SUMMARY
Cryptococcus neoformans (C. neoformans) penetration into the central nervous system (CNS) requires traversal of the blood-brain barrier that is composed of a single layer of human brain microvascular endothelial cells (HBMEC), but the underlying mechanisms of C. neoformans traversal remain incompletely understood. C. neoformans transcytosis of HBMEC monolayer involves rearrangements of the host cell actin cytoskeleton and small GTP-binding Rho family proteins such as Rac1 are shown to regulate host cell actin cytoskeleton. We, therefore, examined whether C. neoformans traversal of the blood-brain barrier involves host Rac1. While the levels of activated Rac1 (GTP-Rac1) in HBMEC increased significantly upon incubation with C. neoformans strains, pharmacological inhibition and down-modulation of Rac1 significantly decreased C. neoformans transcytosis of HBMEC monolayer. Also, Rac1 inhibition was efficient in preventing C. neoformans penetration into the brain. In addition, C. neoformans phospholipase B1 (Plb1) was shown to contribute to activating host cell Rac1, and STAT3 was observed to associate with GTP-Rac1 in HBMEC that were incubated with C. neoformans strain but not with its Δplb1 mutant. These findings demonstrate for the first time that C. neoformans Plb1 aids fungal traversal across the blood-brain barrier by activating host cell Rac1 and its association with STAT3, and suggest that pharmacological intervention of host-microbial interaction contributing to traversal of the blood-brain barrier may prevent C. neoformans penetration into the brain.
Introduction
C. neoformans is encapsulated yeast responsible for life-threatening CNS infections primarily in immunocompromised patients, such as those infected with HIV-1 (Chin et al., 2004, Chuck and Sande 1989, Gordon et al., 2000, Hakim et al., 2000, Perfect and Casadevall, 2002). A 2001 case study in Zambia showed that fatality rates of C. neoformans meningoencephalitis are as high as 100% (despite the fact that 56% of the patients were initially treated with antifungal therapy) (Mwaba et al., 2001). C. neoformans meningoencephalitis was one of the leading causes of death in HIV-1-infected adults in Uganda (French et al., 2002).
Several lines of evidence from experimental mouse models of C. neoformans meningoencephalitis as well as human cases indicate that C. neoformans invasion into the brain follows fungemia with cerebral capillaries serving as portals of entry (Chang et al., 2004, Charlier et al., 2005, Chretien et al., 2002, Lee et al., 1996, Olszewski et al., 2004, Shi et al., 2010). However, the mechanisms involved in C. neoformans traversal of the blood-brain barrier, the essential step required for the development of meningoencephalitis remain incompletely understood.
To better understand C. neoformans traversal of the blood-brain barrier, we developed an in vitro model of the blood-brain barrier with human brain microvascular endothelial cells (HBMEC). Upon cultivation on collagen-coated Transwell inserts, HBMEC exhibit morphological and functional properties of tight junction formation and polarized monolayer (Chang et al., 2004, Kim et al., 2004, Ruffer et al., 2004, Stins et al., 1997), a unique property of the brain capillary endothelial monolayer. C. neoformans penetration into the brain has been shown to involve the transcytosis mechanism as well as the Trojan-horse mechanism (Dromer and Levtiz, 2011), and cryptococal interaction with the HBMEC monolayer is likely to provide the information on the transcytosis mechanism. C. neoformans penetration into the brain was investigated in the mouse model of experimental hematogenous meningoencephalitis, which is likely to represent the blood-brain barrier penetration using the transcellular and/or Trojan-horse mechanisms (Chang et al., 2004, Charlier et al., 2005, Chretien et al., 2002, Cox et al., 2001, Dromer and Levitz, 2011, Kim 2008, Olszewski et al., 2004, Shi et al., 2010).
Using the above-mentioned in vitro and in vivo models, we showed that C. neoformans strains exhibit the ability to traverse the blood-brain barrier and penetrate into the brain (Chang et al., 2004). The traversal of C. neoformans across the blood-brain barrier was shown to involve host cell actin cytoskeleton rearrangements, as demonstrated by transmission and scanning electron microscopy (Chang et al., 2004). Internalization of HBMEC monolayer by C. neoformans was associated with microvilli-like protrusions at the entry site on the surface of HBMEC and there was no change in the integrity of HBMEC monolayer following transcytosis of C. neoformans, as assessed by monitoring of transendothelial electrical resistance (TEER) (Chang et al., 2004). In addition, internalized C. neoformans was found to be located within membrane-bound vacuoles of HBMEC, and C. neoformans transmigrates HBMEC monolayer through an enclosed vacuole without intracellular multiplication (Chang et al., 2004). No free yeast is found in the cytoplasm of HBMEC and also between adjacent HBMEC (Chang et al., 2004). These findings suggest the involvement of host cell actin cytoskeleton rearrangements in C. neoformans internalization and traversal of the blood-brain barrier, but the underlying mechanisms remain incompletely understood (Chang et al., 2004, Kim 2008).
Small GTP-binding Rho family proteins such as Rac1 have been shown to regulate host cell actin cytoskeleton functions (Hall 1998) and pathogenic microbes have been shown to exploit such Rho GTPases for their entry into host cells (Galan and Zhou 2000, Kim 2008, Maruvada and Kim 2012, Nhieu and Sansonetti 1999, Shin and Kim 2006). In the present study, we examined the role of host cell Rac1 in C. neoformans transcytosis of HBMEC monolayer and penetration into the brain, and investigated the mechanisms associated with Rac1-mediated transcytosis of C. neoformans across HBMEC monolayer.
RESULTS
1. C. neoformans activates Rac1 in HBMEC
We have shown that C. neoformans traversal of HBMEC monolayer involves host cell actin cytoskeleton rearrangements (Chang et al., 2004). Host cell Rac1 is an important intracellular molecule that takes part in actin cytoskeleton rearrangement, but its role in C. neoformans transcytosis of HBMEC monolayer has not been studied. Hence, we examined whether Rac1 activation occurs in HBMEC in response to C. neoformans, initially using strain B3501A (serotype D).
Briefly, HBMEC were incubated with strain B3501A for 30 and 60 min, lysed, and the lysates subjected to a pull-down assay using p21-activated kinase (PAK-1) GST beads (see Materials and Methods). Since the protein-binding domain (PBD) of PAK1 captures activated Rac1 (Rac1-GTP), the beads were examined for GTP-Rac1 using Western Blot assay with specific Rac1 antibody (Shin and Kim 2006).
Rac1 activation was detected at 30 min of incubation, which increased at 60 min of incubation (Fig 1A). GTP-Rac1 levels were shown to increase in a time-dependent manner as estimated by densitometric analysis of individual bands and compared them to zero time point. The total amount of Rac1 in the lysates of HBMEC incubated with C. neoformans remained unchanged in the samples.
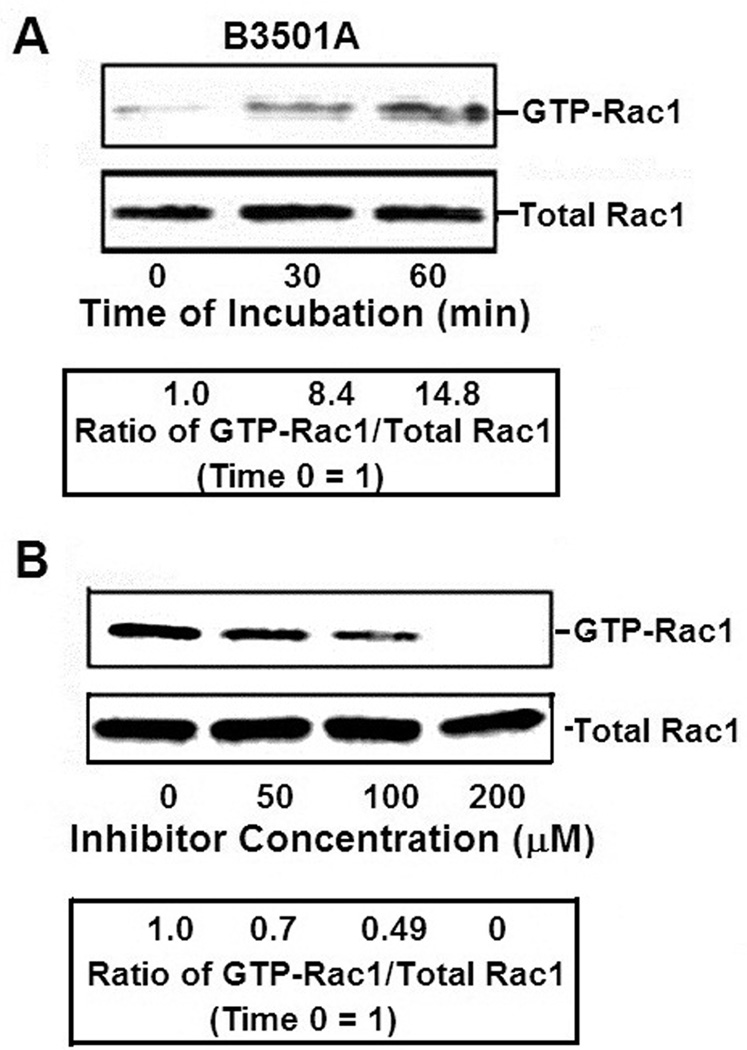
Figure 1. Rac1 activation of HBMEC by C. neoformans.
A. To examine host cell activation of Rac1 by C. neoformans, HBMEC were incubated with C. neoformans serotype D strain B3501A for various time periods. Activated Rac1 (GTP-Rac1) were pulled down by incubation of HBMEC lysates with PBD-GST beads that bind GTP-Rac1 and beads examined for Rac1 in Western Blot assay. Total Rac1 in lysates was examined as a loading control. Band intensities was estimated densitometrically and then represented as a ratio of GTP-Rac1 to total Rac1 assuming Time 0 as 1.
B. The specificity of Rac1 activation was assessed by incubating HBMEC with various concentrations of NSC23766 (a specific inhibitor of Rac1) after which the host cells were incubated with strain B3501A for 30 min and then examined for GTP-Rac1.
Band intensities was estimated densitometrically and then represented as a ratio of GTP-Rac1 to total Rac1 assuming time 0 = 1.
To examine the specificity of Rac1 activation, the activation of Rac1 was studied in the presence of NSC23766 (a specific inhibitor of Rac1) before adding the B3501A cells. The HBMEC were treated with various concentrations of NSC23766, incubated with B3501A for 30 min and then examined for GTP-Rac1. Analysis of Rac1 activation showed that while a linear decrease occurred at 50 µM and 100 µM of NSC23766, activation was completely suppressed at 200 µM (Fig 1B).
Microscopic examination of HBMEC, approximately 18 hours after treatment with up to 200 µM NSC23766, revealed no marked changes in cellular morphology (HBMEC viability was also unaffected, as assessed by live/dead stain, Molecular Probes, Grand Island, NY). Additionally, NSC23766 at 200 µM did not affect the growth of C. neoformans at 37°C during a 9 hr experimental period, as determined by CFUs. We, therefore, used NSC23766 at a concentration of 100 µM for further experiments.
2. Host cell Rac1 contributes to C. neoformans transcytosis of the HBMEC monolayer and penetration into the brain
Since C. neoformans activates Rac1 in HBMEC, the role of Rac1 in C. neoformans transcytosis across the blood-brain barrier was examined using the well-established in vitro model of HBMEC. Briefly, HBMEC treated with NSC23766 or vehicle control (PBS) were incubated with yeast cells of B3501A for determination of transcytosis. As shown in Fig 2A, a significant decrease was observed in transcytosis of B-3501A across the NSC23766-treated HBMEC compared to PBS-treated HBMEC (where transcytosis frequency of strain B3501A ranged between 1.4 % and 8.9 % of total inoculum), suggesting that pharmacological inhibition of Rac1 activation decreased the C. neoformans transcytosis of HBMEC monolayer. It is important to note that HBMEC integrity before and after transcytosis experiments remained unchanged, as assessed by TEER measurements.
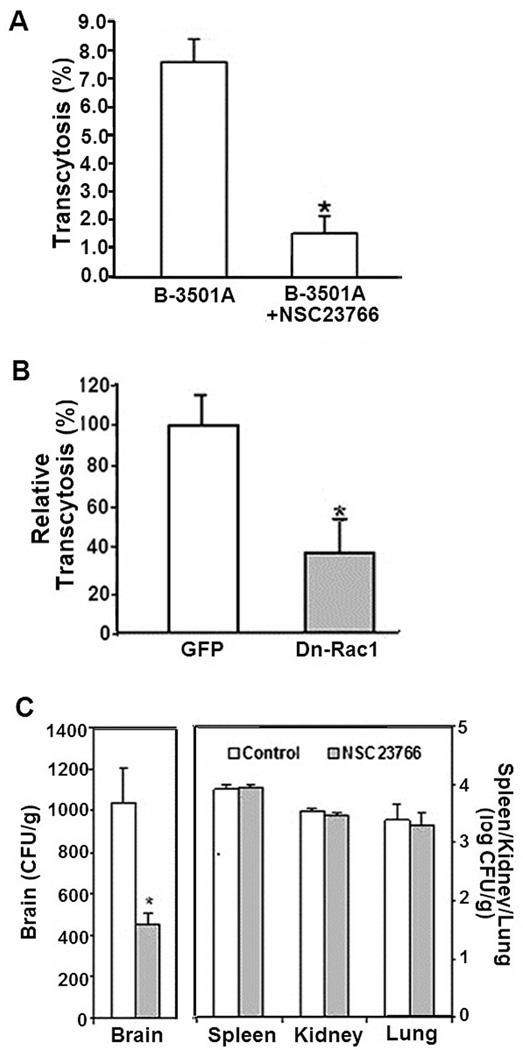
Figure 2. Role of Rac1 in C. neoformans transcytosis across the blood brain barrier.
A. To determine the role of Rac1 in C. neoformans transcytosis, HBMEC monolayers grown on transwells were treated with 100 µM of NSC23766 or vehicle control and incubated with C. neoformans strain B3501A. After 9 hour incubation, transcytosed CFUs were determined as described in Materials and Methods. *p<0.05 compared to vehicle-treated HBMEC
B. HBMEC transfected with adenoviruses expressing dominant-negative Rac1 construct or vector control were examined for transcytosis of C. neoformans strain B3501A and then expressed as relative transcytosis (%) assuming transcytosed yeast numbers in control vector-transfected HBMEC as 100%. *p<0.05 compared to vector-transfected HBMEC
C. To analyze the role of Rac1 in C. neoformans penetration into the brain, mice that received NSC23766 or vehicle control were injected with B-3501A (1×105 cells in 100µl PBS) via the tail vein. 24 hours later, the brains of the mice were removed, homogenized and examined for CFUs by culturing on YPD agar plates. The yeast counts were expressed as CFUs/gm. Kidneys, lungs and spleens were also determined for yeast counts (expressed as CFUs/gm). *p<0.05 compared to PBS-treated animals.
To further confirm the results of C. neoformans transcytosis derived from Rac1 inhibitor-treated HBMEC, the host cells were transfected with adenovirus constructs bearing the dominant-negative construct (N17Rac1) or vector alone. Cells were monitored by fluorescent microscopy for GFP expression of the adenovirus constructs. C. neoformans B-3501A showed significant decrease in transcytosis of HBMEC that were transfected with the dominant-negative Rac1 construct as compared to those that were transfected with vector alone (Fig. 2B). As noted above, HBMEC monolayers were found to maintain their integrity throughout the experiments with no significant changes in TEER values.
Next, the role of Rac1 in C. neoformans penetration into the brain was examined in an in vivo model. Briefly, mice were treated with the Rac1 inhibitor (NSC27366) or vehicle (PBS) and then inoculated with B3501A cells intravenously (see Materials and Methods). CFUs of C. neoformans were significantly reduced in the brains of mice treated with the inhibitor as compared to the control group that received PBS (Fig 2C). In contrast, the CFUs recovered from non-brain organs such as spleens, kidneys and lungs did not significantly differ between the recipients of the inhibitor or PBS. These in vitro and in vivo findings demonstrate that host Rac1 is likely to be involved in the C. neoformans transcytosis of the blood-brain barrier and penetration into the brain.
3. Host Rac1 is involved in HBMEC transcytosis by C. neoformans serotype A strain H99
We next examined whether host Rac1 also plays a role in HBMEC transcytosis by C. neoformans serotype A strain H99 using the method described for serotype D strain. Serotype A strains are most prevalent in clinical as well as environmental isolates of C. neoformans and account for the majority of cryptococcosis cases in AIDS patients (Casadevall and Perfect, 1998).
To examine whether Rac1 activation occurs in HBMEC in response to strain H99, HBMEC treated with NSC23766 or PBS were incubated with H99 for various time periods. Lysates were examined for levels of GTP-Rac1 as well as total Rac1. While GTP-Rac1 levels increased in PBS-treated HBMEC over the time periods, the activation was significantly decreased in the inhibitor-treated cells, demonstrating that the strain H99 activates Rac1 of HBMEC in a time-dependent manner (Fig 3A), similar to that of strain B3501A (Fig 1A).
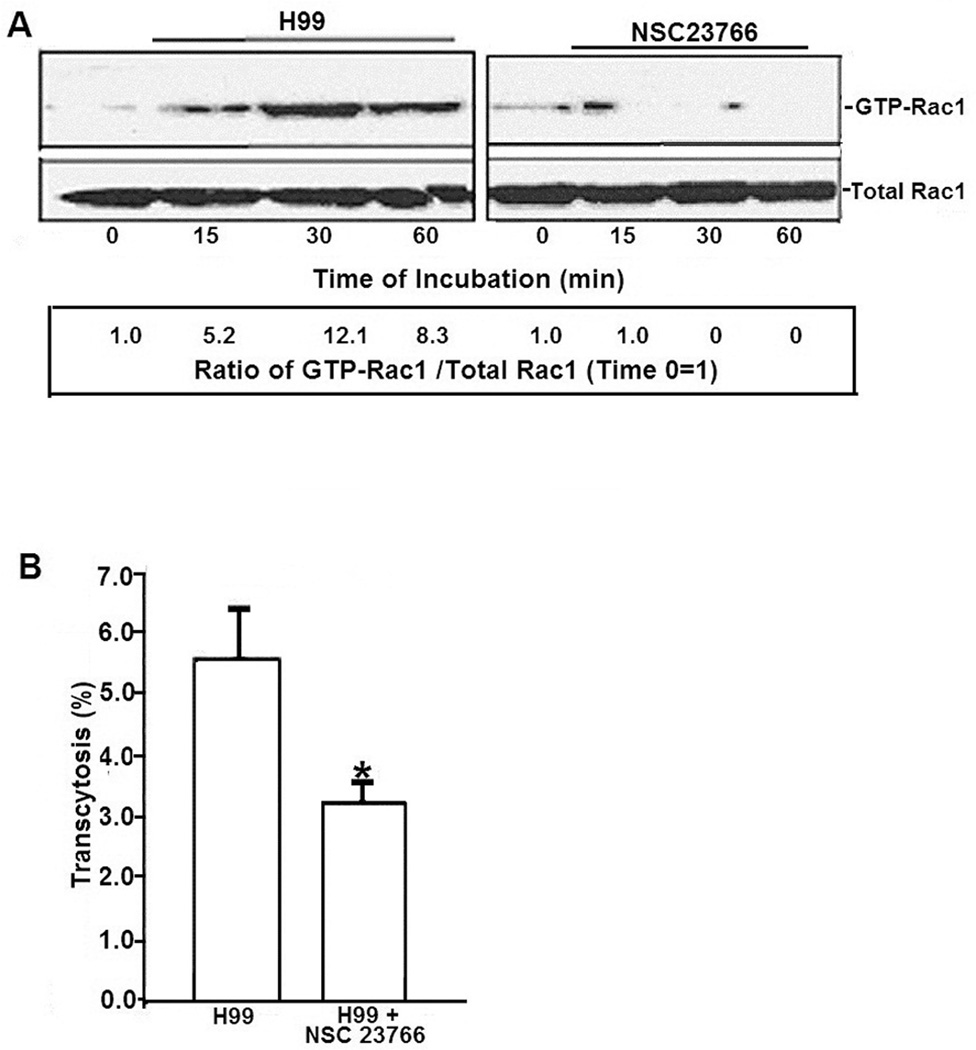
Figure 3. Role of Rac1 in C. neoformans serotype A strain H99 transcytosis of HBMEC monolayer.
A. To examine Rac1 activation of host cells by strain H99, HBMEC treated with Rac1 inhibitor or vehicle control were incubated with strain H99 for various time periods, after which lysates were analyzed for pull-down assays for GTP-Rac1. Numbers represent the ratios of GTP-Rac1 to total Rac1 with control (Time point 0 assumed as 1).
B. The role of Rac1 in strain H99 transcytosis, HBMEC monolayers grown on transwells were treated with NSC23766 or vehicle and incubated with strain H99. After 9 hour incubation, transcytosed CFUs were determined as described in Materials and Methods *p<0.05 compared to vehicle treated HBMEC.
Next, the contribution of Rac1 to transcytosis of strain H99 across the blood-brain barrier was examined using NSC23766. As shown in Fig 3B, the transcytosis of strain H99 was significantly decreased in the inhibitor-treated HBMEC compared to the PBS-treated HBMEC (where transcytosis frequency of strain H99 ranged between 1.1 % and 5.5 % of total inoculum). Taken together, these findings demonstrate that Rac1 activation is involved in C. neoformans transcytosis of HBMEC monolayer by both serotypes A and D strains.
4. Host Rac1 activation and contribution to C. neoformans transcytosis of HBMEC monolayer occurs independent of fungal RAC1
C. neoformans has the RAC1 gene encoding a Rac1 protein that is involved in fungal cytoskeletal architecture (Vallim et al., 2005, Nichols et al., 2007, Shen et al., 2012). Hence, to examine whether cryptococcal Rac1 plays a role in transcytosis across HBMEC, Δrac1 and its parent strain H99 were compared for their ability to transcytose the HBMEC monolayer. Both the Δrac1 mutant and the parent strain exhibited no significant differences in transcytosis of HBMEC monolayer (Fig 4A).
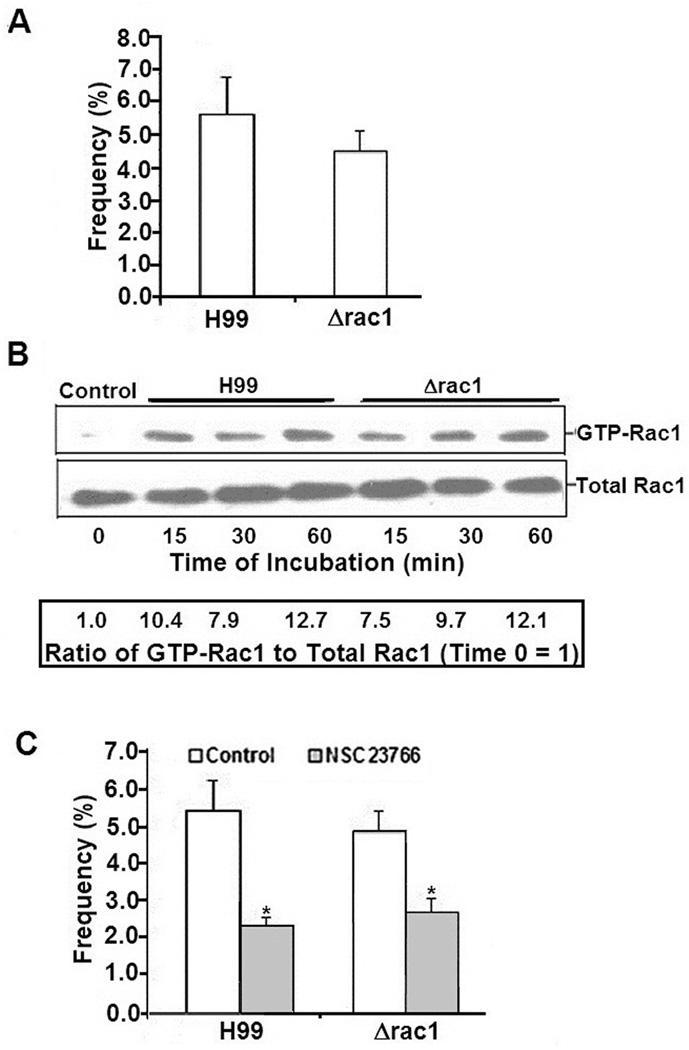
Figure 4. Role of C. neoformans RAC1 in activation of host Rac1 in HBMEC.
A. To determine whether C. neoformans Rac1 played a role in trancytosis, the Δrac1 mutant along with the parent strain H99 were examined for transcytosis across HBMEC monolayers after 9 hour incubation.
B. To analyze the role of C. neoformans Rac1 in activating host Rac1, HBMEC were incubated with strain H99 or Δrac1 mutant for various time periods, after which lysates were analyzed for GTP-Rac1 or total Rac1 in Western Blot assays.
C. To examine differences in transcytosis between strain H99 and the Δrac1 mutant in the presence of Rac1 inhibitor, HBMEC were treated with NSC23766 or vehicle, and then examined for transcytosis after 9 hour incubation. *p<0.05 compared to vehicle-treated HBMEC
To determine whether Rac1 of Cryptococcus was involved in the activation of host Rac1, HBMEC were incubated with either H99 or its rac1 mutant and examined for the levels of GTP-Rac1. Both the parent strain as well as its Δrac1 mutant were equally capable of activating host Rac1 (Fig 4B), suggesting that cryptococcal Rac1 might not be involved in host Rac1 activation as well as host Rac1-mediated cryptococcal transcytosis across HBMEC monolayer.
To further negate the role of cryptococcal Rac1 in host Rac1-mediated transcytosis across HBMEC, both strain H99 and its Δrac1 mutant were incubated with HBMEC that were treated either with NSC23766 or PBS. As shown with the parent strain H99, the Δrac1 mutant showed significantly decreased transcytosis in NSC23766-treated HBMEC as compared to PBS-treated cells, suggesting that the Rac1 inhibitor prevents the HBMEC transcytosis of the Δrac1 mutant in a manner similar to that of the parent strain (Fig 4C). These findings suggest that host Rac1 activation and host Rac1-mediated transcytosis of C. neoformans in HBMEC occur independently of the yeast Rac1.
5. Cryptococcal PLB1 contributes to fungal transcytosis across the blood-brain barrier by activating host Rac1
Previous studies have shown that cryptococcal PLB1 contributes to penetration of yeast cells into the brain (Cox et al., 2001), as shown by the demonstration that the cryptococcal Δplb1 mutant was significantly defective in penetration into the brain following either intravenous or intratracheal administrations of C. neoformans. We, therefore, examined whether cryptococcal PLB1 contributes to fungal transcytosis across the HBMEC monolayer.
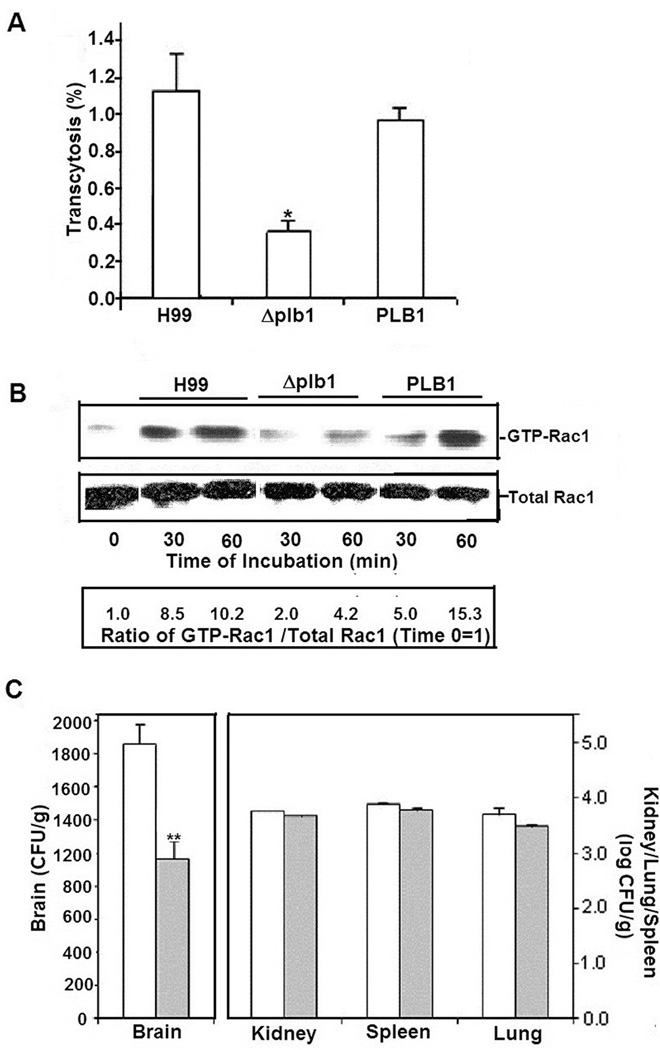
Briefly, HBMEC monolayers were incubated either with strain H99, Δplb1 mutant or the mutant reconstituted with PLB1. While transcytosis of the Δplb1 mutant across the HBMEC monolayer was significantly defective, the defect was restored to the level of the parent strain by reconstitution with a wild type PLB1 gene (Fig 5A). These findings support the notion that cryptococcal Plb1 contributes to the fungal transcytosis of the blood-brain barrier.
Figure 5. Role of C. neoformans PLB1 in activation of host Rac1.
A. To analyze the role of C. neoformans PLB1 in transcytosis, HBMEC were incubated with strain H99, the Δ plb1 mutant or the mutant reconstituted with PLB1. Transcytosed C. neoformans was determind after 9 hour incubation as described in Materials and Methods. *p<0.05 compared to H99 or the reconstituted strain.
B. To analyze the role of PLB1 in activation of host Rac1, HBMEC were incubated with either strain H99, Δ plb1 or the mutant reconstructed with PLB1 for various time points, after which lysates were analyzed for GTP-Rac1 or total Rac1 in Western Blot assays. Numbers represent ratios of GTP-Rac1/ total Rac1 assuming 0 time point as 1.
C. Mice were injected with either strain H99 (n=6) or the Δ plb1 mutant (n=6) via the tail vein. After 24 hours, the brains, spleens, kidneys and lungs were removed, homogenized and cultured for count of CFUs. Yeast numbers were expressed as CFUs per gram of tissue. *p<0.05 compared to H99.
Since Plb1 was shown to affect cryptococcal transcytosis across the HBMEC monolayer, the contribution of the molecule was examined in the activation of host cell Rac1. Briefly, HBMEC were incubated with either the wild type H99, its Δplb1 mutant or its reconstituted PLB1 strain for various time periods and the cell lysates examined for GTP-Rac1.
While HBMEC incubated with either H99 or the PLB1 reconstituted strain showed a time-dependent activation of Rac1, a significant decrease was observed in the levels of GTP-Rac1 in the HBMEC incubated with the Δplb1 mutant (Fig 5B). These findings suggest that cryptococcal PLB1 is likely to play a role in C. neoformans transcytosis of HBMEC monolayer by activating host cell Rac1.
To further confirm the role of cryptococcal PLB1 in penetration into the brain, the Δplb1 mutant or the parent strain H99 was injected intravenously into mice and examined for cryptococcal penetration into the brain. Compared to H99 infected mice, significantly lower CFUs were recovered from the brains of mice infected with the Δplb1 mutant (Fig 5C). However, the CFUs recovered from non-brain organs such as kidneys, spleens or lungs were similar between the recipients of H99 and its Δplb1. Taken together, these findings suggest that cryptococcal PLB1 but not RAC1 is involved in activating host Rac1 and also for transcytosis across HBMEC monolayer.
6. C. neoformans Plb1 is involved in STAT3 association with GTP-Rac1 in HBMEC
To understand a mechanism by which the Plb1 contributes to the host Rac1 activation and fungal transcytosis of HBMEC, we examined the levels of STAT3 associated with GTP-Rac1. STAT3 is a well-known transcriptional activator, but recent studies have reported its possible role in actin cytoskeletal rearrangements through its association with GTP-Rac1 and in aiding the pathogens’ entry into host cells (Simon et al., 2000, Maruvada and Kim 2012). Since the Plb1 was shown to play a role in activating host Rac1, the levels of STAT3 associated with GTP-Rac1 in HBMEC incubated with C. neoformans were examined.
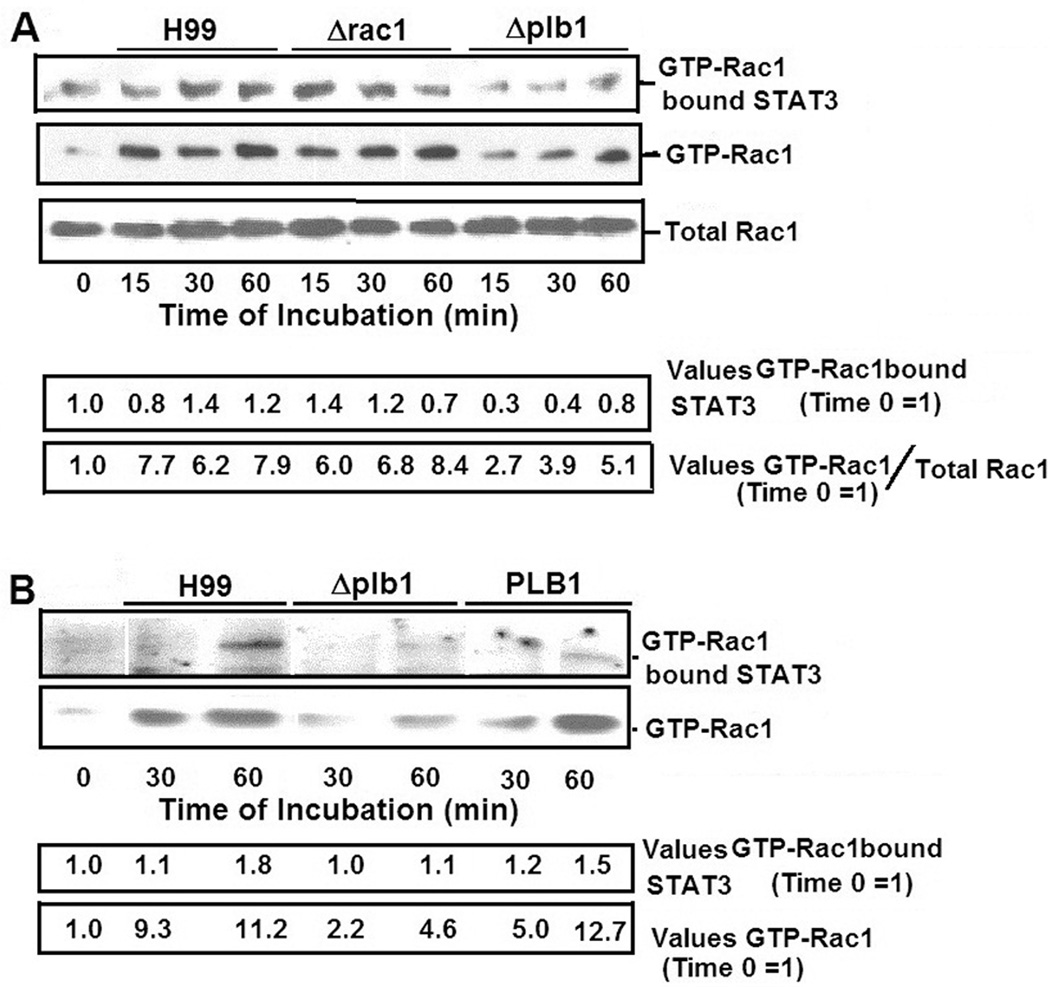
Briefly, the HBMEC were incubated with either the wild type H99 or its Δplb1 mutant for various time periods. Since the Δrac1 mutant did not activate host Rac1, the strain was included as a positive control. PBD-GST beads that were used to bind GTP-Rac1 were washed and examined for GTP-Rac1, total Rac1 and GTP-Rac1 bound STAT3. Western blot analysis showed that while significant amounts of STAT3 were associated with GTP-Rac1 in HBMEC that were incubated with H99 and Δrac1 mutant in a time-dependent manner, the amounts of bound STAT3 were significantly less in HBMEC incubated with the Δplb1 mutant. However, the HBMEC lysates showed similar levels of total Rac1 (Fig 6A).
Figure 6. Analysis of STAT3 association with GTP-Rac1 in lysates of HBMEC incubated with C. neoformans.
A. The lysates of HBMEC incubated with either strain H99, Δrac1 or Δ plb1 for various time periods were examined for the levels of GTP-Rac1 by pull-down assays. Nitrocellulose membranes that were used to resolve GTP-Rac1 in the lysates were examined for STAT3 using a specific monoclonal antibody.
B. In other experiments the nitrocellulose membranes on which GTP-Rac1 was resolved were assessed for levels of GTP-Rac1 bound STAT3. Bands were estimated densitometrically and represented as a ratio of values (STAT3/GTP-Rac1).
To further confirm that STAT3 association with GTP-Rac1 was indeed influenced by the cryptococcal Plb1, the samples of HBMEC incubated with Δplb1 as well as the reconstituted PLB1 strain along with the parent stain H99 (as shown in Fig 5B) were compared for levels of STAT3 associated with GTP-Rac1. As shown above, the amounts of STAT3 associated with GTP-Rac1 was significantly less in HBMEC incubated with the Δplb1 as compared with those incubated with strain H99. Interestingly, the HBMEC that were incubated with the reconstituted PLB1 showed restoration of STAT3 association with GTP-Rac1 to the level of the parent strain H99 (Fig 6B). These findings led us to hypothesize that Plb1 of Cryptococcus plays a role in the transcytosis of the blood-brain barrier through a mechanism involving host cell Rac1 and STAT3.
DISCUSSION
The traversal of C. neoformans across the blood-brain barrier involves host cell actin cytoskeleton rearrangements, as shown by our demonstration that the internalizing yeast was surrounded by microvilli-like projections of HBMEC membranes and C. neoformans transcytosis occurred without any change in the integrity of HBMEC monolayer (Chang et al., 2004).
In the present study, we showed that host Rac1 is involved in C. neoformans traversal of the blood-brain barrier, as demonstrated by (a) host Rac1 activation occurring in response to C. neoformans in HBMEC, (b) prevention of C. neoformans transcytosis of HBMEC monolayer by pharmacological inhibition of Rac1 and transfection with dominant-negative construct of Rac1, as well as (c) decreased C. neoformans penetration into the brain by Rac1 inhibition. Pathogenic microbes have been shown to exploit host cell Rho GTPases for their internalization into host cells (Galan and Zhou 2000, Kim 2008, Nhieu and Sansonetti 1999, Shin and Kim 2006, Maruvada and Kim 2012), but this is the first demonstration that host Rac1 contributes to C. neoformans traversal of HBMEC monolayer and penetration into the brain. The microbe-host interactions involved in host Rac1 contribution to C. neoformans traversal of the HBMEC monolayer and penetration into the brain, however, remain unclear. Although the Rac1 inhibitor (NSC23766) was effective in significant reduction in C. neoformans penetration into the brain, the effect of Rac1 inhibitor on the fungus cannot be unequivocally ruled out, and additional studies are needed to clarify this issue. Also, it remains unclear why blockade of host Rac1 affects C. neoformans penetration into the brain, but not into non-brain organs such as spleen, kidney and lung.
While specific virulence factors have been shown to involve host molecules to aid in the entry of meningitis-causing pathogens into the CNS (Kim 2008), this issue has not been fully examined in C. neoformans traversal of the blood-brain barrier. The genomes of C. neoformans strains have been sequenced, which include strains B-3501A (serotype D) and H99 (serotype A). Serotype A strains are the most prevalent clinical isolates and account for the majority of cryptococcosis cases in AIDS patients, and serotype D strains are predominant in Europe (Casadevall and Perfect, 1998). Functional genomic approaches are likely to identify the cryptococal factors that are involved in Rac1 activation in HBMEC and Rac1-mediated transcytosis of the blood-brain barrier.
We initially hypothesized that cryptococcal Rac1 might be involved in host Rac1 activation and the fungal transcytosis across HBMEC monolayer, but our findings with the Δrac1 mutant did not support the contribution of cryptococcal Rac1 to host Rac1 activation and transcytosis of HBMEC monolayer. In contrast, cryptococcal Plb1 was shown to be involved in host Rac1 activation in HBMEC, as shown by the demonstration that the Δplb1 mutant was defective in Rac1 activation, and this defect was restored to the level of the parent strain in the reconstituted strain with the wild type PLB1. C. neoformans Plb1 is shown to be involved in penetration into the brain (Cox et al., 2001), which was also documented in the present study. However, the mechanisms involved in the cryptococcal Plb1-mediated activation of host Rac1 are unclear. C. neoformans Plb1 has been shown to trigger capsule enlargement (Chrisman et al., 2011), but the time to capsule enlargement is longer (at least overnight or 24 hr incubation) than the time used for transcytosis assay (9 hr). In addition, capsule enlargement is like to impede cryptococcal transcytosis, but our studies with the Δplb1 mutant and the reconstituted strain showed the seemingly contrary results, i.e., Plb1 facilitates C. neoformans transcytosis of HBMEC monolayer and penetration into the brain. Thus, capsule enlargement is less likely to be involved in the Plb1-mediated transcytosis of the blood-brain barrier by C. neoformans. Our pilot data suggests that host cytoplasmic phospholipase A2 is involved in C. neoformans transcytosis of the blood-brain barrier and penetration into the brain. Based on cytoplasmic phospholipase A2 catalytic subunit present in the C- terminal domain of Plb1, we hypothesize that Plb1 might generate specific lipid mediators such as phosphoinositols in HBMEC that could mediate the activation of Rac1. Studies are in progress to elucidate the contribution of cryptococcal Plb1 to host Rac1 activation.
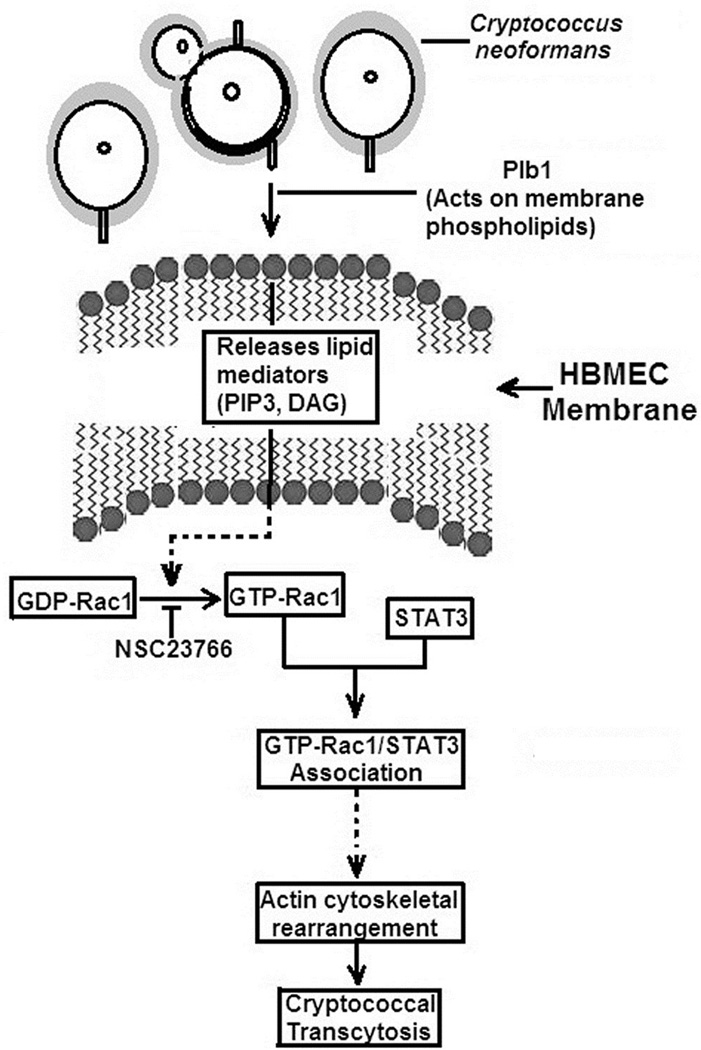
Though STAT3 is a known transcriptional factor and translocated to the nucleus upon activation, our recent data demonstrates that its cytoplasmic functions include its association with GTP-Rac1, role in actin cytoskeletal rearrangements and contribution to pathogen’s entry into host cells (Maruvada and Kim, 2012). In this study, Plb1 of C. neoformans is shown to activate host Rac1 and affect its association with STAT3, suggesting that GTP-Rac1-STAT3 may contribute to C. neoformans transcytosis of the blood-brain barrier, which is highlighted in Fig 7. We hypothesize that cryptococcal Plb1 probably acts on host membrane phospholipids to release lipid mediators that activate host Rac1, which in association with STAT3, regulates C. neoformans transcytosis of HBMEC monolayer and penetration into the brain. Studies are currently in progress to test this hypothesis.
Fig 7. Diagrammatic representation of the host cell signaling pathways activated by C. neoformans Plb1 for transcytosis of HBMEC monolayer.
For transcytosis of HBMEC monolayer, C. neoformans Plb1 is likely to interact with the blood-brain barrier and activate host cell Rac1. Activated Rac1 (GTP-Rac1) in turn interacts with STAT3, which we hypothesize aids in actin cytoskeleton rearrangements involved in C. neoformans transcytosis across HBMEC monolayer. We also hypothesize that the cytosolic phospholipase-like domain present in the C-terminal of Plb1 is likely to act on host cell phospholipids to release lipid mediators, which are involved in activating Rac1.
Taken together, our findings demonstrate that inhibition of host cell signaling molecules involved in C. neoformans traversal of the blood-brain barrier, as shown here with Rac1 inhibition may provide a novel approach for prevention of medically challenging C. neoformans meningoencephalitis. Further determination and characterization of host cell signaling molecules involved in C. neoformans traversal of the blood-brain barrier are likely to identify additional targets for prevention of C. neoformans penetration into the brain.
MATERIALS AND METHODS
Reagents and vectors
NSC23766 (an inhibitor that specifically interferes with Rac1 interaction with upstream guanine nucleoside exchange factors) was prepared as described previously (Gao et al., 2004). Adenoviruses, encoding a dominant-negative Rac1 construct (N17Rac1) bearing a GFP tag were amplified and used for transfection (Shin and Kim 2006). As a vector control, GFP expressing adenovirus from pShuttle-IRES-hrGFP-1 vector (Stratagene, La Jolla, CA) was used as described previously (Shin and Kim 2006). Antibodies to cPLA2, phospho-cPLA2, and Rac1 were obtained from Cell Signaling Technologies (Danvers, MA). GST beads were obtained from Pierce (Rockford, IL). PBD-PAK1 plasmid was a kind gift of Dr. Keith Burridge (North Carolina).
Yeast strains
C. neoformans strains H99 and B-3501A represent serotype A and D strains, respectively, and their genomes have been sequenced (Loftus et al., 2005, Idurnum et al., 2005). Deletion mutants of rac1 and plb1 derived from the H99 strain and their reconstituted strains were previously described (Cox et al., 2001, Vallim et al., 2005). Yeast cells were grown on YPD agar (1% yeast extracts, 2% peptone and 2% dextrose) at 37°C overnight, harvested, washed with PBS and resuspended in Hams-F12/M199 (1: 1, v/v), 5 % heat inactivated fetal bovine serum (FBS) (experimental medium) and 1 % fresh human serum. The number of yeast cells were determined by direct counting from a hemocytometer which was verified by colony forming units (CFUs) on YPD agar, as previously described (Chang et al., 2004).
Mice
9~11 week- old female BALB/cJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All procedures and handling techniques were approved by The Johns Hopkins University Animal Care and Use Committee.
Characterization and culture of HBMEC
HBMEC were isolated and characterized as previously described (Stins et al., 1997). HBMEC were positive for factor VIII-Rag, took up fluorescently labeled acetylated low-density lipoprotein and expressed γ-glutamyl transpeptidase, demonstrating brain endothelial cell characteristics. Cultures were maintained in RPMI-based medium, including 10 % FBS and 10 % NuSerum (BD Biosciences), at 37 °C in a humid atmosphere of 5 % CO2.
Transcytosis of C. neoformans across the HBMEC monolayer
HBMEC were cultured on Transwell polycarbonate tissue-culture inserts with a pore diameter of 12 µm (Corning Costar) for 5 days, as described previously (Chang et al., 2004). Confluency of the HBMEC monolayer was assessed by microscopy as well as by measurement of TEER. The HBMEC monolayers were washed with experimental medium and 107 Cryptococcus cells were added to the upper chamber. Transcytosis of C. neoformans across the HBMEC monolayers was assessed by counting the CFUs present in the samples removed from the lower chamber after 9 hrs of incubation 37° C as previously described (Chang et al., 2004). Transcytosis frequency (%) was determined [(total CFUs recovered from the lower chamber/total number of cryptococcal cells added to the upper chamber) × 100]. Transcytosis frequency in transfected HBMEC was expressed as relative transcytosis (%) compared to transcytosis frequency in control.vector-transfected HBMEC.
Preparation of cell lysates and assays for Rac1 activation
For assessment of Rac1 activation in response to C. neoformans, HBMEC monolayers were incubated with Cryptococcus for various time periods and lysed in modified radioimmuno protection assay (RIPA) buffer containing inhibitors for proteases and phosphatases at 4°C, as previously described (Shin and Kim 2006). For adenovirus infection, 50% confluent HBMEC were infected with dialyzed adenoviral particles (at a ratio of viral particles to HBMEC of 100:1 or multiplication of infection of 100) and incubated for two more days to reach confluency before adding C. neoformans cells. The HBMEC lysates were sonicated, centrifuged and assayed for protein concentration.
For Rac1 activation assay, 400 µg of protein was incubated with 30 µg of PBD-PAK-1 (which captures activated Rac1 or GTP-Rac1) bound to glutathione agarose beads. Beads were washed with the lysis buffer and subjected to SDS-PAGE and Western Blot analysis using a specific Rac1 antibody. Lysates were also examined for total Rac1.
Experimental hematogenous C. neoformans infection in mice
Mice were anesthetized by subcutaneous administration of pentobarbital sodium (50 mg/kg) and received NSC23766 (50 µg/mouse in 100µl PBS) via intraperitoneal administration 30 min before, and 3 hr and 6 hr after tail vein injection of B-3501A (1×105 cells in 100µl PBS). This dose of NSC23766 was shown to inhibit Rac1 activity in mice (Akbar H et al 2007). Control animals were given 100 µl PBS as vehicle control. At 24 hr after B-3501A injection, mouse chest was cut open, and blood from right ventricle was collected and cultured for determination of CFUs. The animals were, then perfused with a mammalian Ringer's solution by transcardiac perfusion through a 23-gauge needle inserted into the left ventricle of the heart under the perfusion pressure of about 100 mmHg, as previously described (Zhu et al., 2010). The perfusate exited through a cut in the right atrium. At 30 min after perfusion of Ringer solution, mice were decapitated. The brains were removed, weighed, homogenized in 2 ml RPMI, and cultured on YPD agar plates to determine CFUs/gm. Kidneys, lungs and spleens were also removed, homogenized, and cultured for determinations of CFUs/gm.
Statistical Analysis
Data are expressed as mean ± SEM. Differences of C. neoformans transcytosis across HBMEC monolayer and C. neoformans penetration into mouse brains, kidneys, spleens, and lungs were determined by Student’s t test. p<0.05 was considered significant.
ACKNOWLEDGEMENTS
This work was supported by the NIH grants AI84984 and NS26310 (KSK). The works by KJ Kwon-Chung were supported by funds from the intramural program of the National Institue of Allergy and Infectious Diseases, NIH, Bethesda, MD. The experiments were approved by the Animal Care and Use Committee of the Johns Hopkins University.
Footnotes
The authors declare no conflicts of interest.
References
- Casadevall A, Perfect JR. Cryptococcus neoformans. Washington, DC: American Society for Microbiology Press; 1998. 1998. [Google Scholar]
- Chang YC, Stins MF, McCaffery MJ, Miller GF, Pare DR, Dam T, et al. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect Immun. 2004;72:4985–4995. doi: 10.1128/IAI.72.9.4985-4995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin S, Rozycki G, Pugatch D, Harwell JI. Aetiology of meningitis in HIV-infected patients in a referral hospital in Phnom Penh, Cambodia. Int J STD AIDS. 2004;15:48–50. doi: 10.1258/095646204322637263. [DOI] [PubMed] [Google Scholar]
- Charlier C, Chretien F, Baudrimont M, Mordelet E, Lortholary O, Dromer F. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood-brain barrier. Am J Pathol. 2005;166:421–432. doi: 10.1016/S0002-9440(10)62265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien F, Lortholary O, Kansau I, Neuville S, Gray F, Dromer F. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J Infect Dis. 2002;4:522–530. doi: 10.1086/341564. [DOI] [PubMed] [Google Scholar]
- Chrisman CJ, Albuquerque P, Guismaraes AJ, Nieves E, Casadevall A. Phsopholipids trigger Cryptococcus neoformans capsule enlargement during interactions with Amoebae and macorphages. PLoS Pathog. 7 doi: 10.1371/journal.ppat.1002047. e1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck SL, Sande MA. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med. 1989;321:794–799. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- Cox GM, McDade HC, Chen SC, Tucker SC, Gottfredsson M, Wright LC. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol Microbiol. 2001;39:166–175. doi: 10.1046/j.1365-2958.2001.02236.x. [DOI] [PubMed] [Google Scholar]
- Dromer F, Levitz S. Invasion of Cryptococcus into the central nervous system (chapter 34) In: Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall, editors. Cryptococcus, from human pathogen to model yeast. American Society of Microbiology; 2011. [Google Scholar]
- French N, Gray K, Watera C, Nakiyingi J, Lugada E, Moore M, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16:1031–1038. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- Galan JE, Zhou D. Striking a balance: modulation of the actin cytoskeleton by Salmonella. Proc Natl Acad Sci USA. 2000;97:8754–8761. doi: 10.1073/pnas.97.16.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SB, Walsh AL, Chaponda M, Gordon MA, Soko D, Mbwvinji M, et al. Bacterial meningitis in Malawian adults: pneumococcal disease is common, severe, and seasonal. Clin Infect Dis. 2000;31:53–57. doi: 10.1086/313910. [DOI] [PubMed] [Google Scholar]
- Hakim JG, Gangaidzo IT, Heyderman RS, Mielke J, Mushangi E, Taziwa A, et al. Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. AIDS. 2000;14:1401–1407. doi: 10.1097/00002030-200007070-00013. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Idnurm A, Bahn Y, Nielsen K, Lin X, Fraser JA, Heitman J. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat Rev Microbiol. 2005;3:753–764. doi: 10.1038/nrmicro1245. [DOI] [PubMed] [Google Scholar]
- Kim KS. Mechanisms of microbial traversal of the blood-brain barrier. Nat Rev Microbiol. 2008;6:625–634. doi: 10.1038/nrmicro1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YV, DiCello F, Hillaire CS, Kim KS. Differential Ca2+ signaling by thrombin and protease-activated receptor-1-activating peptide in human brain microvascular endothelial cells. Am J Physiol Cell Physiol. 2004;286:C31–C42. doi: 10.1152/ajpcell.00157.2003. [DOI] [PubMed] [Google Scholar]
- Lee SC, Dickson DW, Casadevall A. Pathology of cryptococcal meningoencephalitis: analysis of 27 patients with pathogenetic implications. Hum Pathol. 1996;8:839–847. doi: 10.1016/s0046-8177(96)90459-1. [DOI] [PubMed] [Google Scholar]
- Loftus BJ, Fung E, Roncaglia P, Rowley D, Amedeo P, Bruno D, et al. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science. 2005;307:1321–1324. doi: 10.1126/science.1103773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruvada R, Kim KS. IbeA and OmpA of Escherichia coli K1 exploit Rac1 activation for invasion of human brain microvascular endothelial cells. Infect Immun. 2012 doi: 10.1128/IAI.06320-11. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwaba P, Mwansa J, Chintu C, Pobee J, Scarborough M, Portmouth S, Zumla A. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad Med J. 2001;77:769–773. doi: 10.1136/pmj.77.914.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhieu GT, Sansonetti PJ. Mechanism of shigella entry into epithelial cells. Curr Opin Microbiol. 1999;2:51–55. doi: 10.1016/s1369-5274(99)80009-5. [DOI] [PubMed] [Google Scholar]
- Nichols CB, Perfect ZH, Alspaugh JA. A Ras1-Cdc24 signal transduction pathway mediates thermotolerance in the fungal pathogen Cryptococcus neoformans. Mol Microbiol. 2007;63:1118–1130. doi: 10.1111/j.1365-2958.2006.05566.x. [DOI] [PubMed] [Google Scholar]
- Olszewski MA, Noverr MC, Chen G-H, Toews GB, Cox GM, Perfect JR, Huffnagle GB. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am. J. Pathol. 2004;164:1761–1771. doi: 10.1016/S0002-9440(10)63734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect JR, Casadevall A. Cryptococcosis. Infect Dis Clin North Am. 2002;4:837–874. doi: 10.1016/s0891-5520(02)00036-3. [DOI] [PubMed] [Google Scholar]
- Rüffer C, Strey A, Janning A, Kim KS, Gerke V. Cell-cell junctions of dermal microvascular endothelial cells contain tight and adherens junction proteins in spatial proximity. Biochemistry. 2004;43:5360–5369. doi: 10.1021/bi035517c. [DOI] [PubMed] [Google Scholar]
- Shen G, Zhou E, Alspaugh JA, Wang P. Wsp1 is downstream of Cin1 and regulates vesicle transport and actin cytoskeleton as an effector of Cdc42 and Rac1 in Cryptococcus neoformans. Eukaryot Cell. 2012 doi: 10.1128/EC.00011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Li SS, Zheng C, Jones GJ, Kim KS, Zhou H, Kubes P, Mody CH. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus in the brain. J Clin Invest. 2010;120:1683–1693. doi: 10.1172/JCI41963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Kim KS. RhoA and Rac1 contribute to type III group B streptococcal invasion of human brain microvascular endothelial cells. Biochem Biophys Res Commun. 2006;345:538–542. doi: 10.1016/j.bbrc.2006.04.130. [DOI] [PubMed] [Google Scholar]
- Simon AR, Vikis HG, Stewart S S, Fanburg BL, Cochran BH, Guan KL. Regulation of STAT3 by Direct Binding to the Rac1 GTPase. Science. 2000;290:144–147. doi: 10.1126/science.290.5489.144. [DOI] [PubMed] [Google Scholar]
- Stins MF, Gilles F, Kim KS. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol. 1997;76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- Vallim MA, Nichols CB, Fernandes L, Cramer KL, Alspaugh JA. A Rac Homolog Functions Downstream of Ras1 To Control Hyphal Differentiation and High-Temperature Growth in the Pathogenic Fungus Cryptococcus neoformans. Eukaryot Cell. 2005;4:1066–1078. doi: 10.1128/EC.4.6.1066-1078.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Maruvada R, Sapirstein A, Malik KU, Peters-Golden M, Kim KS. Arachidonic acid metabolism regulates Escherichia coli penetration of the blood-brain barrier. Infect Immun. 2010;78:4302–4310. doi: 10.1128/IAI.00624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]