.
To evaluate whether differences in cortical bone mass and microarchitecture were translated into differences in bone strength, femurs were tested in bending. iPTH significantly increased ultimate force, stiffness, and displacement in Postn+/+ mice, but not in Postn−/− mice (Fig. 1 and Table S3). Hence, absence of Postn led to bone compartment-specific alterations in response to iPTH.
Postn Mediates PTH Inhibition of Sost Expression.
The absence of a cortical and particularly periosteal bone response to PTH in Postn−/− mice raised the possibility that Sost expression could be dysregulated in absence of periostin. iPTH significantly increased, respectively decreased, Postn (+49.5%, P < 0.05) and Sost (−41.5%, P < 0.05) mRNA expression in femur cortices of Postn+/+ mice, whereas no changes in either gene expression occurred in Postn−/− mice. Moreover, in Postn+/+ mice, iPTH increased Postn protein expression at the periosteal surface and in osteocytes, but it inhibited sclerostin in osteocytes (Fig. 2 A and C). In contrast, in Postn−/− mice, no difference in Sost staining was observed between iPTH and vehicle (Fig. 2 B and C). Actually, in Postn−/−, Sost was intensively express throughout osteocytic lacunae and canalicular system, both in vehicle and PTH-treated groups (Fig. 2 B and C).
Fig. 2.
Postn and Sost protein expression in response to iPTH. (A) Immunohistochemical staining of Postn in longitudinal sections of the proximal tibia of Postn+/+ mice 24 h after PTH treatment shows increased Postn expression at the periosteum (Ps), but not at the endocortical surfaces (Ec). (B) Immunohistochemical staining of Sost expression in tibia midshaft sections showing decreased expression 24 h after PTH treatment in Postn+/+ but not Postn−/− mice. Note the presence of Sost into tin osteocyte canaliculae (white arrow) that disappeares with PTH treatment in Postn+/+ and not in Postn−/− (black arrow) mice. (C) Immunohistofluorescence of Sost (green) and Postn (red) localization in osteocytes of the proximal tibia. Colocalization appears in yellow.
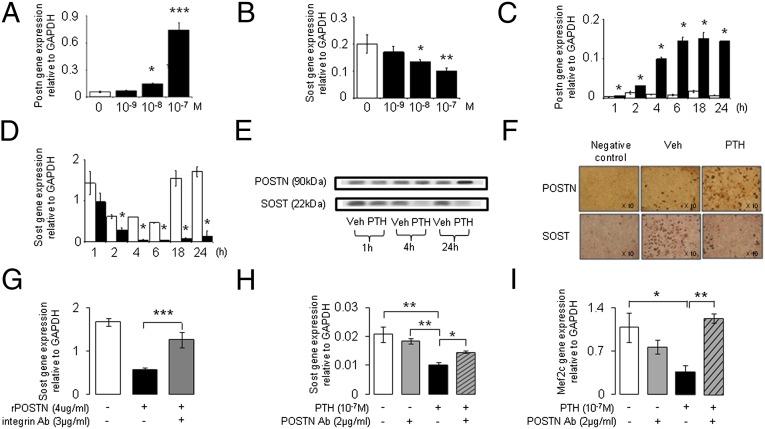
To confirm and expand these in vivo observations, we next investigated the effects of PTH on Postn and Sost expression in UMR-106 osteoblast-like cells. PTH stimulated Postn and inhibited Sost mRNA expression time- and dose-dependently (Fig. 3 A and B). Consistent with the mRNA expression, levels of Postn and Sost protein, respectively, increased and decreased in osteoblasts after exposure to PTH, as analyzed by both Western blot and immunochemistry (Fig. 3 E and F). Furthermore, UMR-106 cells exposed to recombinant Postn showed a significant reduction of Sost mRNA levels and this effect was blocked by a neutralizing antibody against the Postn receptor, integrin αVβ3 (Fig. 3G).
Fig. 3.
Regulation of Postn and Sost gene expression by PTH in UMR-106 cells. (A and B) Dose–response of Postn and Sost gene expression after 24 h of PTH treatment at 10−9, 10−8, and 10−7 M. (C and D) Time-course of Postn and Sost gene expression to PTH 10−7 M (open bars, vehicle; closed bars, PTH). (E) Western blot of Postn and Sost protein expression. (F) Immunocytochemistry of Postn and Sost expression. (G) Effects of recombinant Postn- and integrin-blocking antibodies on Sost mRNA levels. (H) Effects of PTH and Postn-neutralizing antibodies on Sost gene expression. (I) Effects of PTH and Postn-neutralizing antibodies on MEF2C gene expression. All treatments (G–I) tested for 24 h. *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle. Bars show means (± SEM).
In turn, Postn-neutralizing antibodies partially prevented Sost inhibition by PTH (−55%) (Fig. 3H). Inhibition of Sost expression by PTH has been shown to be mediated by a down-regulation of the transcription factor MEF2C in UMR-106 cells (11). Accordingly, Postn-neutralizing antibodies completely blocked PTH-induced inhibition of MEF2C, indicating that Postn regulates Sost expression upstream of MEF2C (Fig. 3I).
Altered PTH-Stimulated β-Catenin Signaling in Postn−/− Mice.
Because Sost down-regulation eventually allows activation of Wnt-β-catenin signaling (20), we next evaluated the consequences of Postn deletion on β-catenin signaling in vivo by generating TOPGAL;Postn reporter mice. Pellets of primary calvaria osteoblasts from TOPGAL;Postn−/− mice showed no X-Gal blue staining, in contrast to Postn+/+ osteoblasts (Fig. 4A). After 14 d in culture, X-Gal staining revealed nuclear and cytosolic expression of β-catenin in a higher number of cells from Postn+/+ vs. Postn−/− (Fig. 4A). Eventually, the number of X-Gal+ cells was significantly increased in response to PTH in Postn+/+ osteoblastic cultures but not in Postn−/−.
Fig. 4.
Altered PTH-stimulated β-catenin signaling in Postn−/− mice. (A, Left) Pellet of calvaria osteoblasts shows X-Gal staining in the Postn+/+ but not Postn−/−; (Right) X-Gal staining of calvaria osteoblasts after 14 d in culture. Nuclear and cytoplasm staining are indicated by an arrow and asterisk, respectively. The histogram shows the effects of PTH and Postn on the numbers of X-Gal+ cells. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. vehicle. Bars show means (± SEM). Open bars, vehicle; closed bars, PTH 10−7 M; shaded bars, recombinant periostin (2 μg/mL). (B) X-Gal staining of midshaft tibia from TOPGAL;Postn+/+ and TOPGAL;Postn−/− mice. (C) Immunohistochemical staining of periostin protein expression (brown) and X-Gal (blue). Ec, endocortical; Ps, Periosteum surfaces.
Next, these results were confirmed in vivo. In TOPGAL;Postn+/+ mice, X-Gal was most prominently expressed in osteocytes and the periosteum and markedly stimulated by iPTH (Fig. 4B). In contrast, in TOPGAL;Postn−/− mice, β-galactosidase activity was weak and not stimulated by iPTH. Moreover, PTH-stimulated Postn expression occurred in the vicinity of X-Gal+ cells, suggesting that Postn may regulate Wnt signaling by autocrine/paracrine mechanisms (Fig. 4C).
Indeed both TOPGAL;Postn+/+ and TOPGAL;Postn−/− osteoblasts responded to recombinant Postn by a modest increase of β-galactosidase activity, indicating that Postn may also directly stimulate Wnt-β-catenin signaling by some direct mechanism (Fig. 4C). In addition, we confirmed that integrin signaling is functional in Postn−/− osteoblasts, as demonstrated by their response to RGD peptides and recombinant Postn, which normally activated P-Src, P-Fak, and P-PkD (Fig. S2).
Anti-Sost Antibodies Partially Rescue PTH Anabolism in Postn−/− Mice.
To further evaluate the relationship between Postn expression and Wnt-β-catenin signaling effects on bone, Postn−/− mice were treated with a Sost-blocking antibody, plus or minus PTH. In Postn+/+, Sost-Ab alone increased femoral BMD, BV/TV, CtBV, and CtTh, as well as osteocalcin levels similarly to PTH alone (Fig. 5). However, the effects of Sost-Ab plus PTH were partially additive but not synergistic (Fig. 5). These observations suggest than a substantial proportion of PTH effects is because of Sost inhibition; alternatively that Sost-Ab does not fully block Sost activity in our experiment.
Fig. 5.
Effects of Sclerostin blocking antibodies (Sost-Ab) on bone response to iPTH. (A) Femur BMD gain. (B) BV/TV of the distal femur. (C) CtTV and CtBV of the midshaft femur. (D) Biomechanical properties of the cortical tibia. (E) Moment of inertia. (F) Difference of seric osteocalcin levels between the end and the beginning of the treatments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 PTH vs. vehicle in pretreatment (Sost-Ab or control-Ab) and genotype (Postn+/+ or Postn−/−). Bars shows mean (± SEM). Open bars, Control-Ab+vehicle; closed bars, Control-Ab+PTH (40 μg⋅kg⋅d); light shaded bars, Sost-Ab+vehicle; dark shaded bars, Sost-Ab+PTH (40 μg⋅kg⋅d).
Sost-Ab also significantly increased femur BMD, BV/TV, CtBV, and CtTh in Postn−/− mice, further indicating that the Wnt-β-catenin signaling pathway of bone formation is functional in these mice (see also Fig. 4). With Sost-Ab, PTH effects were rescued in Postn−/− but only on femur BMD gain and CtTV (Fig. 5C), confirming a role for Postn-mediated Sost inhibition on PTH effects at the periosteum (Fig. 1). The absence of PTH effects, respectively the rescuing effects of Sost-Ab on Postn−/− bone, were independent of mechanical loading, because results from mouse calvaria were consistent with the long bones (Fig. S3).
Altered Osteoblastic Functions and Osteocytic Differentiation in Postn-Deficient Cells.
We next questioned whether PTH-stimulated Postn expression could play an additional direct role on bone formation. For this purpose, we thought to investigate the direct influence of Postn expression on osteoblast functions. Primary osteoblasts from Postn−/− mouse calvariae showed a lower proliferation rate compared with Postn+/+ osteoblasts (Fig. 6A), without evidence of a cell adhesion defect. After 4 d in culture, 24 h of PTH exposure significantly stimulated osteoblast proliferation in Postn+/+ but not in Postn−/− (Fig. 6B) mice. Differentiation was also impaired in Postn−/− osteoblasts, as shown by a reduced alkaline phosphatase (ALP) activity (119.8 ± 4.9 nmol vs. 194.6 ± 9.3 nmol PNP/mg of protein in Postn+/+, P < 0.001) (Fig. 6C). ALP staining and mineralization were also lower in Postn−/− vs. Postn+/+ osteoblasts cultures (Fig. 6 C and D). Moreover, PTH increased ALP staining in Postn+/+ osteoblasts but not in Postn−/− (Fig. 6C). Eventually, Postn−/− osteoblasts presented a decreased migration capacity compared with Postn+/+, consistent with the role of Postn as a cell migration factor (Fig. 6E).
Fig. 6.
Proliferation and differentiation of Postn-deficient osteoblast. (A) Proliferation was evaluated by thymidine 3H in Postn+/+ and Postn−/− mouse calvaria osteoblasts after 2, 4, and 6 d of culture. **P < 0.01, ***P < 0.001 by unpaired t test compared with Postn+/+ mice. (B) Cell proliferation was evaluated after 6 h of PTH treatment (10−7 M) in Postn+/+ and Postn−/− osteoblasts cultures from calvaria. *P < 0.05 unpaired t test compared with vehicle. (C) ALP production and staining in osteoblasts cultures from Postn+/+ and Postn−/− calvaria mice after 14 d. ***P < 0.001 unpaired t test compared with Postn+/+ mice. (D) Mineralization of Postn+/+ and Postn−/− calvaria evaluated by Alizarin red after 21 and 28 d of culture. (E) Scratch-wound test to assess migration of osteoblast. Bars shows mean (± SEM). Open bars, vehicle Postn+/+; hatch open bars, PTH Postn+/+; shaded bars, vehicle Postn−/−; hatch shaded bars, PTH Postn−/−.
As shown in Fig. 7, expression levels of osteoblast differentiation genes were altered in cultures from Postn−/− mouse calvariae, both spontaneously and upon PTH stimulation. Expression levels of Runx2, Osterix (Sp7), Alp1, bGlap, and ATF-4 were lower in Postn−/− vs. Postn+/+ cells (Fig. 7A). Moreover, PTH significantly decreased Runx2, Sp7, MEF2C (−41.6%, −82.3%, and −65.0% vs. vehicle, P < 0.01) and increased Alp1 and bGlap (+218.3% and +507.1% vs. vehicle, P < 0.01) in Postn+/+, with little or no effect in Postn−/− osteoblasts (Fig. 7A). After 28 d, primary osteoblasts cultures began to express osteocytic cell markers, such as in Postn+/+, but virtually not in Postn−/− (i.e., another indication of defective differentiation in absence of periostin). As expected, PTH significantly decreased early (Phex), mature (MEPE), and late osteocytic (Sost) gene expression Postn+/+ (Fig. 7B).
Fig. 7.
Influence of Postn on PTH-regulated osteoblast/osteocyte gene expression. (A) The relative expression of selected osteoblastic (A) and osteocytic (B) genes was determined by quantitative RT-PCR adjusted to Gapdh after 14 and 28 d of culture. (C) Immunohistochemical analysis of apoptotic cells in response to PTH in longitudinal sections of the proximal tibia and in Postn+/+ and Postn−/− calvaria mice after 14 d of culture. White arrow, caspase 3+ cell; black arrow, caspase 3− cell. (Magnification: Upper, 10×; Lower, 20×.) *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. vehicle. Bars shows mean (± SEM). Open bars, vehicle; closed bars, PTH 10−7 M; shaded bars.
Because Postn up-regulation eventually allows PTH-stimulation of Wnt-β-catenin signaling and this pathway mediates PTH antiapoptotic effect on osteoblasts (21, 22), we eventually evaluated apoptosis of Postn−/− osteoblasts. iPTH significantly decreased the expression of proapoptotic gene caspase 3 in Postn+/+ but not in Postn−/− calvaria cultures. Inhibition of caspase 3 by PTH was found in mouse calvaria cultures and on ex vivo sections of proximal tibia in Postn+/+, but was not observed in Postn−/− (Fig. 7C).
Discussion
The main objective of our study was to elucidate the role of Postn in the skeletal response to PTH. Postn deletion in mice resulted in altered cortical bone response to iPTH, with an absence of both Sost inhibition and activation of β-catenin signaling in the periosteum, osteoblasts, and osteocytes. Conversely, Postn directly suppressed MEF2C and Sost gene expression, the latter through integrin αVβ3, and further exerted a moderate direct effect on β-catenin signaling in TOPGAL reporter cells. Moreover, osteoblast proliferation, differentiation, migration, and response to PTH were altered in absence of Postn. These results identify periostin as a regulator of Wnt-β-catenin signaling, a crucial mediator of bone anabolism.
A relationship between Postn and PTH was first suggested by the observation that Postn is regulated by PTH in mouse and humans osteoblasts (16, 23, 24). Our study now demonstrates the functional significance of PTH-stimulated Postn expression with regard to bone formation. It shows that Postn modulates osteoblast functions through both Sost-independent and -dependent mechanisms. In vitro and in vivo Postn mRNA and protein expression were increased by PTH in parallel to the inhibition of Sost expression, indicating that these events are temporally related. Moreover, Sost down-regulation by PTH was partially inhibited by a periostin neutralizing antibody, whereas in Postn−/− mice Sost gene regulation by PTH was definitively abolished. Then, recombinant Postn directly inhibited Sost expression. Ultimately, cortical bone-forming indices were not stimulated by PTH in the absence of Postn, particularly at the periosteum. Interestingly, an interaction between Postn and Sost gene polymorphisms has recently been found in an association study of subjects with extreme-BMD (25). We had previously reported that Postn is necessary for the bone anabolic response to mechanical stimuli (19). At that time we did speculate that the mechanotransduction properties of bone could be impaired because of an alteration of the bone matrix quality, or alternatively, that Postn could directly regulate Sost. The present work demonstrates that Postn directly inhibits Sost expression through its integrin αVβ3 receptor (26, 27). However, in UMR-106 cells, about half of Sost inhibition by PTH appeared independent of Postn. Furthermore, Sost-blocking antibodies did not restore PTH effects in vivo in Postn−/− mice, whereas they did restore the bone anabolic effects of mechanical stimuli (19). Indeed, these observations lead us to propose that PTH down-regulation of Sost in osteocytes is mediated by a two-step process [i.e., through PTH1R activation and cAMP/ERK signaling (probably a rapid and short term effect)], that is independent of Postn (20), then through Postn production and an autocrine/paracrine activation of integrin signaling, leading to a prolonged suppression of Sost expression. These latter observations are consistent with a direct activation of Wnt-β-catenin signaling by PTH in osteoblasts (28, 29).
The polarized expression of Postn in the cortical compartment (i.e., its preferential expression in the periosteum and outer third of the cortex) could also have implications in the differential effects and potency of PTH at the periosteal vs. endocortical surfaces (30–33). At clinically relevant doses, indeed (i.e., less than 40 μg⋅kg⋅d in mice and 20 μg/d in humans), PTH exerts relatively modest (if any) bone anabolic effects on the periosteum, contrasting with its strong endocortical anabolism (34–36). The latter is largely explained by the fact that activation of osteoblast lining cells is potentiated by key growth factors and clastokines normally released at bone resorbing surfaces. These factors may be poorly expressed at periosteal surfaces, in contrast to Postn, which now appears as a major mediator of PTH anabolic effects on the periosteum, as well as osteoblast proliferation and differentiation factors. These observations are in accordance with previous data indicating that knock-down of Postn either by neutralizing antibodies or siRNA induced a defect in the attachment of osteoblastic cells, which affects their differentiation and mineralization processes (23, 37).
In addition, PTH-stimulated increase in osteoblasts numbers appears to be at least partly mediated by its positive effects on cell survival mediated by Wnt-β-catenin (38). Our observations that Postn−/− osteoblasts and osteocytes have higher apoptosis, and that PTH decreased proapoptotic gene expression in Postn+/+ osteoblastic cultures but not in Postn−/− cultures, further suggest that PTH-induced cell survival through β-catenin signaling could also be mediated by Postn.
In conclusion, the essential role of Postn in the periosteal anabolic response to PTH is mediated by both a suppression of Sost expression in osteocyte and direct stimulatory effect of Postn on Wnt signaling and osteoblast functions. In addition, Postn expression in osteocytes near the endocortical ridge could be involved in remodeling processes at this surface. In the absence of Postn, bone formation is impaired and PTH is unable to effectively improve cortical structure and strength. In turn, these results will inform the development of bioassays for circulating periostin (39, 40) to monitor PTH activity on bone and potentially novel therapeutic approaches to improve cortical bone strength.
Materials and Methods
In Vivo PTH Experiment.
Postn-Lac Z knock-in mice (Postn−/−) were generated as reported previously (18). First, 12-wk-old Postn−/− and Postn+/+ female mice were treated with either daily subcutaneous PTH (40 μg⋅kg⋅d) or vehicle for 5 wk. This experiment was repeated in Postn+/+ and Postn−/− mice with concomitant intravenous injection of a Sost-blocking antibody (Sost-Ab, 12 mg⋅kg⋅wk) for 5 wk. MicroCT scans, histomorphometry, immunohistochemistry, gene expression, and mechanical resistance of mouse femur or tibia were performed as previously described (20). We also used the Wnt reporter TOPGAL mice to investigate the role of Postn on β-catenin activation. TOPGAL mice were bred with Postn−/− to generate TOPGAL;Postn−/− and TOPGAL;Postn+/+ reporter mice. The mice were used for immunohistochemistry and primary osteoblast cultures. For treatment regimens and protocols details see the SI Text.
In Vitro PTH Experiments.
Stock cultures of osteoblast-like UMR-106 cells were incubated for 7 d, medium was replaced every 2 d, and cells were exposed to different treatments for 24 h. For regimen detail, RNA extraction, gene expression, Western blot, and immunocytochemistry, see the SI Text. To assess the effects of PTH on osteoblast proliferation and differentiation in absence of Postn, primary osteoblast cultures were isolated from Postn+/+ and Postn−/− newborn calvaria. PTH treatment, osteogenic proliferation and differentiation assay, the qualitative migration test, and quantitative RT-PCR are detailed in the SI Text.
Supplementary Material
Acknowledgments
We thank Dr. Michaela Kneissel (Novartis) for providing the sclerostin-blocking antibodies and for her assistance in designing these experiments, and Madelein Lachize and Juliette Zicchinni for technical assistance. We are grateful to Prof. Lynda Bonewald (University of Missouri) for her valuable advice given during the course of this study. These studies were supported by Swiss National Science Foundation Grant 3100A0-116633/1 and European Commission Grant 201099-TALOS (to S.L.F.); the Riley Children’s Foundation, Indiana University Department of Pediatrics and National Institutes of Health Grant NIH HL60714 (to S.J.C.); and a grant from the European Calcified Tissue Society-Amgen Fellowship (to N.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203085109/-/DCSupplemental.
References
- 1.Kraenzlin ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7:647–656. doi: 10.1038/nrendo.2011.108. [DOI] [PubMed] [Google Scholar]
- 2.Kousteni S, Bilezikian JP. The cell biology of parathyroid hormone in osteoblasts. Curr Osteoporos Rep. 2008;6:72–76. doi: 10.1007/s11914-008-0013-9. [DOI] [PubMed] [Google Scholar]
- 3.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang JC, et al. PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res. 2004;19:235–244. doi: 10.1359/JBMR.0301226. [DOI] [PubMed] [Google Scholar]
- 5.Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11:76–81. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay R, et al. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: Early actions of teriparatide. J Bone Miner Res. 2006;21:366–373. doi: 10.1359/JBMR.051109. [DOI] [PubMed] [Google Scholar]
- 7.Pierroz DD, et al. Are osteoclasts needed for the bone anabolic response to PTH? A study of intermittent PTH With Denosumab or alendronate in knock-in mice expressing humanized RANKL. J Biol Chem. 2010;285:28164–28173. doi: 10.1074/jbc.M110.101964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samadfam R, Xia Q, Goltzman D. Pretreatment with anticatabolic agents blunts but does not eliminate the skeletal anabolic response to parathyroid hormone in oophorectomized mice. Endocrinology. 2007;148:2778–2787. doi: 10.1210/en.2006-1475. [DOI] [PubMed] [Google Scholar]
- 9.Samadfam R, Xia Q, Goltzman D. Co-treatment of PTH with osteoprotegerin or alendronate increases its anabolic effect on the skeleton of oophorectomized mice. J Bone Miner Res. 2007;22:55–63. doi: 10.1359/jbmr.060915. [DOI] [PubMed] [Google Scholar]
- 10.Robling AG, et al. Anabolic and catabolic regimens of human parathyroid hormone 1-34 elicit bone- and envelope-specific attenuation of skeletal effects in Sost-deficient mice. Endocrinology. 2011;152:2963–2975. doi: 10.1210/en.2011-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leupin O, et al. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res. 2007;22:1957–1967. doi: 10.1359/jbmr.070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer I, Loots GG, Studer A, Keller H, Kneissel M. Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res. 2010;25:178–189. doi: 10.1359/jbmr.090730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianchi EN, Ferrari SL. Beta-arrestin2 regulates parathyroid hormone effects on a p38 MAPK and NFkappaB gene expression network in osteoblasts. Bone. 2009;45:716–725. doi: 10.1016/j.bone.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onyia JE, et al. Molecular profile of catabolic versus anabolic treatment regimens of parathyroid hormone (PTH) in rat bone: An analysis by DNA microarray. J Cell Biochem. 2005;95:403–418. doi: 10.1002/jcb.20438. [DOI] [PubMed] [Google Scholar]
- 15.Li X, et al. Determination of dual effects of parathyroid hormone on skeletal gene expression in vivo by microarray and network analysis. J Biol Chem. 2007;282:33086–33097. doi: 10.1074/jbc.M705194200. [DOI] [PubMed] [Google Scholar]
- 16.Reppe S, et al. Gene expression profiles give insight into the molecular pathology of bone in primary hyperparathyroidism. Bone. 2006;39:189–198. doi: 10.1016/j.bone.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Gillan L, et al. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62:5358–5364. [PubMed] [Google Scholar]
- 18.Rios H, et al. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol. 2005;25:11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnet N, et al. The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. J Biol Chem. 2009;284:35939–35950. doi: 10.1074/jbc.M109.060335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer I, Keller H, Leupin O, Kneissel M. Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol Metab. 2010;21:237–244. doi: 10.1016/j.tem.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Jilka RL, et al. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnoke M, Midura SB, Midura RJ. Parathyroid hormone suppresses osteoblast apoptosis by augmenting DNA repair. Bone. 2009;45:590–602. doi: 10.1016/j.bone.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortunati D, et al. Periostin is a collagen associated bone matrix protein regulated by parathyroid hormone. Matrix Biol. 2010;29:594–601. doi: 10.1016/j.matbio.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Shen X, Falzon M. PTH-related protein modulates PC-3 prostate cancer cell adhesion and integrin subunit profile. Mol Cell Endocrinol. 2003;199:165–177. doi: 10.1016/s0303-7207(02)00287-3. [DOI] [PubMed] [Google Scholar]
- 25.Xiao SM, et al. Association of CDX1 binding site of periostin gene with bone mineral density and vertebral fracture risk. Osteoporos Int. 2012;23:1877–1887. doi: 10.1007/s00198-011-1861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci. 2009;66:2219–2230. doi: 10.1007/s00018-009-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashima TG, et al. Periostin, a novel marker of intramembranous ossification, is expressed in fibrous dysplasia and in c-Fos-overexpressing bone lesions. Hum Pathol. 2009;40:226–237. doi: 10.1016/j.humpath.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Ogita M, Rached MT, Dworakowski E, Bilezikian JP, Kousteni S. Differentiation and proliferation of periosteal osteoblast progenitors are differentially regulated by estrogens and intermittent parathyroid hormone administration. Endocrinology. 2008;149:5713–5723. doi: 10.1210/en.2008-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clément-Lacroix P, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci USA. 2005;102:17406–17411. doi: 10.1073/pnas.0505259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau KH, Kapur S, Kesavan C, Baylink DJ. Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. J Biol Chem. 2006;281:9576–9588. doi: 10.1074/jbc.M509205200. [DOI] [PubMed] [Google Scholar]
- 31.Robinson JA, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong VJ, et al. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biol Chem. 2007;282:20715–20727. doi: 10.1074/jbc.M703224200. [DOI] [PubMed] [Google Scholar]
- 33.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: Arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 34.Pierroz DD, et al. Beta-Arrestin2 regulates RANKL and ephrins gene expression in response to bone remodeling in mice. J Bone Miner Res. 2009;24:775–784. doi: 10.1359/JBMR.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouxsein ML, et al. beta-Arrestin2 regulates the differential response of cortical and trabecular bone to intermittent PTH in female mice. J Bone Miner Res. 2005;20:635–643. doi: 10.1359/JBMR.041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrari SL, et al. Bone response to intermittent parathyroid hormone is altered in mice null for beta-Arrestin2. Endocrinology. 2005;146:1854–1862. doi: 10.1210/en.2004-1282. [DOI] [PubMed] [Google Scholar]
- 37.Litvin J, et al. Expression and function of periostin-isoforms in bone. J Cell Biochem. 2004;92:1044–1061. doi: 10.1002/jcb.20115. [DOI] [PubMed] [Google Scholar]
- 38.Weinstein RS, Jilka RL, Almeida M, Roberson PK, Manolagas SC. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology. 2010;151:2641–2649. doi: 10.1210/en.2009-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malanchi I, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 40.Corren J, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.