Abstract
A component in seminal fluid elicits an ovulatory response and has been discovered in every species examined thus far. The existence of an ovulation-inducing factor (OIF) in seminal plasma has broad implications and evokes questions about identity, tissue sources, mechanism of action, role among species, and clinical relevance in infertility. Most of these questions remain unanswered. The goal of this study was to determine the identity of OIF in support of the hypothesis that it is a single distinct and widely conserved entity. Seminal plasma from llamas and bulls was used as representative of induced and spontaneous ovulators, respectively. A fraction isolated from llama seminal plasma by column chromatography was identified as OIF by eliciting luteinizing hormone (LH) release and ovulation in llamas. MALDI-TOF revealed a molecular mass of 13,221 Da, and 12–23 aa sequences of OIF had homology with human, porcine, bovine, and murine sequences of β nerve growth factor (β-NGF). X-ray diffraction data were used to solve the full sequence and structure of OIF as β-NGF. Neurite development and up-regulation of trkA in phaeochromocytoma (PC12) cells in vitro confirmed NGF-like properties of OIF. Western blot analysis of llama and bull seminal plasma confirmed immunorecognition of OIF using polyclonal mouse anti-NGF, and administration of β-NGF from mouse submandibular glands induced ovulation in llamas. We conclude that OIF in seminal plasma is β-NGF and that it is highly conserved. An endocrine route of action of NGF elucidates a previously unknown pathway for the direct influence of the male on the hypothalamo–pituitary–gonadal axis of the inseminated female.
Keywords: neurotrophins, hypothalamus, fertility, neuroendocrine
In a monograph nearly 50 y ago, Thaddeus Mann summarized the natural properties of seminal plasma as a vehicle for sperm transport, a controller of sperm motility and capacitation, and as a stimulant of uterine contractility (1). Notwithstanding Mann’s admonishment to resist the temptation “to assign to every newly discovered chemical constituent of semen a major role in the process of fertilization,” recent isolation of a protein factor in seminal plasma (2–4) suggests an additional role of the ejaculate—as an inducer of ovulation.
The role of the fluid portion of the ejaculate, and the male accessory glands responsible for producing it, has been enigmatic. From an evolutionary perspective, it has been suggested that the male accessory glands likely originated as the machinery for producing a copulatory plug, which has the “chastity effect” of preventing the sperm of other males from entering the female tract, as well as minimizing sperm loss after insemination (5). If this is so, then the persistence of an elaborate accessory gland system in many species in which plug formation does not occur may be viewed as nothing more than an evolutionary vestige.
The first reports of an ovulation-inducing factor (OIF) in semen resulted from the observation that ovulation occurred after intravaginal or intramuscular administration of Bactrian seminal plasma to female Bactrian camels (6, 7). The existence of OIF in seminal plasma was confirmed in later studies involving llamas and alpacas, New World relatives of camels (2). The results of this and subsequent studies have led to the discoveries that OIF exists in the semen of camelids (induced ovulators), is a potent stimulator of luteinizing hormone (LH) secretion, has a dose-dependent effect on ovulation and the form and function of the corpus luteum and acts via a systemic rather than a local pathway at physiologically relevant doses (2, 8–10).
It is not surprising that the discovery of OIF in seminal plasma was made in species categorized as induced ovulators (e.g., rabbits, camelids, and koalas) because factors influencing the occurrence of ovulation can be studied without the confounding effects of spontaneous ovulation. However, results of recent studies support the hypothesis that OIF in seminal plasma is conserved among species, including those considered to be spontaneous ovulators (e.g., cattle, horses, pigs, and mice) (9, 11). Furthermore, OIF in seminal plasma influenced ovarian function in species considered to be spontaneous ovulators. That is, OIF induced ovulation in a prepubertal mouse model (11) and altered ovarian follicular wave dynamics in cows (12).
To characterize the biochemical nature of OIF, attempts were made to ablate the bioactivity of seminal plasma by molecular mass cutoff filtration, treatment with charcoal or heat, and enzymatic digestion with proteinase K or pronase E (3). An in vivo llama ovulation bioassay was used to confirm that OIF is not a steroid, prostaglandin, or gonadotropin releasing hormone (GnRH); rather it is a protein molecule that is resistant to heat and enzymatic digestion with proteinase K and has a molecular mass of more than about 30 kDa. Protein fractions of llama seminal plasma were subsequently isolated and purified by column chromatography and tested for ovulation-inducing bioactivity using the in vivo llama ovulation bioassay (4).
The primary goal of the following series of studies was to determine the identity of OIF in seminal plasma in support of the hypothesis that OIF is a single distinct and widely conserved constituent of seminal plasma. Seminal plasma from llamas and bulls was used as that of species representative of induced and spontaneous ovulators, respectively.
Results
Biochemical Purification and Protein Identification of OIF.
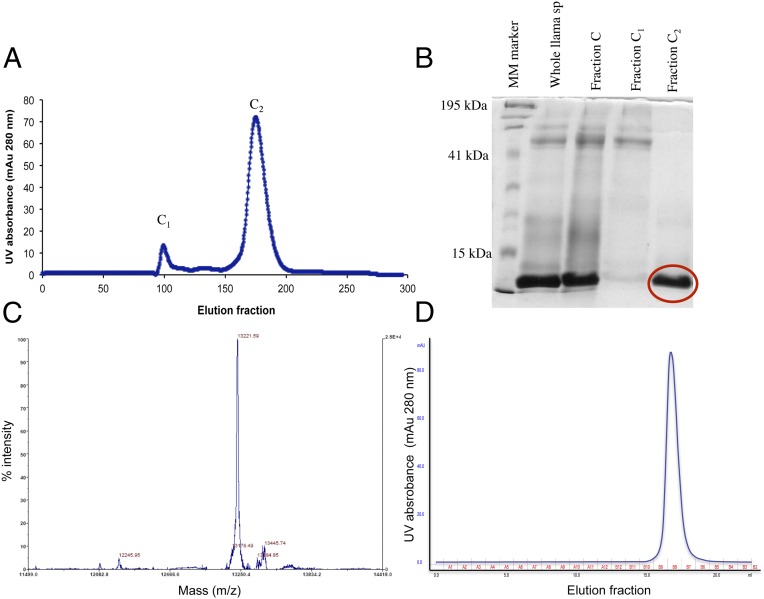
Using an approach similar to that described in our previous report (4), three protein fractions of llama seminal plasma were identified using hydroxylapatite column chromatography (fractions A, B, and C) and a prominent protein band with a mass of ∼14 kDa was identified by SDS/PAGE of fraction C. Fraction C was loaded into a sephacryl gel filtration column for further purification resulting in two distinct subfractions, C1 and C2. Fraction C2 contained a highly purified isolate of a 14-kDa protein (Fig. 1) that was identified previously as OIF by the effect of eliciting a preovulatory LH surge followed by ovulation and corpus luteum formation in >95% of llamas after intramuscular administration (4).
Fig. 1.
Separation and molecular mass of ovulation-inducing factor (OIF) in llama seminal plasma. (A) Separation of fraction C was done using sephacryl gel filtration fast protein liquid chromatography and isocratic elution with PBS. Fraction C was isolated previously by hydroxylapatite column chromatography. (B) Protein band at about 14 kDa on denaturing 12% SDS/PAGE (circled) was the major constituent of fraction C2. (C) Mass spectra (mass-to-charge ratio; m/z) of OIF (fraction C2 isolated from llama seminal plasma) by MALDI-TOF analysis showing a single peak at 13,221 Da, corresponding to the monomeric subunit of the homodimer of β-NGF. (D) Narrow peak appeared at 16.755 mL of elution volume after loading a nondenatured sample of fraction C2 into a calibrated superdex gel filtration column, corresponding to the molecular mass of β-NGF (i.e., homodimer of 26 kDa).
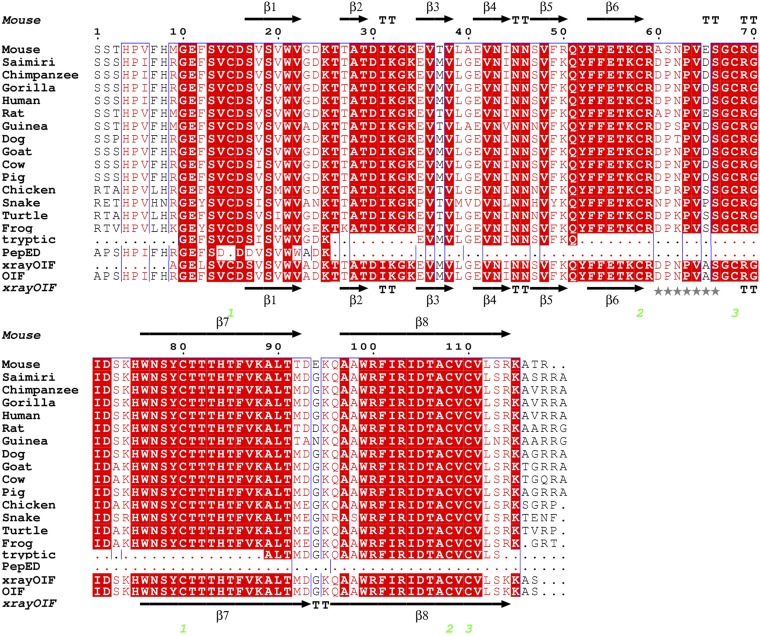
Analysis of the purified nontrypsinated fraction C2, cut from the SDS/PAGE gel, by MALDI-TOF revealed a major peak with a mass of 13,221 Da (Fig. 1). Four tryptic peptides of 12–17 aa, derived from the 14-kDa band of fraction C2 by capillary LC-MS/MS, had peptide sequence homology ranging from 80 to 100% with human, porcine, bovine, and murine sequences of type β nerve growth factor (β-NGF) listed in the database of the National Center for Biotechnology Information (NCBI; http://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins), and aligned using the database of the European Bioinformatics Institute (EBI; www.ebi.ac.uk/Clustal; Tables S1 and S2). A sample of nondenatured C2 fraction was analyzed by Edman degradation to confirm the sequence obtained by LC-MS/MS; a 23-residue segment of C2 bore sequence homology ranging from 73 to 86% of human, porcine, bovine, and murine sequences for NGF listed in the EBI database (Tables S1 and S2 and Fig. 2).
Fig. 2.
Structure-based sequence alignment of ovulation-inducing factor (OIF) from seminal plasma and nerve growth factor (NGF). Sequence alignments of mature OIF (determined by X-ray crystallography sequencing) with NGF from different species (mouse, PDB 1bet; Saimiri boliviensis, Q5ISB0.2; chimpanzee, BAA90438.1; gorilla, Q9N2F0.1; human, PDB 1sg1; rat, P25427.2; guinea pig, P19093.1; dog, AAY16195.1; goat, AFA52664.1; cow, NP_001092832.1; pig, Q29074.1; chicken, P05200.1; snake venom, Q5YF90.1; turtle, ACY72443.1; and frog, P21617.2). Tryptic is the tryptic peptide sequence of llama OIF (residues:10–23, 35–51, 89–99, and 100–113) obtained by LC-MS/MS and PepED is the N-terminal amino acid sequence of llama OIF obtained by internal Edman degradation. Residues are numbered in black and follow the sequence of mouse NGF (PDB 1bet). Solid black arrows indicate β-strands. TT indicates tight turns. Strictly conserved residues are indicated by white letters on a red background. Conservatively substituted residues are indicated by red letters on a white background. The three disulfide bridges between Cys15 and Cys80, Cys58 and C108, and C68 and Cys110 are shown in green numbers. Loops are labeled L1–L4 (blue). Stars indicate flexible loop 3.
The molecular mass of fraction C2 of llama seminal plasma, determined by SDS/PAGE and MALDI-TOF analyses, corresponded to that of the monomeric subunit of β-NGF (i.e., 13 kDa). A narrow peak appeared at 16.755 mL of elution volume after loading nondenatured fraction C2 into a calibrated superdex gel filtration column. The peak corresponded to the molecular mass of β-NGF homodimer (i.e., 26 kDa; Fig. 1). The reducing conditions of SDS/PAGE used in this and the previous study (4) apparently rendered the homodimer into monomers.
X-Ray Sequence and Crystal Structure of OIF from Seminal Plasma.
The four tryptic peptides and Edman chemistry sequence provided only partial sequencing of OIF. X-ray crystallography was used to derive full OIF sequence information based on electron density. A BLAST search in the database of the National Center for Biotechnology Information (NCBI) with the tryptic peptides revealed high sequence identity with β-NGF (Fig. 2). The structure of OIF was solved by molecular replacement using murine β-NGF (PDB code 1bet) (13) as a search model, and subsequently refined to 2.3-Å resolution. Simulated annealing was used throughout the refinement process to eliminate model bias and generate unbiased omit maps (Table S3). Most of the OIF residues (residues 9–117) could be assigned on the basis of shape of the electron density, the partial OIF sequence, identity with homologous β-NGF proteins (Fig. 2), and the chemical environment of the residues. Some residues at the N terminus and loop region (residues 61–66) showed poor or no density. Residues 1–8 were omitted from the structure because there was no density; however, they were assigned to the OIF sequence on the basis of the Edman chemistry sequence. Residue 9 showed only enough density to build in Ala, but it is most likely Arg, based on the LC-MS/MS sequence. Similarly, residue 12 was assigned as Leu, but is likely Phe, based on the LC-MS/MS sequence. Residue 65 was built in as Ala, but NGF homologs have Asp, Glu, or Ser at this position (Fig. 2). The final OIF sequence (residues 1–117; Fig. 2) has a calculated molecular weight of 13,039 Da, which is slightly smaller than the experimental value of 13,221 Da obtained by MALDI-TOF. The difference may be attributed to missing residues at the C terminus (i.e., no observed electron density in the crystal structure due to flexibility) or some ambiguities from crystallographic sequencing.
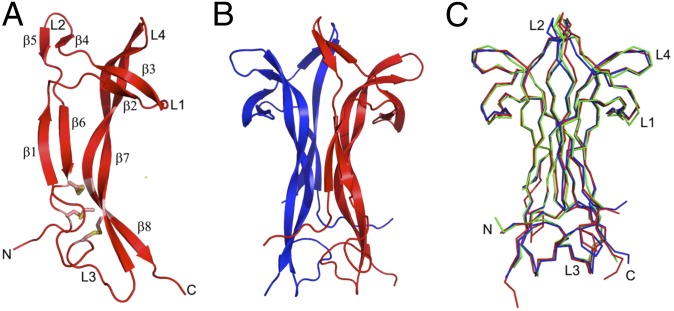
The overall structure of OIF consists of a noncovalently associated biological homodimer related by a noncrystallographic twofold rotation axis (Fig. 3). It exists as a dimer in solution (Fig. 1D) and crystallizes as a dimer. Each monomer has four loops (L1–L4) and eight β-strands joined by three disulfide bridges. High structural similarity exists between OIF and NGF of mice (PBD codes 1btg and 1bet) (13) and humans (PDB codes 1www, 1sg1, and 1sgf) (14). Superposition of the NGF structures onto OIF revealed root-mean-square deviations ranging from 0.8 to 1.2 Å for all overlapping Cα atoms (Fig. 3). This is consistent with the very high sequence identity between OIF and NGF shown in Fig. 2. Thus, the crystal structure and sequence derived from X-ray crystallography confirmed that OIF is β-NGF.
Fig. 3.
Protein structure of ovulation-inducing factor (OIF) from seminal plasma. (A) Monomer of OIF. β-Strands are labeled (β1–β8). Loops are labeled L1–L4. The three disulfide bridges between Cys15 and Cys80, Cys58 and C108, and C68 and Cys110 are shown in stick representation. (B) Biological dimer of OIF, rotated 90° with respect to A. OIF monomers are colored red and blue. (C) Cα superpositions of OIF (blue), mouse NGF (PDB code 1btg; red), and human NGF (1sg1; green). Superpositions of the three dimers reveal high structural similarity between OIF and NGF. Loops of one of the two monomers are labeled L1–L4.
Confirmation by PC12 Bioassay.
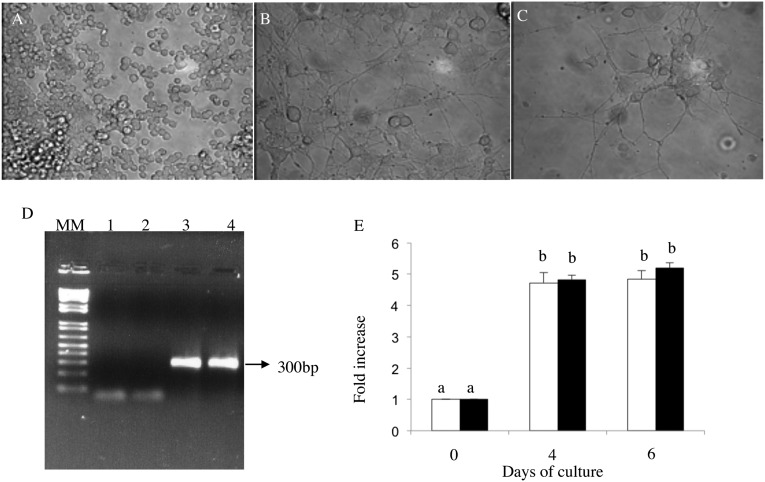
Immortalized rat phaeochromocytoma cells (PC12) have trkA receptors on their cell surface that bind specifically with NGF, and neurite growth in PC12 cells in response to treatment is used as an in vitro bioassay for NGF (15). Treatment of PC12 cells with purified OIF (fraction C2 of llama seminal plasma) induced neurite outgrowth in a fashion similar to that of recombinant mouse NGF after 6 d of in vitro culture (Fig. 4). Further, OIF up-regulated mRNA expression for trkA in PC12 cells similar to that of recombinant mouse NGF after 4 and 6 d of in vitro culture (Fig. 4).
Fig. 4.
Purified OIF (fraction C2 isolated from llama seminal plasma) induced neurite growth and NGF-specific receptor trkA in immortalized rat phaeochromocytoma (PC12) cells in vitro. PC12 cells after 6 d of in vitro culture with (A) no treatment (negative control), (B) treatment with 50 ng/mL of recombinant mouse NGF (positive control), or (C) treatment with 50 ng/mL of purified OIF (fraction C2 from llama seminal plasma). (D) Transcript expression for the NGF-specific receptor trkA in PC12 cells after 6 d of in vitro culture with 50 ng/mL of recombinant mouse NGF or 50 ng/mL of purified OIF. MM: DNA ladder 100–1100 bp. Lanes 1 and 2: Control template without primers. Lane 3: trkA product in PC12 cells treated with rNGF. Lane 4: trkA product in PC12 cells treated with OIF. (E) Response in mRNA for trkA in PC12 cells after 6 d of in vitro culture with 50 ng/mL recombinant mouse NGF (white bar) or 50 ng/mL of purified OIF (black bar). Mean ± SEM of three independent experiments. (a and b) Values with different superscripts are different (P < 0.01).
Immunoblot Analysis of Purified OIF and Whole Seminal Plasma.
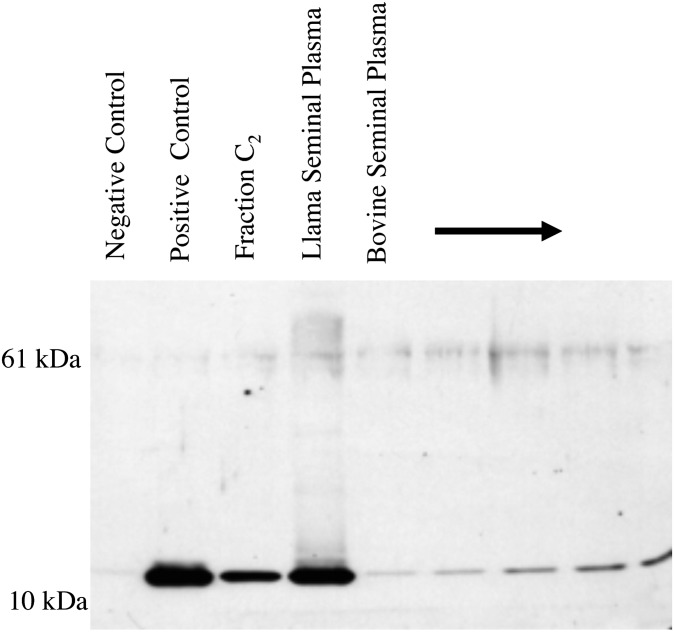
Western immunoblot analysis using a commercial polyclonal antibody against NGF revealed the similarity in immunorecognition between NGF and OIF (fraction C2 from llama seminal plasma; Fig. 5). Further, samples of whole seminal plasma of llamas and bulls displayed a similar staining pattern with a distinct band at ∼13 kDa (i.e., that of the NGF monomer). A less distinct band appeared at just over 60 kDa in immunoblots of whole seminal plasma and was interpreted as pro-NGF (16).
Fig. 5.
Immunoblot analysis of the seminal plasma of llamas and bulls with a polyclonal mouse anti-NGF. Negative control: cytochrome C (300 ng). Positive control: recombinant mouse NGF (300 ng). Fraction C2: OIF purified from llama seminal plasma (300 ng). Whole llama seminal plasma (800 ng total protein). Whole bovine seminal plasma (0.8, 1.6, 3.2, 4.8, and 6.4 μg total protein, respectively).
Ovulation-Inducing Effect of NGF.
In replicate 1, the proportion of llamas that ovulated in response to intramuscular treatment with OIF (250 μg fraction C2 of llama seminal plasma), β-NGF (250 μg from mouse submandibular glands), or saline (negative control) was 4/4, 2/4, and 0/4, respectively. An i.v. route of administration of the same treatments in replicate 2 resulted in an ovulation rate of 4/5, 4/5, and 0/5, respectively. Combined among replicates, the proportion of llamas that ovulated was similar in the OIF- and NGF-treatment groups, both of which were higher than in the saline-treated group (8/9, 6/9, 0/9; P < 0.01).
Discussion
Nerve growth factor belongs to a family of neurotrophins that includes brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4). All of the neurotrophins exist in nature as homodimers with a molecular mass of 26–27 kDa (17). That OIF is NGF explains the paradoxical results of two previous studies regarding the molecular mass of the bioactive fraction of OIF. Seminal plasma filtered to fractions containing less than ∼30 kDa molecules failed to induced an ovulatory response in llamas (3), yet the fraction digested to less than about 19 kDa by proteinase K (as determined by denaturing SDS/PAGE) retained ovulation-inducing activity (4). The apparent contradiction may be attributed to the breakage of the homodimer into monomers less than 19 kDa, by the denaturing conditions of the SDS-PAGE. Given the retention of bioactivity, it is unlikely that proteinase K actually rendered seminal NGF into its monomers.
Originally discovered in mouse sarcoma, cobra venom, and submandibular salivary glands of adult mice, NGF has been characterized classically by its role in promoting survival and growth of sensory (dorsal root) and sympathetic neurons, and cells of the adrenal medulla (18). However, NGF has subsequently been identified in a variety of nonneuronal cells including tissues of both the male and female reproductive organs. Early purification experiments revealed that bovine seminal plasma is a rich source of NGF (19) and is likely produced primarily by the vesicular glands (20). It has also been detected in the prostate gland of guinea pigs, rabbits, and bulls (19). Identification of NGF and its receptor in the testis and epididymis of mice, and in human and bovine ejaculated spermatozoa have led to the suggestion that NGF may play a role in sperm viability and fertilizing ability (21, 22).
The NGF protein and its receptor trkA have also been identified in the ovary of rodents (23), sheep (24), cattle (25), and humans (26). Synthesis of NGF and its receptor were found in both granulosa and theca cells in cows and women (25, 27). Vascular cell proliferation in cultured neonatal rat ovaries (either directly or through synthesis of vascular endothelial growth factor) was induced by treatment with NGF (28), suggesting a role in the maintenance of follicular and luteal vasculature. As well, NGF stimulated estradiol secretion from cultured human granulosa cells (directly and by increasing the formation of FSH receptors (27)). Up-regulation of trkA and NGF mRNA was detected in granulosa and theca cells, respectively, at the first preovulatory surge of gonadotropins at puberty in rats, and the use of NGF antibodies or a trkA blocker at the time of the preovulatory LH surge inhibited ovulation (23). An increase in NGF and trkA in follicular cells after the preovulatory LH surge was associated with disruption of gap junctions among follicular cells preceding ovulation (29).
The abundance of NGF in seminal plasma and the effects of seminal plasma on ovarian function strongly support the idea of an endocrine mode of action (i.e., systemic distribution with distant target tissues). This is in sharp contrast to the notion that NGF is produced by target cells and has paracrine/autocrine actions (described above). Support for an endocrine mechanism is found in the following observations: (i) intramuscular administration of seminal plasma resulted in ovulation in 33/35 (94%) llamas and alpacas compared with 0/35 (0%) given saline over four separate in vivo studies (2, 8); (ii) ovulation rate was highest after intramuscular administration of seminal plasma, intermediate after intrauterine treatment with endometrial curettage (to mimic postcoital endometrial inflammation) (8), lower after intrauterine administration without curettage, and nil after intrauterine administration of saline with or without curettage (93, 67, 24, and 0%, respectively) (2, 8); (iii) intramuscular or intrauterine administration of seminal plasma resulted in a surge in circulating concentrations of LH beginning within 1 h and peaking within 3 h of treatment, followed by ovulation at 30 h (2); (iv) administration of purified OIF from seminal plasma had a dose-dependent effect on LH release, ovulation, and corpus luteum (CL) form and function, and the effect was evident at physiologically relevant doses (i.e., as little as 1/100th of that present in an ejaculate) (10); (v) trkA receptors are present in gonadotrophs (30) and OIF (NGF) purified from seminal plasma induced LH release from cultured pituitary cells of llamas and cows (31); and (vi) the in vivo LH- and ovulation-inducing effects of OIF purified from seminal plasma were ablated by pretreatment of females with a GnRH antagonist (i.e., the hypothalamus is a target for OIF/NGF) (32).
A mechanism of action that involves GnRH neurons in the hypothalamus (32) implies that OIF/NGF in seminal plasma crosses the blood–brain barrier of the inseminated female. The implication is consistent with results of studies in which I125-labeled NGF was detected in the hypothalamus after i.v. administration in mice (33), and in which serum NGF activated the hypothalamo–pituitary–adrenal axis in rats by acting within the CNS (34). The responsiveness of GnRH neurons to NGF is apparently unknown. The anterior pituitary is not protected by the blood–brain barrier and support for the notion of a direct effect of OIF/NGF at the level of the pituitary is found in studies in which LH release was elicited by seminal plasma treatment of in vitro cultures of anterior pituitary cells of rats (35), llamas, and cattle (31). The NGF protein and its trkA receptor were detected in 75 and 44%, respectively, of LH-containing gonadotrope cells of the rat anterior pituitary (30). This is consistent with a pituitary route of action; however, it remains unclear whether the LH response of pituitary gonadotropes is the result of increased synthesis of LH, increased release, or both. Binding of OIF/NGF to trkA receptors induces phosphorylation of phospholipase C (PLC)-γ and Shc leading to activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase, in turn leading to activation of early growth response protein-1 (Egr-1). Expression of Egr-1 (originally identified as a nerve growth factor response gene product) (36) within the pituitary gland is limited to the gonadotrope and somatotrope subpopulation (30). As a member of the gene family that recognizes the GC-rich nucleotide sequence in the promoter region of the LHβ gene, Egr-1 has a profound effect on LHβ gene expression (37), thus providing a plausible pathway for OIF/NGF regulation of LH secretion.
Nerve growth factor is highly conserved among species (Fig. 2), consistent with results of recent studies that demonstrate that OIF in seminal plasma is conserved among species (9, 11, 38). Not only was ovulation induced in llamas by conspecific and heterospecific seminal plasma (camelid, bull, stallion, boar, and rabbit), but administration of llama seminal plasma affected ovarian function in females of unrelated species (mice and cows) (11, 21). However, compared with llama seminal plasma, that of bulls, stallions, and boars induced a lower incidence of ovulation in female llamas. This may be due to differing quantities or isoforms of OIF present in each species (Fig. 5) or to slight variations in amino acid sequences affecting receptor binding (Figs. 2 and 3).
Two isoforms of NGF have been isolated from mouse submaxillary glands. One is a dimer of two identical subunits linked together by noncovalent bounds with a molecular mass of 26 kDa, known as 2.5S-NGF or β-NGF (18). The second is a higher molecular mass isoform known as 7S-NGF complex was originally reported to have a molecular mass of 140 kDa, and is composed of three different protein structures termed α, β, and γ (39). The 2.5S-NGF is a product of proteolytic cleavage of the β subunit from the larger 7S-NGF complex and is biologically active in stimulating neurite outgrowth. Early observations that higher molecular weight forms of NGF also have biological activity (18) have been confirmed more recently. Isoforms of NGF have been identified with molecular masses ranging from 16 to 60 kDa (16, 40). Using human and mouse monoclonal antibodies against β-NGF, a 60-kDa pro-NGF was identified in several different commercial β-NGF preparations, as well as in recombinant NGF and in dorsal root ganglia of rats (16). In the present study, a less distinct band appeared at just over 60 kDa in immunoblots of whole seminal plasma of llamas and bulls (representative of two general categories of mammals considered to be induced and spontaneous ovulators) and was interpreted as an unprocessed proform of NGF. Proforms of NGF are most prominent in the central and peripheral nervous system; that is, little or no β-NGF is found in these tissues (41). The relevance of a given NGF isoform and its relative stability, binding properties, and physiological effects on the reproductive system remain to be determined.
Conclusions
Ovulation-inducing factor (OIF) is nerve growth factor (NGF) in seminal plasma. It is surprising that its effects in the female were not identified earlier, given the abundance of NGF in seminal plasma. This realization helps justify the existence of the elaborate male accessory gland system as more than an evolutionary vestige among species. Nerve growth factor is highly conserved, and identification of OIF as NGF explains recent discoveries of the effects of seminal plasma on gonadotropin release and ovarian function in a variety of species. The purification yield of the mature protein, β-NGF, from llama seminal plasma is high, and purification of large quantities permitted the discovery that NGF from the ejaculate has an important role in regulating gonadotropin release and ovarian function in the female. The notion of an endocrine route of action of NGF on reproductive function is unique and elucidates a direct pathway for the influence of the male on the hypothalamo–pituitary–gonadal axis of the female. Identification of OIF as NGF in llama seminal plasma represents a unique sequence for the family Camelidae and the only seminal plasma-derived NGF to be isolated, characterized, and fully sequenced in any species. Crystallographic study of purified OIF from seminal plasma provided the opportunity to determine the structure of natural NGF (described previously only in the mouse, human, and cow) and has laid the foundation for examining structure–function relationships of proforms of NGF and receptor recognition sites. Purification of the mature and proforms of the protein in quantity from seminal plasma will enable in vivo study of the role of NGF in reproductive and neurologic health and disease.
Materials and Methods
Ejaculates were collected from llamas by artificial vagina and from bulls by electroejaculation. The seminal plasma was separated by centrifugation from the cellular portion of the ejaculate and samples were stored frozen. Seminal plasma samples were pooled within species after thawing for the purposes of determining the identity of OIF. Llama seminal plasma was loaded on a hydroxylapatite chromatography column and three protein fractions (designated A, B, and C) were eluted. In fraction C, a major 14-kDa protein detected by SDS/PAGE was subsequently loaded on a gel filtration column yielding two subfractions, C1 and C2. A purified protein band cut from SDS/PAGE of fraction C2 was used to determine molecular mass by MALDI-TOF and protein identification by LC-MS/MS. Samples of the nondenatured fraction of C2 were loaded on a calibrated Superdex-75 HiLoad 16/60 gel filtration column to confirm the molecular mass. Sequences of tryptic (LC-MS/MS) and nondenatured peptides (Edman degradation) derived from fraction C2 were compared with known protein sequences listed in the database of the European Bioinformatics Institute. To determine the protein structure, a sample of OIF was screened against 1,536 mixture solutions, and conditions were optimized to produce crystals for X-ray diffraction. Diffraction data were collected on beamline 08ID-1 and the structure was solved by molecular replacement using murine β-NGF structure as the search model. Neurite development in immortalized rat PC12 was used as an in vitro bioassay for NGF-like properties of OIF. Up-regulation of an NGF-specific receptor (i.e., mRNA for trkA) was compared in PC12 cells cultured in vitro with NGF vs. OIF. Samples of fraction C2 of llama seminal plasma, as well as whole seminal plasma from llamas and bulls were examined by Western blot analysis using a polyclonal mouse anti-NGF as the primary antibody. Lastly, the ovulatory response of female llamas was examined by ultrasonography after treatment with OIF (fraction C2 of llama seminal plasma), β-NGF (from mouse submandibular glands), or saline. (An expanded version of Materials and Methods can be found in SI Materials and Methods.)
Acknowledgments
We thank Orleigh Bogle, Manuel Palomino, and Miriam Cervantes for assistance with animal data collection and Lata Prasad for assistance with X-ray diffraction data collection. This research was performed at the University of Saskatchewan and supported by grants from the Natural Sciences and Engineering Research Council of Canada, the Alpaca Research Foundation, the Chilean National Science and Technology Research Council (Fondecyt 1120518), the Saskatchewan Health Research Foundation, and the Canadian Institutes of Health Research. X-ray crystallography was done at the Canadian Light Source, Saskatoon, Saskatchewan.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 4EFV).
1Deceased October 5, 2009.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206273109/-/DCSupplemental.
References
- 1.Mann T. Biochemistry of Semen and of the Male Reproductive Tract. Frome, UK: Butler, Tanner Ltd; 1964. p. 493. [Google Scholar]
- 2.Adams GP, Ratto MH, Huanca W, Singh J. Ovulation-inducing factor in the seminal plasma of alpacas and llamas. Biol Reprod. 2005;73:452–457. doi: 10.1095/biolreprod.105.040097. [DOI] [PubMed] [Google Scholar]
- 3.Ratto MH, Huanca W, Adams GP. Ovulation-inducing factor: A protein component of llama seminal plasma. Reprod Biol Endocrinol. 2010;8:44. doi: 10.1186/1477-7827-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratto MH, Delbaere LTJ, Leduc YA, Pierson RA, Adams GP. Biochemical isolation and purification of ovulation-inducing factor (OIF) in seminal plasma of llamas. Reprod Biol Endocrinol. 2011;9:24. doi: 10.1186/1477-7827-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedford JM. Enigmas of mammalian gamete form and function. Biol Rev Camb Philos Soc. 2004;79:429–460. doi: 10.1017/s146479310300633x. [DOI] [PubMed] [Google Scholar]
- 6.Chen BX, Yuen ZX, Pan GW. Semen-induced ovulation in the bactrian camel (Camelus bactrianus) J Reprod Fertil. 1985;74:335–339. doi: 10.1530/jrf.0.0740335. [DOI] [PubMed] [Google Scholar]
- 7.Xu YS, Wang HY, Zeng GQ, Jiang GT, Gao YH. Hormone concentrations before and after semen-induced ovulation in the bactrian camel (Camelus bactrianus) J Reprod Fertil. 1985;74:341–346. doi: 10.1530/jrf.0.0740341. [DOI] [PubMed] [Google Scholar]
- 8.Ratto MH, Huanca W, Singh J, Adams GP. Local versus systemic effect of ovulation-inducing factor in the seminal plasma of alpacas. Reprod Biol Endocrinol. 2005;3:29. doi: 10.1186/1477-7827-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratto MH, Huanca W, Singh J, Adams GP. Comparison of the effect of ovulation-inducing factor (OIF) in the seminal plasma of llamas, alpacas, and bulls. Theriogenology. 2006;66:1102–1106. doi: 10.1016/j.theriogenology.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 10.Tanco VM, Ratto MH, Lazzarotto M, Adams GP. Dose-response of female llamas to ovulation-inducing factor from seminal plasma. Biol Reprod. 2011;85:452–456. doi: 10.1095/biolreprod.111.091876. [DOI] [PubMed] [Google Scholar]
- 11.Bogle OA, Ratto MH, Adams GP. Evidence for the conservation of biological activity of ovulation-inducing factor in seminal plasma. Reproduction. 2011;142:277–283. doi: 10.1530/REP-11-0042. [DOI] [PubMed] [Google Scholar]
- 12.Tanco VM, Van Steelandt MD, Ratto MH, Adams GP. Effect of purified llama ovulation-inducing factor (OIF) on ovarian function in cattle. Theriogenology. 2012 doi: 10.1016/j.theriogenology.2012.03.036. 10.1016/ j.theriogenology.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 13.McDonald NQ, et al. New protein fold revealed by a 2.3-A resolution crystal structure of nerve growth factor. Nature. 1991;354:411–414. doi: 10.1038/354411a0. [DOI] [PubMed] [Google Scholar]
- 14.Bax B, Blundell TL, Murray-Rust J, McDonald NQ. Structure of mouse 7S NGF: A complex of nerve growth factor with four binding proteins. Structure. 1997;5:1275–1285. doi: 10.1016/s0969-2126(97)00280-3. [DOI] [PubMed] [Google Scholar]
- 15.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinshagen M, et al. Commercial recombinant human beta-nerve growth factor and adult rat dorsal root ganglia contain an identical molecular species of nerve growth factor prohormone. J Neurochem. 2000;74:2127–2133. doi: 10.1046/j.1471-4159.2000.0742127.x. [DOI] [PubMed] [Google Scholar]
- 17.Kolbeck R, Jungbluth S, Barde YA. Characterisation of neurotrophin dimers and monomers. Eur J Biochem. 1994;225:995–1003. doi: 10.1111/j.1432-1033.1994.0995b.x. [DOI] [PubMed] [Google Scholar]
- 18.Angeletti RH, Bradshaw RA. Nerve growth factor from mouse submaxillary gland: Amino acid sequence. Proc Natl Acad Sci USA. 1971;68:2417–2420. doi: 10.1073/pnas.68.10.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper GP, Glanville RW, Thoenen H. The purification of nerve growth factor from bovine seminal plasma. Biochemical characterization and partial amino acid sequence. J Biol Chem. 1982;257:8541–8548. [PubMed] [Google Scholar]
- 20.Hofmann HD, Unsicker K. The seminal vesicle of the bull: A new and very rich source of nerve growth factor. Eur J Biochem. 1982;128:421–426. [PubMed] [Google Scholar]
- 21.Ayer-LeLievre C, Olson L, Ebendal T, Hallböök F, Persson H. Nerve growth factor mRNA and protein in the testis and epididymis of mouse and rat. Proc Natl Acad Sci USA. 1988;85:2628–2632. doi: 10.1073/pnas.85.8.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, et al. Detection of nerve growth factor (NGF) and its specific receptor (TrkA) in ejaculated bovine sperm, and the effects of NGF on sperm function. Theriogenology. 2010;74:1615–1622. doi: 10.1016/j.theriogenology.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 23.Dissen GA, et al. A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinology. 1996;137:198–209. doi: 10.1210/endo.137.1.8536613. [DOI] [PubMed] [Google Scholar]
- 24.Barboni B, et al. Preovulatory rise of NGF in ovine follicular fluid: Possible involvement in the control of oocyte maturation. Microsc Res Tech. 2002;59:516–521. doi: 10.1002/jemt.10230. [DOI] [PubMed] [Google Scholar]
- 25.Dissen GA, et al. Direct effects of nerve growth factor on thecal cells from antral ovarian follicles. Endocrinology. 2000;141:4736–4750. doi: 10.1210/endo.141.12.7850. [DOI] [PubMed] [Google Scholar]
- 26.Abir R, et al. Presence of NGF and its receptors in ovaries from human fetuses and adults. Mol Hum Reprod. 2005;11:229–236. doi: 10.1093/molehr/gah164. [DOI] [PubMed] [Google Scholar]
- 27.Salas C, et al. Nerve growth factor-dependent activation of trkA receptors in the human ovary results in synthesis of follicle-stimulating hormone receptors and estrogen secretion. J Clin Endocrinol Metab. 2006;91:2396–2403. doi: 10.1210/jc.2005-1925. [DOI] [PubMed] [Google Scholar]
- 28.Julio-Pieper M, Lara HE, Bravo JA, Romero C. Effects of nerve growth factor (NGF) on blood vessels area and expression of the angiogenic factors VEGF and TGFbeta1 in the rat ovary. Reprod Biol Endocrinol. 2006;4:57. doi: 10.1186/1477-7827-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayerhofer A, et al. Involvement of nerve growth factor in the ovulatory cascade: trkA receptor activation inhibits gap junctional communication between thecal cells. Endocrinology. 1996;137:5662–5670. doi: 10.1210/endo.137.12.8940397. [DOI] [PubMed] [Google Scholar]
- 30.Patterson JC, Childs GV. Nerve growth factor and its receptor in the anterior pituitary. Endocrinology. 1994;135:1689–1696. doi: 10.1210/endo.135.4.7925133. [DOI] [PubMed] [Google Scholar]
- 31.Bogle OA, Ratto MH, Adams GP. Ovulation-inducing factor (OIF) induces LH secretion from pituitary cells. Anim Reprod Sci. 2012 doi: 10.1016/j.anireprosci.2012.06.006. 10.1016/j.anireprosci.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Silva ME, et al. Cetrorelix suppresses the preovulatory LH surge and ovulation induced by ovulation-inducing factor (OIF) present in llama seminal plasma. Reprod Biol Endocrinol. 2011;9:74. doi: 10.1186/1477-7827-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loy R, Taglialatela G, Angelucci L, Heyer D, Perez-Polo R. Regional CNS uptake of blood-borne nerve growth factor. J Neurosci Res. 1994;39:339–346. doi: 10.1002/jnr.490390311. [DOI] [PubMed] [Google Scholar]
- 34.Taglialatela G, Perez-Polo JR. Developmental profile of the hypothalamo-pituitary-adrenal axis response to nerve growth factor. Neurosci Lett. 1994;182:231–234. doi: 10.1016/0304-3940(94)90804-4. [DOI] [PubMed] [Google Scholar]
- 35.Paolicchi F, Urquieta B, Del Valle L, Bustos-Obregón E. Biological activity of the seminal plasma of alpacas: Stimulus for the production of LH by pituitary cells. Anim Reprod Sci. 1999;54:203–210. doi: 10.1016/s0378-4320(98)00150-x. [DOI] [PubMed] [Google Scholar]
- 36.Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987;238:797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- 37.Brown P, McNeilly AS. Transcriptional regulation of pituitary gonadotrophin subunit genes. Rev Reprod. 1999;4:117–124. doi: 10.1530/ror.0.0040117. [DOI] [PubMed] [Google Scholar]
- 38.Silva ME, et al. Is an ovulation-inducing factor (OIF) present in the seminal plasma of rabbits? Anim Reprod Sci. 2011;127:213–221. doi: 10.1016/j.anireprosci.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Varon S, Normura J, Shooter EM. Reversible dissociation of the mouse nerve growth factor protein into different subunits. Biochemistry. 1968;7:1296–1303. doi: 10.1021/bi00844a008. [DOI] [PubMed] [Google Scholar]
- 40.Paoletti F, et al. 2011 Conformational plasticity of proNGF. PLoS ONE 6(7): e22615. Doi:10.1371/journal.pone.0022615. [Google Scholar]
- 41.Pedraza CE, et al. Pro-NGF isolated from the human brain affected by Alzheimer’s disease induces neuronal apoptosis mediated by p75NTR. Am J Pathol. 2005;166:533–543. doi: 10.1016/S0002-9440(10)62275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]