Abstract
Standard tuberculosis (TB) treatment includes an initial regimen containing drugs that are both rapidly bactericidal (isoniazid) and sterilizing (rifampin and pyrazinamide), and ethambutol to help prevent the emergence of drug resistance. Antagonism between isoniazid and pyrazinamide has been demonstrated in a TB treatment mouse model. Because isoniazid’s bactericidal activity is greatest during the initial two treatment days, we hypothesized that removing isoniazid after the second day would increase the effectiveness of the standard regimen. To test this hypothesis, we developed a mouse model to measure the early bactericidal activity (EBA) of drug regimens designed to analyze the essentiality of both isoniazid and pyrazinamide during the first 14 d of therapy. Our results clearly indicate that discontinuation of isoniazid after the second day of treatment increases the EBA of standard therapy in the mouse model, whereas omitting pyrazinamide during the first 14 d was detrimental. Substitution of moxifloxacin for isoniazid on day 3 did not increase the EBA compared with only removing isoniazid after day 2. Our data show that a mouse model can be used to analyze the EBA of TB drugs, and our findings support pursuing clinical trials to evaluate the possible benefit of removing isoniazid after the first 2 treatment days.
Keywords: chemotherapy, Mycobacterium
Although tuberculosis (TB) has largely disappeared from many regions of the world, globally this disease is the leading cause of mortality due to bacterial infection, causing nearly 2 million deaths each year (1). The current standard of TB treatment requires daily administration of multiple drugs for at least 6 mo (2). Several unique characteristics of the etiologic agent Mycobacterium tuberculosis may account for this required duration of treatment. M. tuberculosis is a true human pathogen in that treatment of TB is meant to eliminate every tubercle bacillus in the body, in contrast to other opportunistic bacterial pathogens which can also be part of the normal human microbiota. Furthermore, as M. tuberculosis is an extremely slow-growing organism (doubling time of ∼20 h), the sterilization process does not occur rapidly.
The standard drug treatment of TB unfolds in two phases (3), an initial phase known as the “bactericidal phase” and a continuation phase known as the “sterilizing phase.” The former phase aims at killing the vast majority (99%) of actively metabolizing and replicating bacilli without selecting drug-resistant mutants, whereas the latter phase aims at eliminating the remaining bacilli, referred to as nonreplicating persisters. The key bactericidal TB drug (4, 5) is isoniazid (INH), which is responsible for killing rapidly growing bacilli during the first 2 d of treatment (6). Beyond these 2 d, the remaining persisting bacilli are killed in a process of slow sterilization, dependent on the two sterilizing drugs, rifampin (RIF) and pyrazinamide (PZA). It is on these concepts that the standard 6-mo short-course chemotherapy of TB is based. In M. tuberculosis, the emergence of drug-resistant bacilli depends on selection of preexisting mutants within the bacterial population (7). Thus, the RIF–INH–PZA combination is supplemented with ethambutol (EMB) to decrease the risk of selecting RIF-resistant mutants in the case of initial INH-resistance (2).
However, the combination of INH with PZA has an antagonistic effect in the mouse model and likely in humans (8). Therefore, we hypothesized that discontinuing the use of INH after its maximum bactericidal effect during the initial 2 d would relieve its antagonism with PZA and result in increasing the antimicrobial potency of the RIF–INH–PZA–EMB regimen. As the fluoroquinolone moxifloxacin (MXF) has demonstrated bactericidal activity close to that of INH (9, 10) but without antagonism with PZA (11), we further hypothesized that the substitution of MXF for INH after the initial 2 d of treatment would be beneficial. To test these hypotheses, we modeled a human early bactericidal activity (EBA) study using a mouse model of TB, demonstrating that the EBA for the standard TB treatment in mice mimics the EBA in humans. We used this model system to conduct a 14-d mouse EBA study to examine the effect of discontinuation of INH after the first 2 d of TB treatment (Study 1). The positive effects observed from the removal of INH then kindled the hypothesis that it may be of additional benefit to postpone the use of PZA in early treatment to take full advantage of the bactericidal potency of INH by removing the INH–PZA antagonism. To address this issue, we conducted a second EBA study (Study 2) in which we tested the respective roles of INH and PZA in another 14-d EBA study in the mouse model.
Results
Study 1.
Mice were infected by aerosol with M. tuberculosis in two successive runs. The day after infection (day −17), the mean lung log10 CFU counts for the two runs were 2.65 ± 0.09. Eighteen days after infection, on day 0 (just before starting treatment), the mean lung log10 CFU counts had increased by more than 4 log10 to 6.90 ± 0.36. All untreated mice remained alive, and their lung CFU counts remained stable around 7 log10. All treated mice also remained alive, but their lung CFU counts steadily declined as shown in Fig. 1A. After 2 d of treatment with RIF–INH–PZA–EMB, the mean CFU counts were reduced by 0.25 log10, although this was not statistically significant (P > 0.05).
Fig. 1.
Decline in bacterial load during the EBA of drug regimens with and without INH after 2 d of treatment. (A) log10 CFU counts with SD. (B) Regression lines for CFU data. R, rifampin; H, isoniazid; Z, pyrazinamide; E, ethambutol; M, moxifloxacin.
In mice continued on treatment with RIF–INH–PZA–EMB, the mean CFU counts declined by 0.40 log10 on day 4, by 0.96 log10 on day 7, by 1.28 log10 by day 11, and by 1.78 log10 on day 14 compared with the day 0 CFU counts. On average the daily decline in log10 CFU counts was 0.127, actually similar to that observed during the first 2 d (Table 1). In mice treated with RIF–EMB–PZA or RIF–MXF–PZA–EMB after the initial 2 d of RIF–INH–PZA–EMB, the decline in lung CFU counts was similar for both groups but more rapid than that observed in mice continued on treatment with RIF–INH–PZA–EMB. The difference between RIF–INH–PZA–EMB and both RIF–EMB–PZA and RIF–MXF–PZA–EMB reached statistical significance on days 11 (P < 0.05) and 14 (P < 0.01). The overall decline in the log10 CFU counts from day 2 and day 14 was 1.53 for RIF–INH–PZA–EMB, 2.04 for RIF–EMB–PZA, and 1.98 for RIF–MXF–PZA–EMB, i.e., a mean daily log10 reduction (EBA) of 0.127, 0.170, and 0.165, respectively (Fig. 1B).
Table 1.
Early bactericidal activity (EBA) of drug regimens with and without isoniazid after the second day of treatment in study 1
| Drug regimens |
|||
| EBA | 2RHZE/12RHZE | 2RHZE/12RZE | 2RHZE/12RMZE |
| EBA0–2 | 0.125 | 0.125 | 0.125 |
| EBA2–14 | 0.127 | 0.170 | 0.165 |
| EBA0–14 | 0.130 | ||
E, ethambutol; H, isoniazid; M, moxifloxacin; R, rifampin; Z, pyrazinamide.
Study 2.
Mice were infected by aerosol with M. tuberculosis in three successive runs. The day after infection (day −13), the mean lung log10 CFU counts for the three runs were 4.29 ± 0.22. Fourteen days after infection, on day 0 of treatment, the mean lung CFU counts had increased by 3.3 log10 to 7.59 ± 0.19. In contrast with the first study and in relation to the high number of implanted CFU, all untreated control mice died within 3–4 wk after infection. All treated mice survived, but their lung CFU counts declined more irregularly (Fig. 2A) than in study 1, likely in relation to the high bacillary burden and the actively replicating state of the bacilli on treatment initiation.
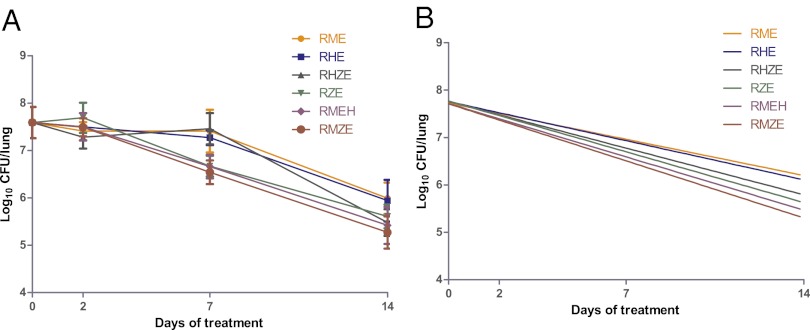
Fig. 2.
Decline in bacterial load during the 14-d EBA of drug regimens with and without INH and PZA. (A) log10 CFU counts with SD. (B) Regression lines for CFU data. R, rifampin; H, isoniazid; Z, pyrazinamide; E, ethambutol; M, moxifloxacin.
After 2 d of treatment, the mean lung CFU counts had declined by 0.18, 0.34, and 0.81 log10 in RIF–MXF–EMB–INH-, RIF–INH–PZA–EMB-, and RIF–EMB–INH-treated mice, respectively; it had declined by 0.24 and 0.17 log10 in RIF–EMB–MXF- and RIF–MXF–PZA–EMB-treated mice, respectively, and had increased by 0.11 log10 in RIF–EMB–PZA-treated mice (Fig. 2A). Although there was a trend in favor of mice treated with INH-containing regimens, the SDs of the means were large and differences between drug regimens did not reach statistical significance.
After 7 d of treatment and compared with the day 0 lung CFU counts, the mean counts had declined by 0.93, 0.13, and 0.32 log10 in RIF–MXF–EMB–INH-, RIF–INH–PZA–EMB-, and RIF–EMB–INH-treated mice, respectively; and declined by 0.18, 1.05, and 0.92 log10 in RIF–EMB–MXF-, RIF–MXF–PZA–EMB-, and RIF–EMB–PZA-treated mice, respectively, almost nullifying the differences seen at day 2. It is of interest to note that the bactericidal activities of RIF–MXF–PZA–EMB and RIF–EMB–PZA, both of the regimens without INH, and RIF–MXF–EMB–INH, a regimen with the two bactericidal drugs INH and MXF and no PZA, were taking the lead at day 7. However, as for day 2 results, the SDs of the mean CFU counts were large and differences between drug regimens did not reach statistical significance.
After 14 d of treatment, the trend observed on day 7 was confirmed. With a 2.32 log10 decline from day 0 CFU counts, RIF–MXF–PZA–EMB ranked first, followed by RIF–MXF–EMB–INH with 2.17 log10 decline, and RIF–INH–PZA–EMB with 2.11 log10 CFU decline (Fig. 2B). The remaining positions were RIF–EMB–PZA with 1.98, RIF–EMB–INH with 1.65, and RIF–EMB–MXF with 1.60 log10 CFU declines. The differences between the first three regimens and the last two regimens, RIF–EMB–INH that contained no PZA, and RIF–EMB–MXF that contained neither INH nor PZA, were statistically significant (P < 0.01). The mean daily log10 reduction (EBA) for the first 14 d for each treatment group is presented in Table 2.
Table 2.
Comparative bactericidal activity, expressed as EBA, of drug regimens in study 2
| Drug regimens |
||||||
| EBA | RHZE | RMZE | RHE | RZE | RME | RMEH |
| EBA0–14 | 0.15 | 0.17 | 0.12 | 0.14 | 0.11 | 0.16 |
E, ethambutol; H, isoniazid; M, moxifloxacin; R, rifampin; Z, pyrazinamide.
Discussion
This mouse EBA study was initiated to test the hypothesis that INH has its greatest bactericidal effectiveness during the first 2 d of treatment and then does not contribute to EBA because of antagonism with PZA, and in fact, may have a detrimental effect. When INH was withdrawn from the RIF–INH–PZA–EMB regimen after the initial 2 d and mice were given RIF–PZA–EMB or RIF–PZA–MXF–EMB onwards, the EBA0–14 was significantly better (P < 0.01) than with RIF–INH–PZA–EMB (Fig. 1). Such a finding supports the concept that after the first days of treatment the antagonism between INH and PZA (8) has a negative impact on the response to chemotherapy. It is thus conceivable to improve the effectiveness of TB treatment by a more rational use of available drugs. In such a perspective, we are not suggesting to simply discontinue INH administration to patients after the initial two days because INH, in addition to its bactericidal role, has also a role in protection against acquired resistance to other drugs, especially RIF. INH may be replaced after the first 2 d by another bactericidal drug, such as MXF, which does not exhibit antagonism with PZA (11) to prevent emergence of resistance even though it did not increase the bactericidal potential of the drug combination in our study 1. Removal of INH after only 2 d of treatment could have significant public health implications in that two days of exposure to INH would likely result in minimal emergence of INH resistance during primary treatment, as even monotherapy with INH for 2 d has never been shown to select for INH-resistant bacilli (5). Thus, using INH for only the first 2 d of treatment renders INH useful for retreatment; furthermore, this could also result in increased utility of INH for latent M. tuberculosis infection and significantly decreased severe hepatotoxicity. However, it is also possible that INH or another bactericidal drug may add to the activity of the regimen after 14 d, and the role of such drugs later during treatment will require further investigation.
Our second mouse EBA study was performed to determine whether it would be beneficial to postpone the use of the sterilizing drug PZA until day 14 with or without INH. The results provide a clear response: All PZA-containing regimens (i.e., RIF–MXF–PZA–EMB, RIF–INH–PZA–EMB, and RIF–EMB–PZA) were more bactericidal than their counterparts without PZA, i.e., RIF–EMB–MXF and RIF–INH–EMB. Precisely, the treatment with the RIF–MXF–PZA–EMB combination resulted in a more rapid mean log10 daily CFU fall over the 14-d extended EBA than treatment with RIF–EMB–MXF (0.17 and 0.11, respectively), and even than treatment with RIF–INH–EMB–PZA (0.17 and 0.15, respectively). The only PZA-deprived regimen almost as bactericidal as the best regimen, RIF–MXF–PZA–EMB, was the RIF–MXF–EMB–INH regimen that contained the two bactericidal drugs, INH and MXF. One may tentatively conclude that the sterilizing process, mainly caused by RIF and PZA, begins as early as the bactericidal process. To optimize it, sterilizing drugs should be given from treatment initiation in combination with drugs that do not negatively interfere with them, as is the case with INH and PZA. However, the impact of initiating PZA administration only after the first 2 d, immediately following a 2-d INH treatment, has yet to be determined.
One may wonder why it is not beneficial and even detrimental to postpone the use of the sterilizing drug PZA after the initial bactericidal phase of TB treatment. Among many possible reasons, the following two reasons are proposed: First, in immune-competent mice or humans receiving the regular daily treatment for drug susceptible disease, organisms cannot replicate after the first intake of bactericidal drugs. They are either killed or held in a state of drug tolerance (persistence). Therefore, the bacteria become fully susceptible to the sterilizing drugs, especially PZA, of which the main characteristic is to be active only or mainly on nonreplicating organisms. The second reason is related to the mechanism of action of PZA. It is likely that PZA is hydrolyzed intracellularly by the bacterial pyrazinamidase to pyrazinoic acid, which accumulates within the bacterial cell where it can cause poisonous-like cellular damage (12) and inhibition of transtranslation (13). Quite obviously, accumulation is favored by the nonreplicating state of the bacilli and cannot take place in actively replicating ones where the poisonous-like damage is permanently diluted. To administer PZA on treatment initiation is therefore beneficial on the condition that bacterial cell replication is totally stopped by the drugs given in combination with PZA.
The studies reported here demonstrate that EBA studies can reliably be performed in the mouse model. In several technical aspects, they provided very interesting information. First of all, in both studies, the 14-d log10 killing effect of the standard RIF–INH–PZA–EMB combination was close to 2 (precisely, it was 1.78 for the first mouse EBA study and 2.11 for the second mouse EBA study; Tables 1 and 2). Notably, such killing is of the same amplitude as the killing reported in several recent EBA studies conducted among patients treated daily for 14 d with RIF–INH–PZA–EMB (14–16). The early response of M. tuberculosis to daily RIF–INH–PZA–EMB is therefore similar in humans and in mice, and suggests that similar drug exposure levels and metabolic states of the bacilli exist in both mouse and human lungs during the first 14 d of treatment. Indirectly, it also suggests that the treatment response of bacilli in the human sputum samples is reflecting that inside the lung cavity, at least during the initial 14 d of treatment. More practically, it suggests that the results of EBA studies performed in mice are likely to be predictive of the results of EBA studies performed in humans, thus indicating that this mouse model could be a powerful tool in the EBA analysis of novel TB drugs and drug regimens. This model could also be used to analyze the early drug interactions on tubercle bacilli, a subject that remains to be explored for a better understanding of the mechanisms involved in drug killing and for optimizing the antimicrobial response. The second issue of interest is the fact that, in both of the mouse EBA studies, we did not observe the steep decline in CFU counts during the first 2 d followed by a more moderate reduction thereafter as observed in humans treated with RIF–INH–PZA–EMB. On the contrary, the decline in the first 2 d was limited and irregular, and the reduction over the 14 d was rather monophasic. It is possible that the huge load of extracellular organisms concentrated in the lung cavities allows for a more rapid decrease in CFUs in human sputum samples than the more dispersed load of intracellular organisms in the mouse lungs, or that the steep initial CFU decline observed in humans results from additional effects such as the rapid decrease in productive coughing consequent to the microbial killing (17). In view of this possibility, the mouse model may underestimate the contribution of INH to 2-d EBA in human TB treatment. Another technical issue is the number of time points to be included for the EBA performance. In our first mouse EBA study, CFU counts were performed on days 0, 2, 4, 7, 11, and 14. Because the workload required to perform lung CFU counts from so many mice at these time points was very high and the data from days 4 and 11 were not very informative, we reduced the time points for CFU counts to days 0, 2, 7, and 14 in the second study. Although assessments based on day 14 were the most informative, we feel that the more repeated time points as in our first study have “buffered” CFU count variations and therefore appear advisable.
In conclusion, the current EBA studies performed in the mouse provided, at least in the assessment of the standard RIF–INH–PZA–EMB regimen, results similar to those obtained in humans. As expected, they confirmed that INH gives its maximum bactericidal effect during the first 2 d of standard treatment and not beyond, and the antagonism between INH and PZA between 2 and 14 d of treatment has also been confirmed. More surprisingly, they demonstrated that the sterilizing effect of PZA begins as soon as drug combination therapy is initiated. In a broader perspective, our results also highlight the importance of continued optimization of multidrug regimens, as the improved use of existing drugs has the potential to enhance treatments for infectious (and other) diseases, benefitting individual patients as well as the public health of the general community.
Materials and Methods
All experimental procedures were performed at the Johns Hopkins University Center for Tuberculosis Research (Baltimore, MD).
Antimicrobials.
INH, RIF, PZA, and EMB were purchased from Sigma. MXF was provided by Bayer. All drugs were either dissolved or suspended in distilled water before oral gavage. Stock solutions were prepared weekly, as described (18). All antibiotic solutions were stored at 4 °C.
Bacterial Strain.
M. tuberculosis H37Rv was passaged in mice, frozen in 1-mL aliquots, and stored at −80 °C before use. For each infection, an aliquot was thawed and subcultured in Middlebrook 7H9 broth supplemented with 10% oleic acid–albumin–dextrose–catalase (Difco) and 0.05% Tween 80 (Sigma).
Aerosol Infection.
In both studies, female outbred Swiss mice (Charles River) aged 4–6 wk old were used to recapitulate differences in outbred hosts (i.e., humans). The mice were infected by the aerosol route using the inhalation exposure system (Glas-Col) and a log-phase broth culture (Difco 7H9 with 10% oleic acid–albumin–dextrose–catalase and Tween 80) with an optical density at 600 nm of ∼1.0. After aerosol infection, mice were randomized into treatment groups to have 15 mice per group per time point. Untreated mice were killed either (i) on the day after infection (n = 5) to determine the numbers of CFUs implanted in the lungs or (ii) on the day of treatment initiation (n = 15) to determine pretreatment baseline CFU counts. Quantitative lung cultures were performed on selective 7H11 plates (Becton-Dickinson), as described (18). All procedures involving animals were approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Drug Treatment.
In both studies, drug regimens were given for 14 d, once daily, 7 d per week, in 0.2-mL doses by esophageal canula (gavage). For managerial reasons, treatment began 18 d after infection in study 1, and 14 d after infection in study 2. Drugs were administered at the following doses: INH, 10 mg/kg; RIF, 10 mg/kg; PZA, 150 mg/kg; and MXF and EMB, 100 mg/kg. Drug dosages were chosen to produce serum area under the concentration curve (AUC) in mice equivalent to the AUC obtained with currently recommended human dosages (11, 19). RIF was given 1 h before administration of the other drugs to avoid any adverse pharmacokinetic interaction (20–22).
In study 1, all mice received the standard RIF–INH–PZA–EMB regimen for the first 2 d. Then they received RIF–INH–PZA–EMB, RIF–EMB–PZA, or RIF–MXF–PZA–EMB for the following 12 d.
In study 2, for day 1 to day 14, mice received one of the following regimens: (i) no treatment and were the untreated negative control group; (ii) RIF–INH–PZA–EMB, the standard positive control; (iii) RIF–EMB–INH, to assess the impact of not giving PZA during the initial 14 d of treatment; (iv) RIF–EMB–PZA, to assess the impact of not giving INH during the initial 14 d of treatment. The comparison between regimens iii and iv would permit prioritizing the role of INH and PZA during the initial 14 d of treatment; (v) RIF–EMB–MXF, a regimen totally deprived of INH and PZA to assess the potential of MXF as an alternative bactericidal drug; (vi) RIF–MXF–EMB–INH, the standard regimen in which PZA is replaced by a second bactericidal drug to assess the impact of adding INH to RIF–EMB–MXF; (vii) RIF–MXF–PZA–EMB, a key regimen to assess the impact of replacing from the day of treatment initiation INH by another bactericidal drug with no known antagonism with PZA.
Assessment of Treatment Efficacy.
The relative bactericidal activity of each drug regimen was assessed on the basis of the decline in lung CFU counts. In study 1, 15 mice from each treatment group were killed after 0, 2, 4, 7, 11, and 14 d of treatment. In study 2, 15 mice from each treatment group were killed after 0, 2, 7, and 14 d of treatment. The reduction in the number of time points was justified by the increased number of regimens included in study 2 and the fact that CFU counts from the day-4 and day-11 time points were not considered as really informative in study 1. Lungs were removed under aseptic conditions, placed in PBS, homogenized, and plated on selective 7H11 plates, as described (8). Plates were incubated for 4 wk at 37 °C before CFU counts were determined.
Statistical Analysis.
The sample size of 15 outbred Swiss mice per time point and treatment group was decided to recapitulate as much as possible EBA studies conducted in humans. The CFU count for each individual mouse was based on the dilution of the lung homogenate that yielded the number of colonies closest to 50. At a given time point, it was usually the same dilution for mice of the same treatment group. The mean of the 15 individual CFU counts at each time point was calculated and log10 transformed before analysis. The overall reduction in the log10 CFU counts from initiation to completion of treatment was calculated for each treatment group, and the EBA, in accordance with its standard definition (4–6), was expressed as the mean daily log10 reduction of CFU counts in total mouse lungs. Group means for experimental treatment groups were compared with that of the standard treatment control by two-way ANOVA with Bonferroni posttests. Group proportions were compared with the standard treatment regimen using Fisher’s exact test and adjusting for multiple comparisons. All analyses were performed with GraphPad Prism version 4.01 (GraphPad).
Acknowledgments
This work was supported by the Howard Hughes Medical Institute; National Institutes of Health (NIH) Grants AI 37856, AI 36973, and AI 79590; and a supplement from the NIH Division of AIDS.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.World Health Organization 2011. Global tuberculosis control: WHO report 2011 (WHO Press, Geneva)
- 2.World Health Organization 2009. Treatment of tuberculosis: Guidelines. (WHO Press, Geneva), 4th Ed.
- 3.Canetti G. The eradication of tuberculosis: Theoretical problems and practical solutions. Tubercle. 1962;43:301–321. doi: 10.1016/s0041-3879(62)80071-3. [DOI] [PubMed] [Google Scholar]
- 4.Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121:939–949. doi: 10.1164/arrd.1980.121.6.939. [DOI] [PubMed] [Google Scholar]
- 5.Donald PR, Diacon AH. The early bactericidal activity of anti-tuberculosis drugs: a literature review. Tuberculosis (Edinb) 2008;88(Suppl 1):S75–S83. doi: 10.1016/S1472-9792(08)70038-6. [DOI] [PubMed] [Google Scholar]
- 6.Jindani A, Doré CJ, Mitchison DA. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med. 2003;167:1348–1354. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- 7.David HL. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl Microbiol. 1970;20:810–814. doi: 10.1128/am.20.5.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida D, et al. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob Agents Chemother. 2009;53:4178–4184. doi: 10.1128/AAC.00830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pletz MW, et al. Early bactericidal activity of moxifloxacin in treatment of pulmonary tuberculosis: a prospective, randomized study. Antimicrob Agents Chemother. 2004;48:780–782. doi: 10.1128/AAC.48.3.780-782.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson JL, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006;10:605–612. [PubMed] [Google Scholar]
- 11.Nuermberger EL, et al. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am J Respir Crit Care Med. 2004;169:421–426. doi: 10.1164/rccm.200310-1380OC. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: A review. Int J Tuberc Lung Dis. 2003;7:6–21. [PubMed] [Google Scholar]
- 13.Shi W, et al. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science. 2011;333:1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diacon AH, et al. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob Agents Chemother. 2010;54:3402–3407. doi: 10.1128/AAC.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diacon AH, et al. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2011;15:949–954. doi: 10.5588/ijtld.10.0616. [DOI] [PubMed] [Google Scholar]
- 16.Diacon AH, et al. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet. 2012 doi: 10.1016/S0140-6736(12)61080-0. 10.1016/S0140-6736(12)61080-0. [DOI] [PubMed] [Google Scholar]
- 17.Loudon RG, Spohn SK. Cough frequency and infectivity in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1969;99:109–111. doi: 10.1164/arrd.1969.99.1.109. [DOI] [PubMed] [Google Scholar]
- 18.Nuermberger E, et al. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother. 2006;50:2621–2625. doi: 10.1128/AAC.00451-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, et al. Treatment of tuberculosis with rifamycin-containing regimens in immune-deficient mice. Am J Respir Crit Care Med. 2011;183:1254–1261. doi: 10.1164/rccm.201012-1949OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhillon J, Dickinson JM, Sole K, Mitchison DA. Preventive chemotherapy of tuberculosis in Cornell model mice with combinations of rifampin, isoniazid, and pyrazinamide. Antimicrob Agents Chemother. 1996;40:552–555. doi: 10.1128/aac.40.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickinson J, Guy A, Mitchison DA. Bioavailability of rifampin in experimental murine tuberculosis. Antimicrob Agents Chemother. 1992;36:2066–2067. doi: 10.1128/aac.36.9.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grosset J, Truffot-Pernot C, Lacroix C, Ji B. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother. 1992;36:548–551. doi: 10.1128/aac.36.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]