Abstract
Infant iron deficiency anemia (IDA) occurs spontaneously in monkey populations as it does in humans, providing a model for understanding effects on brain and behavior. A set of 34 monkey infants identified as IDA (hemoglobin <11 g/dL) over a 5-year period at the California National Primate Research Center (CNPRC) was compared to a set of 57 controls (hemoglobin >12 g/dL) matched for age and caging location. The infants had participated in a Biobehavioral Assessment conducted at 3–4 months of age at CNPRC that included measures of behavioral and adrenocortical response to a novel environment. IDA males differed from control males in two factors (“activity”, “emotionality”) derived from observational data taken on the first and second day of the exposure to the novel environment. In the male infants, IDA was associated with less restriction of activity in the novel environment on both days and less emotionality on the second day (p<.05). IDA males also displayed less response to approach by a human (human intruder test) than did control males. IDA females did not differ from controls. Adrenocortical response was not significantly affected. These findings may be relevant to functional deficits in human infants with IDA that influence later behavior.
Keywords: iron deficiency anemia, human intruder, plasma cortisol, rhesus monkey, infant, behavioral inhibition, temperament
Introduction
Studies in humans have demonstrated that iron deficiency anemia (IDA) during the first 2 years of life is associated with impaired cognitive and motor development that can persist despite the correction of the anemia (Lozoff et al., 2007). In addition to cognitive and motor impacts, research in humans indicates that affect, or emotionality, is influenced by IDA in infants and toddlers (Lozoff et al., 1985; Lozoff et al., 1986; Rahmanifar et al., 1993; Lozoff et al., 1996; Lozoff et al., 1998; Williams et al., 1999; Lozoff et al., 2003; Wachs et al., 2005; Lozoff et al., 2007; Wachs et al., 2008). These studies used observational rating scales or measured behaviors reflecting emotion during cognitive testing. In children, IDA is often associated with a variety of dietary, environmental and psychosocial deprivations that complicate simple causal inferences (Schneider et al., 2008). For instance, the mother of an anemic infant may herself have poor nutritional status that influences interaction with her infant. Although design and statistical tools are used to control for confounders, isolation of an effect of infant IDA on affect or emotion is difficult to achieve in human studies (McCann and Ames, 2007). Thus we developed a model to study the relationship between anemia and affect in infants by using controlled dietary iron deprivation in rhesus monkeys. Monkey infants have long been a model for study of emotional behavior in infants (Kalin, 2004; Maestripieri and Wallen, 2008). Dietary iron deprivation during gestation or lactation was found to have long lasting consequences for affective aspects of behavior of monkey infants (Golub et al., 2006; Golub et al., 2007). In that study, the infants were raised in the primate nursery rather than by their mothers so that postnatal iron exposure could be controlled. This raised the question of whether these results from prenatal iron deprivation would generalize to mother-reared monkeys recognized as anemic in infancy. To help answer this question we turned to a database available on infant behavior in a large colony of rhesus monkeys. Information on IDA was obtained from complete blood counts (CBCs) conducted at the same time as behavioral evaluation. Although causal relationships cannot be determined in this type of study, it more closely parallels the research designs used to study developmental effects of IDA in humans.
The database used in this study was produced in conjunction with management of the rhesus monkey colony at the California National Primate Research Center (CNPRC). The Biobehavioral Assessment (BBA) database contains the results of an evaluation of temperament in 1482 3-month old rhesus infants (Capitanio et al., 2006) collected over a 5-year period. Infants experimentally deprived of dietary iron during prenatal or postnatal development in our previous studies (Golub et al., 2006; Golub et al., 2007) were evaluated with the BBA. Infant monkeys considered for the present study were not subjected to controlled dietary iron deprivation. Also, unlike infants raised in the nursery in our previous study, they lived outdoors in large social groups and were separated from their mothers and placed in an indoor environment only for the BBA. A complete blood count (CBC) taken at the time of the BBA was used to select a group of infants with IDA from this background. They were compared to control infants with good iron status matched for date of birth, gender and birth location. Records from the BBA were then compared for the two groups.
Methods
Biobehavioral assessment overview
The California National Primate Research Center (CNPRC) maintains a colony of approximately 5000 rhesus macaques (Macaca mulatta) housed in outdoor half acre corrals, smaller outdoor “corn cribs,” indoor caging, and indoor nurseries. Each year, the majority of available 3-to-4-month-old infants in the colony participate in a 25-h Biobehavioral Assessment (BBA) program designed to characterize behavioral and physiological responsiveness in young animals. More than 2000 infants have been tested since 2001. An electronic database has been prepared with data from the BBA.
The procedures used to generate the BBA data used in this report have been described previously (Capitanio et al., 2005; Capitanio et al., 2006; Golub et al., 2006). Infants between 90 and 120 days of age were separated from their dams and relocated from their home cages to individual indoor cages (Holding Cage, 60 × 65 × 79 cm, Lab Products, Inc., Maywood, NJ) in the morning on the day of testing. Five to 8 infants, comprising a single cohort, were tested at the same time. The Holding Cage contained a cloth diaper, a stuffed terry cloth duck and a novel manipulable object (approximately 4 × 9 cm) containing activity sensors. Infants were provided with water, a fruit-flavored drink, commercial monkey diet and fresh fruit.
The commercial diet fed to the monkeys in outdoor corrals and during BBA participation (Lab Diet #5038, Purina Mills International, St. Louis MO) contained 230 ppm iron as iron carbonate. Infants were tested in the same predetermined random order for each test throughout the 25-h period (Table 1). For most assessments, they were transferred to a test cage in an adjacent room and videotaped. Tapes were later scored using The Observer software (Noldus Information Technology) for frequency and duration of behaviors. Plasma cortisol was measured in 4 blood samples taken during the 25-h period (see below). At the conclusion of testing on the second day, infants were returned to their mothers and released back to their home enclosures. Behavioral data obtained by observers at the time of the BBA and video-recorded human intruder data were available for this study. Data from the other videorecorded tests (preferential look, video playback of response to social stimuli) have not yet been scored for all animals. Intra- and inter-observer reliabilities are checked annually, and exceed 85% agreement.
Table 1.
Outline of the Biobehavioral Assessment
| Assessment | Location | Duration | Time |
|---|---|---|---|
| Day 1 | |||
| Relocation from home cage to test room; first novel object (Actiwatch) in cage | 0900 hr | ||
| Holding cage observation | Holding cage | 5 min focal observation | 0915 hr |
| Blood sample 1: “Response to relocation,” CBC |
Test room | 1100 hr | |
| Preferential look: Visual recognition memory, data not reported |
Test cage | 6 min session | ~1130 hr |
| Video playback: Response to social stimulus, data not reported |
Test cage | 10 min session | ~1230 hr |
| Human Intruder: Response to human frontal stare or profile |
Test cage | 4 min session | ~1400 hr |
| Blood sample 2: “Adaption to relocation”, followed by dexamethasone injection (500 ug/kg, i.m) |
Test room | 1600 hr | |
| First novel object (Actiwatch) replaced with second novel object | Holding cage | 1630 hr | |
| Day 2 | |||
| Holding cage observation | Holding cage | 5 min focal observation | 0700 hr |
| Blood sample 3: “Response to dexamethasone”, followed by ACTH injection (2.5 IU, i.m.) |
Test room | 0830 hr | |
| Blood sample 4: “Response to ACTH” |
Test room | 0900 hr | |
| Temperament rating: Overall behavioral characteristics during test period |
Test room | ~930 hr | |
| Return to mothers and home cage | ~1000 hr |
Assurance of compliance with animal codes
All procedures were conducted according to the Guidelines for Use and Care of Laboratory Animals of the National Research Council and according to CNPRC SOPs. The CNPRC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Experimental protocols were approved prior to implementation by the University of California, Davis IACUC.
Subject selection
Subjects were selected from animals tested between 2001 and 2005 based on the CBC data from blood samples obtained on the first day of the BBA. Only animals from the outdoor half-acre corrals were considered, resulting in 695 possible subjects. Each animal was then classified as either iron deficient anemic (IDA, hemoglobin <11 g/dL and mean corpuscular volume (MCV) ≤65 fL)) or control (hemoglobin ≥12 g/dL and MCV ≥68 fL. Iron deficient non-anemic animals were classified with hemoglobin ≥12 g/dL and MCV ≤ 65 fL but were not used in this analysis. These parameters are commonly used to identify iron deficiency anemia in both humans and nonhuman primates (Bicknese et al., 1993; Looker et al., 1997; Domellof et al., 2002; Cook et al., 2003). In the absence of information about genetic disorders, anemia characterized with low hemoglobin and MCV is presumed to be due to iron deficiency. For each of the 34 IDA animals identified, 2 matched controls were chosen based on sex, date of birth (DOB) and specific corral location at the time of testing. A control with the closest DOB to the IDA animal was used. If no matched control was available from the same corral within 30 days of the IDA animal’s DOB, then a control of the same sex with the closest DOB from any corral was used. 11 of the 68 matched controls were subsequently excluded to balance the sample. Infants born by C-section or in the indoor colony were excluded, as all IDA infants were born vaginally and outdoors. Any animals tested prior to 107 days of age were removed to balance the male/female ratio for IDA and controls. Dam’s weight, cage rank and reproductive history were obtained from the CNPRC colony database.
Blood sampling
Blood samples (0.5 or 1.0 mL) were obtained by femoral venipuncture at 4 timepoints during the 25-h period. The first sample was also used for the CBC analysis on which the hematology selection criteria were based. Immediately following sample #2, animals received an injection of dexamethasone (American Regent Laboratories, Inc., Shirley, NY) (500 ug/kg, i.m.), and following blood sample #3 animals were injected with adrenocorticotropic hormone (ACTH) (Amphastar Pharmaceuticals, Inc. Rancho Cucamongo, CA) (2.5 IU, i.m.). Samples for cortisol analysis were transferred to EDTA tubes and centrifuged to separate plasma which was frozen (−80° C) until assayed for cortisol concentration by RIA (Diagnostic Products Corp., Los Angeles, CA) through the CNPRC Endocrine Core. See Table 1 for blood sampling schedule.
Holding cage observation
Observations to assess the effects of separation and relocation were conducted by a live observer for 5 min per animal at the beginning (Day 1) and end (Day 2) of the 25-h period using the predetermined random order. An experienced observer sat 2.6 m away and recorded behaviors using The Observer software. Behaviors included activity states (such as lie, crouch, locomote) and events including vocalizations, self-directed behaviors and facial gestures (Table 2). Owing to slight variations in the length of the observation periods, duration measures were converted to a proportion of the total observation time, and frequencies of states and events were converted to a rate per 60 sec.
Table 2.
Behaviors and definitions for Holding Cage behavior scoring.
| Behavior | Definition |
|---|---|
| States (frequency and duration) | |
| Sit | Hindquarters are on the floor; includes shifting weight slightly one step |
| Lie | Relaxed posture with body resting on a horizontal surface |
| Stand | Torso in a stationary position and weight is supported by 3 or 4 legs; can include steps taken that only involve one or two feet |
| Locomote | Directed movement from one location to another |
| Crouch | Ventral surface close to floor: head at or below the level of the shoulders |
| Sleep | Eyes closed |
| Pace | Repetitive rapid movement over the same path |
| Rock/sway | Unbroken rhythmic movements of the upper body while the animal is sitting or entire body if the animal is lying down |
| Hang | Holding onto ceiling, sides or front of the cage; all 4 limbs off of floor. |
| Events (frequency only) | |
| Scratch | Common usage |
| Self-groom | Using hands or lips to pick through or part own fur |
| Self-manipulate | Masturbation, pulling, tugging or pushing at self |
| Self-clasp | Hand or feet closed on fur or some body part |
| Self-bite | Discrete biting action, usually directed to limbs and often accompanied by a threat face |
| Stroke | Very gently bringing the hand or foot across the side of head or face |
| Suck | Insertion into mouth of fingers, toes and other body parts |
| Back flip | Tossing body up and backwards in a circular motion in the air |
| Convulsive jerk | Sudden and somewhat violent contractions of the limbs and trunk |
| Cage shake/bounce | Holding onto cage and shaking it, generating a lot of noise |
| Coo | Medium-pitched, moderately intense, clear call |
| Screech | Intense, very high pitched |
| Gecker | Staccato cackling sounds |
| Bark | Gruff, abrupt low-pitched vocalization |
| Lipsmack | Rapid lip movement usually with pursed lips, accompanied by a smacking sound |
| Threat | Scored with at least two or more of the following: open mouth stare, head bob, ear flaps, bark vocalizations |
| Fear grimace | Exaggerated grin with teeth showing |
| Yawn | Wide open mouth displaying teeth |
| Tooth grind | Loud gnashing of teeth |
| Motor stereotypy | Scored with any of the following: Repeated movement, of a head flip, sway (side to side motion while standing or hanging), or up and down motion of the body. |
| Environmental explore | Discrete manipulation by hand or mouth with the physical environment or objects in the cage |
| Eat | Common usage |
| Drink | Common usage |
Exploratory and confirmatory factor analyses of the holding cage behavior data were conducted independently by JPC using data from over 1482 infants collected over a 5-year period using MPlus statistical software (Muthén and Muthén, 2001). Because some variables that we considered important were extremely skewed (e.g., for eat, 83.6% of the sample had scores of zero), we dichotomized these variables under the assumption that whether the behavior was displayed (1=yes, 0=no) was more important than the exact number of times. The initial exploratory factor analysis, performed on the 2001 data set, used weighted least squares with robust standard errors (WLSMV) procedure for estimation and extraction of factors (WSLMV is the recommended procedure for a mix of continuous and dichotomous variables) followed by promax rotation. A two-factor solution provided a satisfactory fit to the data (e.g., root mean square error of approximation = 0.044 [RMSEA <=.05 is considered a “close fit” of the model to data]). Based on this solution, separate confirmatory factor analyses were performed on data from subsequent years. Minor changes were made to the models, but fit was excellent based on traditional fit statistics (both the comparative fit index [CFI] and Tucker Lewis index [TLI] ranged from 0.947 to 0.966; RMSEA ranged from 0.050 to 0.061; WRMR ranged from 0.790 to 0.893 [excellent fit is indicated by CFI and TLI >= 0.95, and by WRMR < 0.90: Muthén and Muthén, 2001]). Finally, we constructed scales based on the factor analyses by summing z-scores for the items that loaded on a given factor. Internal consistency reliabilities were computed and the scales were adjusted to maximize reliability. Cronbach’s alphas ranged from 0.6 to 0.8 (note that having dichotomized variables in scales attenuates internal consistency reliabilities). The final scales were then z-scored and labeled “activity” and “emotionality.” Data reported here for the Holding Cage observations reflect the animals’ z-scores for the Day 1 Activity and Emotionality composites and for the Day 2 Activity and Emotionality composites.
Novel Objects
Response to a novel object was assessed by placing a small plastic object containing an Actiwatch sensor in the living cage. The first object (a black hollow cylinder with ridges on the surface, approximately 4 × 9 cm) was present in the cage when infants were first relocated. This object was switched out following the second blood draw in the afternoon of the first day and replaced with a white plastic cylinder of the same dimensions. The sensor in the objects measured the amount of force exerted on the object.
The data were computed as the number of 15 sec periods in 5-min blocks of time (i.e., a maximum score of 20 for each 5-min block) in which there was any force exerted on the object. The 5 min blocks of time were grouped into 10 intervals which covered the 25-h period. Each animal could have a variable number of 5-min blocks within each interval due to being transferred to the test cage for the behavioral assessments (inadvertent movement of the objects during capture of the infants from the Holding Cage for behavioral testing was removed from the novel object data). Data were summarized for each interval using the following endpoints: the mean number of 15-sec periods of contact per 5-min block; the number of 5-min blocks of contact (i.e., did the animal contact the object at all during the 5-min block?); the number of 5-min blocks in which the animal ignored the object; and the proportion of 5-min blocks in which the object was ignored (calculated by dividing the number of ignored 5-min blocks by the number of 5-min blocks of valid data).
Human Intruder
The goal of the Human Intruder test was to assess the responsiveness of the infant to standardized and graded conditions of challenge. A technician dressed in protective clothing first sat approximately 1 m away and presented a profile for 1 min. She then moved to within 0.3 m and continued to present a profile for 1 min. Next the technician moved back to the 1 m position and attempted to maintain eye contact for 1 min. For the last trial she again moved to within 0.3 m of the cage and attempted to maintain eye contact for 1 min. These 4 conditions were designated profile far, profile near, stare far and stare near. The 4-min test session was videorecorded and later scored using The Observer. The ethogram for this test included all behaviors listed in Table 2, with the following exceptions that reflect the differences in the testing situation: eat and drink were not scored as no food or water was available; “active,” defined as “whole body movement; step; jump,” was scored instead of locomotion; and motor stereotypy was scored as a state rather than an event. The animal’s location in the cage (right or left, which corresponded to near or far from the intruder) was also scored. The durations were converted to the proportion of time observed, and the frequencies were converted to a rate per 60 sec. Over the years, 3 different women served as the “intruders”, but no evidence was found of differential responsiveness.
Temperament Ratings
Just prior to the infant’s reunion with its mother, the technician who performed the testing rated the overall temperament of each animal during the 25-h test period. A list of 16 adjectives describing affect quality (Table 3) were rated on a Likert-like scale of 1 to 7, with 1 reflecting a total absence of the behavior and 7 reflecting an extremely large amount of the behavior. As with the Holding Cage observations (above), ratings on each adjective were z-scored across all subjects tested within a given birth year, and exploratory and confirmatory factor analyses of the temperament ratings for 1284 infants were conducted by JPC. All procedures were identical (e.g., exploratory factor analysis on one sample, examination of fit criteria, confirmatory factor analysis with other samples, examination of scale reliability), with the exception that maximum likelihood was the method of estimation for these continuously distributed variables. A model with four factors fit the data very well in the exploratory (RMSEA = 0.055) and in the confirmatory models (CFI and TLI ranged from 0.914 to 0.952; RMSEA ranged from 0.078 to 0.084; the standardized root mean square residual (SRMR) ranged from 0.048 to 0.059 [for SRMR, values < .08 indicate a good fit: Muthén and Muthén, 2001]). Factor scores were calculated by summing the z-scores for all adjective items loading on a given factor, and then z-scoring each scale. Cronbach’s alpha values for the scales across the five years ranged from 0.6 to 0.9. The four scales, named for the adjective with the highest factor loading, were: Vigilant (vigilant, NOT depressed, NOT tense, NOT timid), Gentle (gentle, calm, flexible, curious), Confident (confident, bold, active, curious, playful), Nervous (nervous, fearful, timid, NOT calm, NOT confident).
Table 3.
Temperament rating adjectives and definitions
| Adjective | Definiton |
|---|---|
| Active, energetic | Moves about a lot, distance traveled by walking, running, climbing or jumping. Not lethargic. |
| Aggressive | High frequency of displays; threats |
| Bold | Is daring, not restrained or tentative. Not timid, shy or coy |
| Calm, equable | Reacts in an even, calm way; is not easily disturbed. Not agitated. Restful, peaceful |
| Confident | Behaves in a positive, assured manner, not restrained or tentative |
| Curious, exploratory, inquisitive | Readily explores new situations, seeking out or investigating novel situations |
| Depressed | Subject appears isolated, withdrawn, sullen, brooding, and has reduced activity |
| Fearful | Fear grins, retreats readily from others or from outside disturbances |
| Flexible | Adapts to situations. Is able to accommodate new ways of doing things |
| Gentle | Subject responds to technicians in an easy-going, kind, and considerate manner. Subject is not rough or threatening. |
| Nervous, anxious, not calm | Jittery, anxious, seems to be anxious about everything |
| Playful | Engages in self-play (hanging, swinging, jumping), or object play |
| Slow | Moves and sits in a relaxed manner; moves slowly and deliberately, not easily hurried |
| Tense | Shows restraint in posture and movement; carries the body stiffly, which suggests a shrinking tendency, as if trying to pull back and be less conspicuous |
| Timid | Subject is easily alarmed and is hesitant to venture into new situations |
| Vigilant, alert | Ready, attentive, watchful, notices with special attention. Not oblivious to surroundings |
Clinical response
In addition to the temperament ratings, food and water consumption and incidence of diarrhea during the test period were rated using the same 7-point scale as for temperament. Body weights were taken on Day 1 of testing, and any other health concerns were reported to veterinarians for assessment and treatment.
Statistics
Most endpoints, including behavior durations, were analyzed by analysis of variance (ANOVA) or repeated measures analysis of variance (RMANOVA). Commercial statistical packages used for analysis were JMP and SAS (SAS, Cary, NC). Distribution of individual behavior frequencies were often skewed to the right with a high number of 0 frequencies. They were either transformed to a bimodal scale and analyzed by chi-square or used to construct composite indices by adding like items (e.g., facial expressions). Endpoints from a given behavioral test were examined for effects of sex and sex by group interactions using a two factor (group, sex) ANOVA or RMANOVA with an interaction term. One-factor ANOVAs were done separately for the two sexes if interactions were identified in any of a set of related endpoints. Post hoc tests on repeated measures in RMANOVA were conducted using the Profile algorithm in SAS or JMP, which contrasts adjacent measures in an ordered series of repeated measures. Temperament ratings were analyzed by chi-square. Statistical significance was recognized as alpha <.05.
Results
Characteristics of anemic infants and their mothers
Mothers of the anemic infants tended to be older and more parous than those of controls, but differed significantly only in the percent of the group that had previously raised a live infant, which was larger for the IDA than for the control dams (Table 4). IDA infants showed a clear pattern of iron deficiency anemia and did not overlap with controls in hemoglobin measures (Table 5). Sex did not influence most hematological parameters but an interaction between sex and group was seen for MCV (F(1,98)=4.94, p=.03) with IDA males having significantly lower MCV than IDA female infants (p<.05, Tukey’s LSD). As intended by the matching procedure, the 2 groups did not differ in gender, age, or weight.
Table 4.
Comparison of dams’ backgrounds
| IDA n=34 | Control n=57 | |
|---|---|---|
| Age at delivery (years) | 8.29 ± 0.67a | 7.22 ± 0.52 |
| Cage rank (% low/med/high) | 35/38/29 | 36/32/32 |
| Conceptions (#) | 3.88 ± 0.59 | 3.04 ± 0.46 |
| Prior live births (#) | 3.56 ± 0.55 | 2.72 ± 0.42 |
| Infants reared for at least 7 days (#) | 2.47 ± 0.39 | 1.93 ± 0.30 |
| Dams with at least one neonatal death (%) | 9.7 | 5.6 |
| Dams rearing an infant for the first time (%) | 21.4* | 58.3 |
Mean ± SEM
p<.05
Table 5.
Comparison of infant hematology.
| IDA | Control | |
|---|---|---|
| N | 34 | 57 |
| % Male | 41.2 | 43.9 |
| Age at testing (days) | 117.9 ± 1.2a | 115.9 ± 0.9 |
| Weight at testing (kg) | 1.1 ± 0.03 | 1.05 ± 0.02 |
| RBC (× 106/μl) | 5.44 ± 0.1 ** | 5.7 ± 0.04 |
| Hemoglobin (g/dL) | 9.8 ± 0.2*** | 12.9 ± 0.1 |
| Hematocrit (%) | 32 ± 0.5*** | 40 ± 0.3 |
| MCV (fL) | 59 ± 1*** | 71 ± 0.3 |
| MCH (pg) | 18.2 ± 0.3*** | 22.9 ± 0.1 |
| MCHC (pg/fL) | 31.0 ± 0.1*** | 32.3 ± 0.1 |
| Platelets (× 105/μl) | 6.50 ± 0.30*** | 4.84 ± 0.23 |
| CD4+/CD8+ | 2.5 ± 0.1* | 2.9 ± 0.1 |
Mean ± SEM
p<.05, compared to controls
p<.01, compared to controls
p<.0001, compared to controls
RBC= red blood cell; MCV=mean corpuscular volume; MCH= mean corpuscular hemoglobin; MCHC= mean corpuscular hemoglobin concentration.
Cortisol response
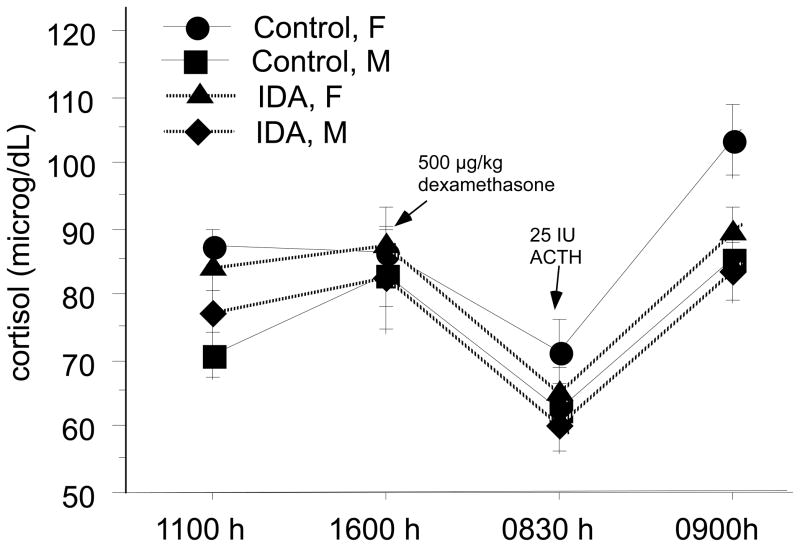
There was no effect of IDA on plasma cortisol (Figure 1) using 2-factor (group, sex) RMANOVA (F(1,92)=.26, n.s.), nor was there a significant interaction between group and sex. Over all time points, the 2 sexes differed in plasma cortisol (F(1,92)=5.22, p=.025), with females having higher cortisol values than males. The analysis also demonstrated a significant effect of timepoint (F(3,90)=99.40, p<.0001). Contrasts between successive timepoints demonstrated a nonsignificant increase between the first and second timepoints (F(1,92)=3.78, p= .0548), and a strongly significant decrease between the second and third timepoints (after dexamethasone injection, F(1,92)=41.72, p<.0001) and a strongly significant increase between the third and fourth timepoints (after ACTH injection, F(1,92)=282.73, p<.0001). There were no significant interactions of timepoint with group (F(1,90)=1.63, p=.188) or sex (F(1,90)=2.32, p=.081), and the three way interaction was not significant (F(1,90)=2.38, p=.075).
Figure 1.
Plasma cortisol response of control and IDA infants. The sample at 1100 h of Day 1 shows the initial response to separation from the home cage and placement in a novel environment, while the sample at 1600 h day 1 likely reflects adaptation to the novel environment. The 0830 h sample on Day 2 represents the response to dexamethasone injection given after the Day 1 1600 h sample, and the 0900 h sample shows the response to ACTH injection given after the 0830h sample. There were no statistically significant differences between groups in cortisol response at any timepoint.
Holding Cage observations (Day 1, Day 2)
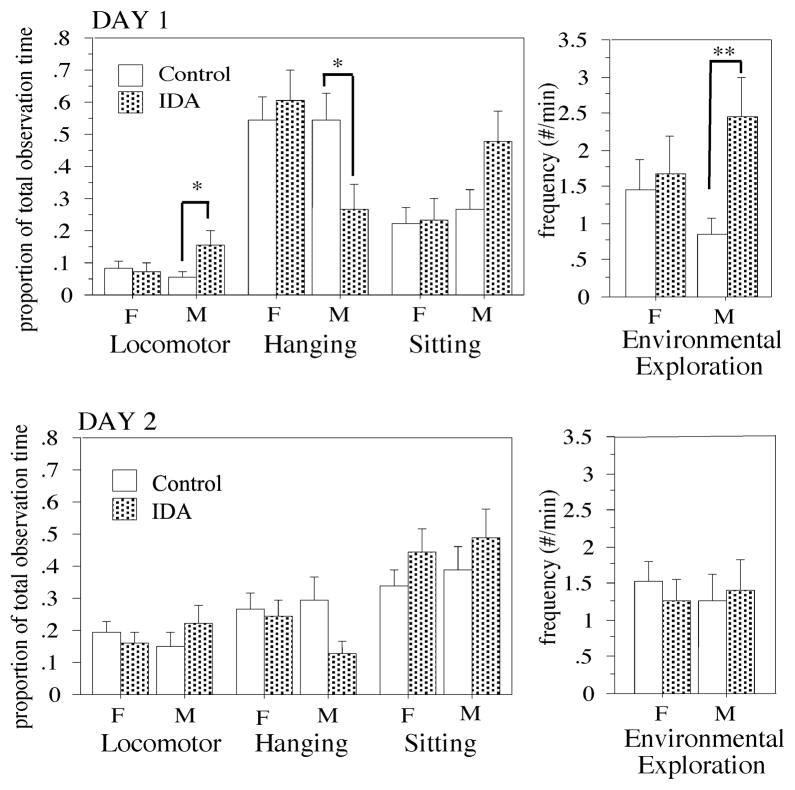
Observations taken in the holding cage during a 5-min period near the beginning and end of the BBA were summarized as the duration of 3 activity states and the frequency of environmental exploration (Figure 2). Examination of sex effects and group by sex interactions indicated that it was appropriate to examine the sexes separately for group effects on Day 1 (sex by group interactions, Day 1 F(1,87)=2.53–5.31, p=.04–.11; Day 2 F(1,86)=.01–1.72, p=.19–.94). IDA males differed from control males in having a greater duration of motor activity (F(1.87)=5.86, p=.020), a lower duration of hanging from top and sides of cage (F(1.87)=4.67, p=.037), a longer duration of sitting (F(1.87)=4.00, p=.053) and a higher incidence of exploratory activity (F(1.87)=10.92, p=.002) during the Day 1 observations, which were conducted within the first hour after the infants were separated from mother and transferred to the novel environment. Female IDA and control infants did not differ on these measures on Day 1. No effects of IDA were identified for these measures taken during the Day 2 observations, near the end of the 25-h session. Also, no sex or group by sex interactions were seen on Day 2.
Figure 2.
Behavior in the Holding Cage situation. The duration for 3 states and the frequency for one behavior (environmental explore) during a 5-min period on Day 1 (top graph) and Day 2 (bottom graph) are compared between groups within sex. F=female, M=male. *p<.05, **p<.01 compared to like-sexed control. For definitions of behaviors see Table 2. “Hanging” is considered an escape response since the infants are located off the ground, a location they often go to when frightened.
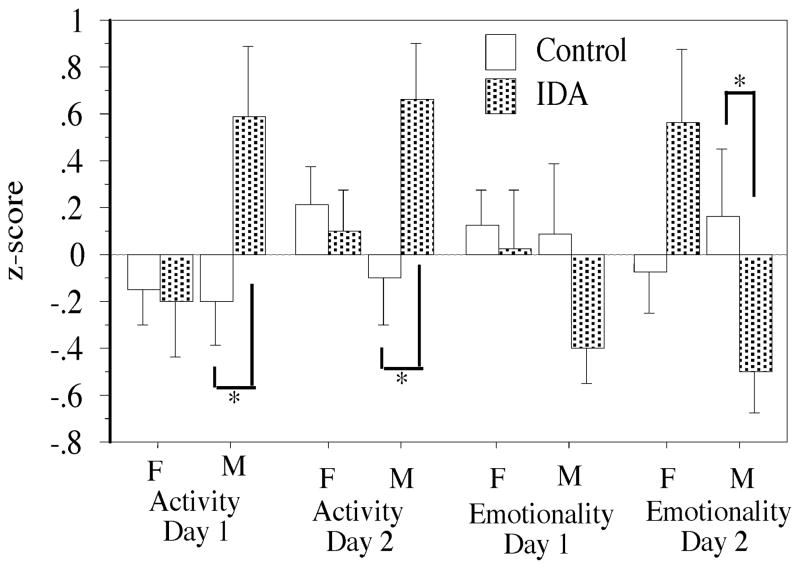
Examination of the composite indices of behavior that were derived from factor analyses (see above) indicated potential sex by group interactions on both days (Activity Day 1, F(1,86)=2.41, p=.12; Activity Day 2, F(1,85)=2.88, p=.09; Emotionality Day 1, F(1,86)=.35, p=.55; Emotionality Day 2, F(1,85)=6.99, p=.01). There were no effects of IDA on these composite indices for females (F(1,49)=.0005–2.06, p=.16–.98). Male IDA infants, by contrast, had higher z-scores than control males for the Activity factor for both Day 1 (F(1,37)=4.13, p=.049) and Day 2 (F(1,37)=4.32, p=.045) and lower z-scores for the Emotionality factor on Day 2 (F(1,37)=4.97, p=.03) (Figure 3).
Figure 3.
Composite z-scores derived from Holding Cage observations by exploratory and confirmatory factor analysis of a larger set of infants participating in the BBA. F=female, M=male. *p<.05 compared to like-sexed control.
Novel object contact
Contact with the novel objects was automatically recorded and summarized as the percent of 5-min periods in which the infants contacted the objects at least once. These scores were generally greater in the IDA infants than in controls over the 25-h period. Omitting the dark period of the light cycle, when infants contacted the object during less than 5% of the 5-min periods, contact averaged 70±3% of the 5-min periods for the IDA infants and 62±2% for controls (F(1,82)=4.09, p=.046) . This was due primarily to the difference in contact time with the second novel object on Day 2 (74±4% vs 61±3%, F(1,82)=6.83, p=.011); differences were not significant on Day 1. Groups did not differ in the number of 15-sec periods with contact within the 5-min periods, which averaged 3.0±0.2. There were no sex or sex by group effects on novel object contact.
Human intruder
Behavior of the infant monkeys showed definite changes during the 4 successively presented conditions of the human intruder tests (profile far, profile near, stare far, stare near). In particular, some behavioral states decreased in duration across conditions (sit, F(3,88)=23.87, p<.0001; crouch, F(3,88)=2.82, p=.04), while others increased (active, F(3,88)=42.70, p<.0001; hang, F(3,88)=9.49, p<.0001; stand, F(3,88)=18.99, p<.0001). There were no significant group or sex effects for these variables, but a group by sex interaction was indicated for “sit” (F(1,87)=5.82, p=.018). When sexes were analyzed separately for “sit”, a significant effect of group was found in the males (F(1,37)=7.83, p=.008), with IDA males showing less time in the “sit” behavior state than control males. Females did not differ in duration of “sit” (F(1,50)=.21, p=.65). IDA did not significantly influence the incidence of species-typical emotional behaviors (bark, scream, and coo vocalizations; lipsmack, fear grimace and threat facial expressions) recorded during the human intruder test. The number of infants displaying a vocalization or facial expression behavior at least once in the 1-min period increased significantly across the 4 conditions (profile far=6%, profile near=7%, stare far=30%, stare near=68%, χ2=127, df=3, p<.0001). The percent of the IDA group showing these behaviors was lower than the percent of control infants, but these differences were not statistically significant. The number of infants exhibiting body postures/movements indicative of distress (convulsive jerk, cling, scratch, self-bite, tooth grind) decreased across the first 3 conditions but increased again in the “stare near” condition. The number of IDA infants demonstrating these behaviors was also lower than but not statistically different from controls.
The individual species-typical emotional behavior occurring in the most infants (66%) in response to the human intruder was threat (see Table 2 for description) under the “stare near” condition. This behavior was also highly sex dependent (males 65%, females 39%, χ2=5.50, df=1, p=.03) and demonstrated a sex-dependent effect of IDA. 21% of IDA males demonstrated threat as compared to 64% of control males (χ2=6.51, df=1, p=.01). In contrast, more IDA females demonstrated threat (35%) than control females (19%), although group differences were not significant.
Analysis was conducted on a distress index that was previously found to differ in response to the human intruder conditions depending on prenatal iron deprivation of nursery-reared infants (Golub et al. 2006). The index was the sum of frequency of the following behaviors: cage shake, convulsive jerk, self-clasp, crouch, motor stereotypy, self-bite, scratch, tooth grind, screech vocalization. In the present data for mother-reared infants there was a significant effect of condition (RMANOVA, F(3,85)=12.65, p<.001) on the distress index. The frequency of distress-related behaviors increased from the least to the most challenging condition. Analysis by group of the contrast between successive conditions demonstrated that; (1) neither group increased their distress index from “profile far” to “profile near”, (2) the control group (but not the IDA group) increased from “profile near” to “stare far” (F(1,56)=4.87, p=.031) and (3) both groups increased from the “stare far” to the “stare near” conditions (control, F(1,56)=12.89, p=.0007; IDA, F(1,31)=6.55, p=.016).
Temperament ratings
Distributions of some temperament category scores (aggression, depressed) were skewed to the right with the majority of animals given the lowest score (1). These categories were scored as binary variables (1, >1). The other categories were analyzed by chi-square as the number of infants given each rating score. None of the temperament categories showed significant effects of group with both sexes combined. Three temperament categories demonstrated a significant effect of sex: active (χ2 =14.23 p=.027), nervous (χ2 =9.7 p=.044), tense (χ2 =12.60 p=.050). When the effect of group was examined separately for each sex for these three categories, IDA males were found to differ from control males on scoring of the active category (χ2 =13.19, p=.022) with more IDA males receiving lower scores. No group differences were identified for the factor clusters derived from multivariate analysis.
Discussion
Table 6 summarizes the endpoints in this study that distinguished IDA and control infants. Integrating across endpoints, behavior of males was more influenced by IDA than that of females, and the major difference from control can be characterized as less fear-elicited response to the test, both in terms of fear-elicited behavior (hanging, threat) and also fear-elicited inhibition of behavior (activity, touching strange object).
Table 6.
Summary of statistically significant differences between IDA and control infants on the Biobehavioral Assessment
| Assessment | Endpoint | Group | sex |
|---|---|---|---|
|
| |||
| Novel object (Actiwatch) | # 5-min periods with contact | IDA>control | ND |
|
| |||
| Holding cage observation, Day 1 | motor activity | IDA>control, M | ND |
| hanging | IDA<control, M | ND | |
| sitting | IDA>control, M | ND | |
| exploratory activity | IDA>control, M | ND | |
| activity factor z score | IDA>control, M | ND | |
|
| |||
| Holding cage observation, Day 2 | activity factor z score | IDA>control, M | ND |
| emotionality factor z score | IDA<control, M | ND | |
|
| |||
| Plasma cortisol | 4 samples | ND | M<F |
|
| |||
| Human Intruder | threat behavior, “stare near” | IDA<control, M | M>F |
| increase in distress index | IDA<control, M | M>F | |
|
| |||
| Temperament rating | “active” rating | IDA<control, M | M<F |
ND= no difference; M= male, F=female
Behavioral inhibition associated with strange people, places and objects shows a distinct pattern of maturation in many species, including humans, nonhuman primates and rodents. Kalin et al. have described the development of fear-related behaviors in infant rhesus elicited with the “human intruder” paradigm, a test that is similar to one of the components of the BBA (Kalin et al., 1991). They discussed two aspects of fear-related behavior, (1) fear related behavioral inhibition elicited by the presence of the stranger and (2) agonistic behavior elicited by direct eye contact (stare) of the stranger. They found that these elicited behaviors developed at 9–12 weeks of age, the age of testing used in the current study. Thus, it is possible that reduced behavioral inhibition (more activity) and lower level of emotional/agonistic responding seen in the IDA males might be interpreted as delayed behavioral development. Alternatively, these behavioral characteristics might reflect long term characteristics of emotional responsiveness (temperament).
In humans, behavioral inhibition is a major dimension of infant temperament that persists into later life. Inhibited reach (Rothbart, 1988) and inhibited social approach (Kagan, 1997) mature at distinct developmental stages in human infants and seem to parallel monkey fear-related behavioral inhibition. Temperament is thought to have its origins in genetic factors as well as early environmental influences (Rogers et al., 2008). Infants identified as “inhibited” and “uninhibited” in the first year of life maintain their characteristic approach/withdrawal into childhood (Broberg et al., 1990; Aksan and Kochanska, 2004) and adolescence. Studies of adults characterized as behaviorally inhibited as infants have demonstrated a lower response of the amygdala to novelty as reflected in fMRI (Schwartz et al., 2003). Children and adolescents at the extremes of “inhibited” vs “uninhibited” temperament dimension are at greater risk of different types of childhood behavior disorders (Biederman et al., 2001; Fox et al., 2001). Although an emphasis has been put on extreme inhibition as a precursor of anxiety disorders, greater risk for aggressive and delinquent behaviors is found in “uninhibited” children. Also of interest is the overlap between behavior inhibition, childhood behavior disorders and later conduct disorders and criminality (Copeland et al., 2007).
Research in humans indicates a characteristic affective pattern in IDA infants and toddlers (Lozoff et al., 1985; Lozoff et al., 1986; Rahmanifar et al., 1993; Lozoff et al., 1996; Lozoff et al., 1998; Williams et al., 1999; Lozoff et al., 2003; Wachs et al., 2005; Lozoff et al., 2007). Infants are characterized as being wary, fearful and solemn and demonstrating decreased activity and positive affect (Lozoff et al. 2007). Further, a recent study of rhesus monkeys identified as anemic at 6 mos of age (Lubach and Coe, 2008) reported that these 10–12 month old rhesus were more distractible and less object oriented during testing. This affective pattern clearly contrasts to the less inhibited behavior seen in IDA monkey infants in the present study. It is important to note that the test situations used for eliciting affective responses in monkey infants in this study were probably much more distressing than those used for humans. Separation from mother, social group and outdoor housing, and relocation to the indoor caging used in the present study can be considered highly stressful as compared to the common human test situation where the infant is observed and tested at home or in the laboratory with its mother present. Thus, the behavioral profile demonstrated here is relevant mainly to responsiveness during highly stressful situations. It may also be that species differences are important, that other differences in the way assessments were conducted are relevant, or that factors associated with developmental IDA as assessed from hematology in the human situation are different than those in our study.
A more important consideration in comparing our study in monkeys to human studies may be the timing of the iron deprivation relative to the stage of brain development. Monkey infants, like human infants (Chantry et al., 2007), are known to have a high incidence of anemia (around 20%) during the latter period of breast feeding without any experimental interventions (Anderson et al., 1983; Bicknese et al., 1993; Kriete et al., 1995). Six to eight months of age is usually the time when IDA is manifest in rhesus monkey infants but milder IDA may be seen earlier. A study of 143 rhesus infants weaned primarily from dams housed indoors at 3–4 months of age identified an incidence of 19% IDA (Bicknese et al., 1993). This study used MCV<66 as the IDA criterion; their IDA group had mean hemoglobin levels of 11.4 g/dL and MCV of 64.7 fL as compared to hemoglobin of 9.8 g/dL and MCV of 59 fL in the present study. Thus our IDA group may represent a segment of the population not often captured in human studies or previous studies with rhesus monkeys. It may be that this population had a deficiency that preceded birth and was initiated during an earlier period of brain development thus leading to a different behavioral outcome. In our first study with dietary iron deprivation, anemia was induced in the dam in the third trimester (Golub et al., 2006) but did not persist into the postnatal period because the infants were transferred to an adequate iron diet at birth. The mother-reared infants in the current study, already severely anemic at 3 months of age, may also have experienced prenatal iron deprivation and thus belong to a similar population in terms of the time of insult to the developing brain.
While IDA influenced behavior of male infants, female IDA infants did not show statistically significant differences from controls. This was the case whether or not the endpoints showed sex differentiation in the control population. Although male and female infants were selected and matched by the same criteria, the sample of males may have been more anemic. While IDA males did not differ from IDA females in hemoglobin or hematocrit, these values were somewhat lower in males and MCV was significantly lower in IDA males than IDA females. Thus the sex-specific effect of IDA could be due in part to more severe IDA in the males.
In a previous study we demonstrated altered behavioral response in the BBA in 3–4 month old rhesus monkey infants who were deprived of dietary iron either prenatally or postnatally. Only the prenatally deprived infants showed differences from controls. In order to achieve these dietary restrictions, the infants were raised in the nursery where they could be fed a controlled iron formula. The response to the BBA was modified by the much different rearing conditions in the nursery, which had acclimated the iron-deprived infants to indoor housing, cages very similar to those used for the BBA, and substantial daily human presence and interaction, but limited their experience with adult monkeys. For instance, the cortisol response of the outdoor reared IDA infants to the test situation (80±2, n=90) was considerably higher than that of the nursery reared iron-deprived infants (60±4, n=38), a result that has been found for field-cage- versus nursery-reared infants regardless of iron status (Capitanio et al., 2005). Also, the nursery raised infants did not demonstrate any threat behaviors in the “stare near” condition of the human intruder test, while 66% of the corral-raised infants showed this behavior. In addition the sample sizes in the controlled iron deprivation experiment were smaller. Nonetheless, a number of parallels could be seen in the BBA test results of the nursery reared iron deprived infants and the male mother-reared infants selected as IDA on the basis of their hematology (see Table 6, 7). Both sets of infants were more active (less inhibited) and less emotional in the home cage observations in the BBA. Both sets of infants contacted the play object provided in their cage more often than their respective controls. This supports the thesis that poor developmental iron status produces essentially similar qualitative profiles of behavioral responsiveness under different social rearing environments. This comparison is relevant to an understanding of the origin of behavioral differences seen in infants identified as IDA. The fact that such infants have the same characteristics as infants deprived of iron prior to birth but not suffering from anemia at the time of testing, supports the interpretation that iron deprivation during prior brain development, rather than lack of iron at the time of the behavioral assessment, is the cause of the behavioral differences.
Table 7.
Summary of differences between prenatally iron deprived (ID) and iron sufficient (IS) rhesus monkey infants on the Biobehavioral Assessment. Data from Golub et al. 2006.
| Assessment | Endpoint | Group |
|---|---|---|
|
| ||
| Novel object (Actiwatch) | sensor counts/5 min | ID>IS |
|
| ||
| Holding cage observation, day 1 | motor activity | ID>IS |
| lying down | ID<IS | |
| exploratory activity | ID>IS | |
| activity factor z score | No Data | |
|
| ||
| Holding cage observation, day 2 | activity factor z score | No Data |
| emotionality factor z score | No Data | |
|
| ||
| Plasma cortisol | 4 samples | ND |
| decrease sample 1–2 | ID<IS | |
|
| ||
| Human Intruder: | threat behavior, “stare near” | no threat behaviors |
| increase in distress index | ID<IS | |
|
| ||
| Temperament rating: | “fear” rating | ID<IS, M |
In summary, a group of 34 3-month old rhesus monkeys with iron deficiency anemia were identified from among 679 infants raised by their mothers in outdoor social caging. These infants were characterized as showing less fear-induced behavioral inhibition than matched controls in a standardized test situation. This is a similar behavior pattern as seen in 3-month old monkey infants who were born to mothers who had IDA in late gestation and were raised in the primate nursery. However, it differs from the affective characteristics of human infants identified with IDA in late lactation who have been described as being more fearful and inhibited. This may be due to different behavioral syndromes induced by iron deprivation that occurs at different periods of brain development.
Acknowledgments
The authors appreciate the expert work of Laura del Rosso, Laura Calonder, and Carmel Stanko, who conducted the BioBehavioral Assessment testing, and the animal technical staff at CNPRC. Investigators from the NIH Program Project “Brain and Behavior in Early Iron Deficiency” assisted in establishing the IDA criteria and provided information and comments on the manuscript. Supported by RR000169 to the California National Primate Research Center, RR019970 (John Capitanio, PI), and P01HD39386 (Betsy Lozoff, PI).
Contributor Information
Casey E. Hogrefe, Email: cehogrefe@ucdavis.edu.
John P. Capitanio, Email: jpcapitanio@ucdavis.edu.
Keith F. Widaman, Email: kfwidaman@ucdavis.edu.
References
- Aksan N, Kochanska G. Links between systems of inhibition from infancy to preschool years. Child Dev. 2004;75:1477–1490. doi: 10.1111/j.1467-8624.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- Anderson J, Keen C, Lonnerdal B. Iron deficiency in outdoor corral housed juvenile rhesus monkeys. Lab Anim Sci. 1983;33:494. [Google Scholar]
- Bicknese EM, George JW, Hird DW, Anderson JH. Prevalence and risk factors for iron deficiency anemia in weanling rhesus macaques. Lab Anim Sci. 1993;43:434–438. [PubMed] [Google Scholar]
- Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, Herot C, Friedman D, Snidman N, Kagan J, Faraone SV. Further evidence of association between behavioral inhibition and social anxiety in children. Am J Psychiatry. 2001;158:1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- Broberg A, Lamb ME, Hwang P. Inhibition: its stability and correlates in sixteen- to forty-month-old children. Child Dev. 1990;61:1153–1163. [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Mankinger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev Psychobiol. 2005;46:318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, DelRosso L, Roberts JA. Nursery rearing and biobehavioral organization. In: Sackett G, Ruppenthal G, Elias K, editors. Nursery Rearing of Nonhuman Primates in the 21st Century. New York: Springer; 2006. pp. 191–213. [Google Scholar]
- Chantry CJ, Howard CR, Auinger P. Full breastfeeding duration and risk for iron deficiency in U.S. infants. Breastfeed Med. 2007;2:63–73. doi: 10.1089/bfm.2007.0002. [DOI] [PubMed] [Google Scholar]
- Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101:3359–3364. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Miller-Johnson S, Keeler G, Angold A, Costello EJ. Childhood psychiatric disorders and young adult crime: a prospective, population-based study. Am J Psychiatry. 2007;164:1668–1675. doi: 10.1176/appi.ajp.2007.06122026. [DOI] [PubMed] [Google Scholar]
- Domellof M, Dewey KG, Lonnerdal B, Cohen RJ, Hernell O. The diagnostic criteria for iron deficiency in infants should be reevaluated. J Nutr. 2002;132:3680–3686. doi: 10.1093/jn/132.12.3680. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Dev. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL. Iron deprivation during fetal development changes the behavior of juvenile rhesus monkeys. J Nutr. 2007;137:979–984. doi: 10.1093/jn/137.4.979. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Capitanio JP, Lozoff B. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotoxicol Teratol. 2006;28:3–17. doi: 10.1016/j.ntt.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J. Temperament and the reactions to unfamiliarity. Child Dev. 1997;68:139–143. [PubMed] [Google Scholar]
- Kalin NH. Studying non-human primates: a gateway to understanding anxiety disorders. Psychopharmacol Bull. 2004;38(Suppl 1):8–13. [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: ontogeny and context-dependent selective expression. Child Dev. 1991;62:1175–1183. [PubMed] [Google Scholar]
- Kriete MF, Champoux M, Suomi SJ. Development of iron deficiency anemia in infant rhesus macaques. Lab Anim Sci. 1995;45:15–21. [PubMed] [Google Scholar]
- Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. Jama. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Klein NK, Prabucki KM. Iron-deficient anemic infants at play. J Dev Behav Pediatr. 1986;7:152–158. [PubMed] [Google Scholar]
- Lozoff B, Wolf AW, Jimenez E. Iron-deficiency anemia and infant development: effects of extended oral iron therapy. J Pediatr. 1996;129:382–389. doi: 10.1016/s0022-3476(96)70070-7. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Wolf AW, Urrutia JJ, Viteri FE. Abnormal behavior and low developmental test scores in iron-deficient anemic infants. J Dev Behav Pediatr. 1985;6:69–75. [PubMed] [Google Scholar]
- Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron-deficiency anemia. Child Dev. 1998;69:24–36. [PubMed] [Google Scholar]
- Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112:846–854. [PubMed] [Google Scholar]
- Lozoff B, Corapci F, Burden MJ, Kaciroti N, Angulo-Barroso R, Sazawal S, Black M. Preschool-aged children with iron deficiency anemia show altered affect and behavior. J Nutr. 2007;137:683–689. doi: 10.1093/jn/137.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubach GR, Coe CL. Selective impairment of cognitive performance in the young monkey following recovery from iron deficiency. J Dev Behav Pediatr. 2008;29:11–17. doi: 10.1097/DBP.0b013e31815f24a9. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Wallen K. Nonhuman primate models of developmental psychopathology: problems and prospects. In: Cicchetti D, Walker E, editors. Neurodevelopmental Factors in Psychopathology. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- McCann JC, Ames BN. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr. 2007;85:931–945. doi: 10.1093/ajcn/85.4.931. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. MPlus User’s Guide. 2. Los Angeles, CA: Muthén & Muthén; 2001. [Google Scholar]
- Rahmanifar A, Kirksey A, Wachs TD, McCabe GP, Bishry Z, Galal OM, Harrison GG, Jerome NW. Diet during lactation associated with infant behavior and caregiver-infant interaction in a semirural Egyptian village. J Nutr. 1993;123:164–175. doi: 10.1093/jn/123.2.164. [DOI] [PubMed] [Google Scholar]
- Rogers J, Shelton SE, Shelledy W, Garcia R, Kalin NH. Genetic influences on behavioral inhibition and anxiety in juvenile rhesus macaques. Genes Brain Behav. 2008 doi: 10.1111/j.1601-183X.2007.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK. Temperament and the development of inhibited approach. Child Dev. 1988;59:1241–1250. [PubMed] [Google Scholar]
- Schneider JM, Fujii ML, Lamp CL, Lonnerdal B, Dewey KG, Zidenberg-Cherr S. The use of multiple logistic regression to identify risk factors associated with anemia and iron deficiency in a convenience sample of 12-36-mo-old children from low-income families. Am J Clin Nutr. 2008;87:614–620. doi: 10.1093/ajcn/87.3.614. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Wachs TD, Kanashiro HC, Gurkas P. Intra-individual variability in infancy: structure, stability, and nutritional correlates. Dev Psychobiol. 2008;50:217–231. doi: 10.1002/dev.20284. [DOI] [PubMed] [Google Scholar]
- Wachs TD, Pollitt E, Cueto S, Jacoby E, Creed-Kanashiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Dev Psychobiol. 2005;46:141–153. doi: 10.1002/dev.20049. [DOI] [PubMed] [Google Scholar]
- Williams J, Wolff A, Daly A, MacDonald A, Aukett A, Booth IW. Iron supplemented formula milk related to reduction in psychomotor decline in infants from inner city areas: randomised study. Bmj. 1999;318:693–697. doi: 10.1136/bmj.318.7185.693. [DOI] [PMC free article] [PubMed] [Google Scholar]