Summary
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most frequent causes of hospital- and community-associated infections. Resistance to the entire class of β-lactam antibiotics, such as methicillin and penicillin, makes MRSA infections difficult to treat. Hospital-associated MRSA strains are often multi-drug resistant, leaving only lower efficiency drugs such as vancomycin as treatments options. Like many other S. aureus strains, MRSA strains produce a series of virulence factors, such as toxins and adhesion proteins. Recent findings have shed some new light on the molecular events that underlie MRSA epidemic waves. Newly emerging MRSA clones appear to have acquired phenotypic traits that render them more virulent or able to colonize better, either via mobile genetic elements or adaptation of gene expression. Acquisition of Panton-Valentine leukocidin genes and increased expression of core genome-encoded toxins are being discussed as potentially contributing to the success of the recently emerged community-associated MRSA strains. However, the molecular factors underlying the spread of hospital- and community-associated MRSA strains are still far from being completely understood, a situation calling for enhanced research efforts in that area.
Introduction
Staphylococcus aureus is a dangerous pathogen, responsible for a multitude of human infections around the world (Lowy, 1998). Many S. aureus infections present as moderately severe infections of the skin or respiratory tract, but S. aureus may also cause more dramatic forms of disease that may be life-threatening, such as necrotizing fasciitis or necrotizing pneumonia. Considerable efforts have been undertaken to decipher the importance that specific molecular determinants have in defining S. aureus virulence and interaction with the host.
From a clinical point of view, a major problem that physicians have to face when treating S. aureus infections is antibiotic resistance. Resistance to the first antibiotic, penicillin, emerged in the 1940s (Barber et al., 1948). In 1942, penicillin-resistant S. aureus was detected (Rammelkamp et al., 1942). Mechanistically, resistance to penicillin is due to an enzyme called penicillinase, which was found even before the introduction of penicillin into clinical use (Abraham et al., 1940). Penicillinase cleaves the β-lactam ring that is characteristic of β-lactam antibiotics such as penicillin and its derivatives. Already in the 1950s, penicillinase-containing strains of S. aureus were pandemic in hospitals and the community (Roundtree et al., 1956). Nowadays, most infectious S. aureus isolates are resistant to penicillin.
To overcome the problem with penicillin-resistant S. aureus, the semisynthetic antibiotic methicillin was developed, which is derived from penicillin, but resistant to β-lactamase inactivation. Methicillin was introduced by Beecham in 1959; but already about one year later, methicillin-resistant S. aureus was detected in the United Kingdom (Jevons et al., 1963). Unlike in the case of resistance to penicillin, the mechanism underlying methicillin resistance protects the bacteria from the entire class of β-lactam antibiotics including penicillins, cephalosporins, and carbapenems.
S. aureus epidemics occur in waves of antibiotic resistance (Chambers et al., 2009). The first epidemic penicillin-resistant strains were replaced by the so-called “archaic” MRSA strains first found in the United Kingdom. This epidemic was largely restricted to Europe. Starting in the 1980s, novel lineages of MRSA emerged, leading to a worldwide pandemic of MRSA that is still ongoing. Nowadays, many industrialized countries report that methicillin-resistant strains account for at least 25 – 50% of infectious S. aureus isolates in hospitals (Diekema et al., 2001). In contrast, some countries such as The Netherlands and the Scandinavian countries historically have low MRSA infection rates (often < 1 %), most likely owing to rigid search-and-destroy and surveillance policies, as well as restraint in antibiotic prescription. In fact, a recent Japanese study indicates that high antibiotic consumption rates lead to increased MRSA burden over time (Nakamura et al., 2012). While for a long time MRSA infections were limited to hospitalized patients, the most recent epidemic MRSA wave, beginning in the mid to late 1990s, is characterized by the emergence of community-associated MRSA (CA-MRSA) with the capacity to infect otherwise healthy individuals.
This review will give an overview over virulence and colonization strategies of S. aureus with a focus on MRSA strains. While for the most part, these strategies do not appear to be different between methicillin-susceptible S. aureus (MSSA) and MRSA strains, there are some examples of virulence and colonization determinants that were found to play significant roles in the pathogenic success of specific MRSA clones. In particular, recent findings give first insight into the potential molecular underpinnings of the rise and disappearance of MRSA during epidemic waves. Furthermore, considerable efforts have been undertaken to understand the molecular factors defining the increased potential of CA-MRSA, as compared to hospital-associated (HA-) MRSA, to spread and cause disease.
Genetics of methicillin resistance in S. aureus
Methicillin resistance in staphylococci is due to the acquisition of a mobile genetic element (MGE) called the staphylococcal cassette chromosome mec (SCCmec) (Katayama et al., 2000). SCCmec is a DNA fragment ranging from 21 to 67 kb in size, depending on the SCCmec type (Hiramatsu et al., 2001). The number of SCCmec types is constantly increasing with the discovery of new elements; currently, there are 11. All SCCmec types include the mecA gene, which codes for the low-affinity penicillin binding protein PBP2a (Hartman et al., 1981), as factor necessary for methicillin resistance. Resistance is due to the fact that β-lactam antibiotics cannot inhibit PBP2a, in contrast to other S. aureus PBPs. SCCmec elements also include ccr genes for integration and excision from the chromosome (Hiramatsu et al., 2001). Other parts of SCCmec elements vary in composition. For example, they may contain additional antibiotic resistance genes. While it has been often stressed that SCCmec elements do not contain virulence genes, this view had to be reversed when it was found recently that a peptide toxin, PSM-mec, is encoded in close vicinity to the mecA gene in several SCCmec types (Queck et al., 2009).
MRSA clones
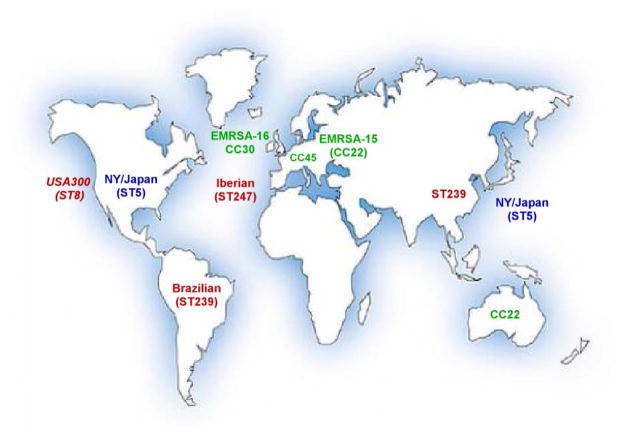
Different MRSA clones have emerged ever since the detection of the first MRSA strain in 1959 (DeLeo et al., 2009). Notably, almost all MRSA clones detected worldwide belong to only five clonal complexes (CCs): 5, 8, 22, 30, and 45 (Fig. 1). The first (archaic) MRSA clone (strain COL, sequence type [ST] 250) harbored the SCCmec element of type 1 and belonged to CC 8. Many important MRSA clones that emerged later, during the MRSA pandemic in the 1980s, belong to the same CC, but had new SCCmec types (types 2 and 3). These included the Iberian (EMRSA-5, ST247) clone, a descendant of COL, and the Brazilian/Hungarian (EMRSA-1, ST239) clone. Further major MRSA clones are the New York/Japan clone (ST5, USA100) and the “pediatric” clone (ST5), both of which belong to CC5.
Fig. 1. Important global MRSA clones.
Shown are MRSA lineages or clones that are currently predominant in large geographical locations around the world. Most clones belong to clonal complexes 5 (shown in blue) or 8 (shown in red). Other predominant clones belong to CCs 22, 30, and 45. Although spreading globally, pronounced numbers of CA-MRSA infections are only seen in the U.S., where almost all CA-MRSA infections are caused by clone USA300 (CC8, shown in red).
Multi-drug resistance
Many MRSA clones have acquired resistance to additional antibiotics, such as erythromycin, clindamycin, ciprofloxacin, tetracycline, etc. (Shorr, 2007). It is alarming that multi-drug-resistant strains of S. aureus are often only susceptible to vancomycin, an antibiotic with considerably lower efficiency compared for example to β-lactams. Furthermore, rare cases of highly vancomycin-resistant MRSA strains (VRSA) have been reported (2002). Fortunately, these did not spread substantially, possibly owing to increased fitness cost associated with high-level resistance to vancomycin. The majority of CA-MRSA strains have not acquired resistance to additional antibiotics, with the exception of limited outbreaks of multi-drug-resistant CA-MRSA (Diep et al., 2008).
Colonization
In the hospital, contaminated fomites and medical devices may play a role as intermediate sources of MRSA infection, but ultimately these originate from patients or hospital personnel that carry MRSA. The anterior nares are the most frequent site of S. aureus carriage; and an association between S. aureus nasal carriage and disease has been noted already in 1931 (Wertheim et al., 2005). Since then, several studies have confirmed the hypothesis that most S. aureus infections originate from strains that colonize the nose (von Eiff et al., 2001, Wertheim et al., 2004). While it has been emphasized that there are S. aureus colonization sites in the body other than the nose, such as the perineum and throat, the roles of these sites as sources of infection are poorly understood. Of note, it is often debated to eradicate MRSA carriage to minimize the potential for MRSA infection. However, studies evaluating the outcome of MRSA eradication produced widely divergent results. In a recent systematic survey it was concluded that mupirocin is effective in the short-term eradication of MRSA, such as for presurgical treatment in hospitals (Ammerlaan et al., 2009).
Interestingly, only about 20% of individuals in the population are persistent nasal carriers of S. aureus, while 30% are intermittent carriers, and about 50% are never colonized (Wertheim et al., 2005). The fact that carriage rates differ among ethnic groups and are higher in patients with certain diseases underlines the importance of host factors determining S. aureus colonization. However, the molecular underpinnings of these differences are not understood, and therefore, in the following there will be a focus on bacterial factors associated with colonization.
Surface-anchored S. aureus binding proteins that interact with human matrix molecules (microbial surface proteins recognizing adhesive matrix molecules, MSCRAMMs) likely play a role in nasal colonization, particularly when the mucin layer is breached and matrix proteins are exposed. Clumping factor B and S. aureus surface proteins G and X (SasG, SasX) have been demonstrated to bind to nasal epithelial cells (O'Brien et al., 2002, Roche et al., 2003, Li et al., 2012). The recently described SasX protein is of particular interest, because it has been linked to an MRSA epidemic wave (Li et al., 2012). SasX is encoded on an MGE occurring predominantly in ST239 MRSA strains, which are the most frequent source of MRSA infections in Asia. The frequency of the sasX gene among invasive ST239 strains in Asia has increased significantly over the last decade. Given that it was shown to contribute to nasal colonization, biofilm formation, and immune evasion and virulence in animal infection models (see below), it is likely that sasX is a major factor responsible for the spread and pathogenic success of ST239. Notably, sasX and its distribution exemplify how the spread of a colonization and virulence factor by horizontal gene transfer may drive an MRSA epidemic wave.
Several surface polymers of S. aureus, such as teichoic acids, have been shown to determine the capacity of S. aureus to colonize the nose (Weidenmaier et al., 2004), but it is not understood how this occurs on a molecular level. Furthermore, S. aureus has a series of mechanisms providing resistance to antimicrobial peptides, which form an important part of innate immune defense on epithelia (Peschel, 2002). Finally, it has been stressed that bacterial interference may have an influence on nasal colonization. However, there is no clear evidence yet to suggest that MRSA epidemic waves are caused by the direct interference of competing MRSA strains, differential expression of surface polymers involved in colonization, or mechanisms of immune evasion. Nevertheless, scenarios are easy to imagine, in which MRSA strains use such mechanisms to displace other strains.
Role of biofilms
Biofilms are surface-attached bacterial agglomerations embedded in extracellular matrix. Staphylococci are known as especially good biofilm formers, which is due primarily to the production of a series of surface molecules that promote extracellular matrix formation (Otto, 2008). Biofilms provide significant protection from antibiotics and host defenses, in addition to enabling the bacteria to remain attached to biotic or abiotic surfaces. Thus, biofilms may contribute to prolonged infection and colonization, and the spread of MRSA in hospital and community settings. Whether S. aureus colonies in the nose can be considered biofilms is debatable, but the physiological status during colonization may be comparable in many aspects to that in biofilms. The strategy of colonizing or biofilm-forming S. aureus to remain in or on human epithelia in relative “silence” is opposed to an aggressive status of active toxin production during acute S. aureus disease. It is noteworthy in that regard that many colonizing S. aureus isolates were shown to be defective in the global virulence regulator Agr (Shopsin et al., 2008). Interestingly, there is evidence to suggest that increased capacity to form biofilms and adhere to epithelial cells may have been linked for example to the spread of the so-called Brazilian MRSA clone (ST239) (Amaral et al., 2005), the likely predecessor of the Chinese sasX-positive ST239 strains.
Diseases caused by MRSA
The most common diseases caused by MRSA in the hospital are not different from those caused by MSSA and mostly include skin, soft-tissue, respiratory, bone, joint, and endovascular infections, and sepsis (Lowy, 1998). Furthermore, together with coagulase-negative staphylococci, S. aureus is the most common cause of infections on indwelling medical devices. The mortality rate of severe, invasive MRSA infections is about 20% and it has been estimated that MRSA infections are the leading cause of death by a single infectious agent in the US, exceeding deaths caused by HIV/AIDS (Klevens et al., 2007). Notably, MRSA as opposed to MSSA infection is associated with significantly higher costs, due to prolonged stay in the hospital, and mortality (Hanberger et al., 2011).
Virulence
Virulence of S. aureus is multi-factorial, dependent on a series of toxins, adhesion, immune evasion and other virulence determinants; the same is true for MRSA strains. In the following, determinants that have been linked to the virulence of specific MRSA strains will be highlighted; and it will be discussed which factors are responsible for the pathogenic success of CA-MRSA strains in particular.
Toxins
Differences in the toxin repertoire between S. aureus clones are common and due to the fact that many S. aureus toxins and other virulence determinants are encoded on MGEs, whose presence varies considerably between strains. Such MGE-encoded toxins include superantigens such as toxic shock syndrome toxin (TSST), some leukotoxins such as Panton-Valentine leukocidin, and exfoliative toxins. In contrast, α-toxin, γ-toxin, some leukotoxins, and phenol-soluble modulins (PSMs) are produced by most strains. Nevertheless, differential expression of such core-genome encoded toxin genes may also cause significant differences in the pathogenic potential between S. aureus strains. A particularly dramatic difference is seen in mutants that are functionally defective in Agr, which controls the expression of many S. aureus toxin genes (Novick et al., 1993).
Among hospital-associated MRSA strains, there are a few examples of lineages that are characterized by harboring specific toxin genes. For example, TSST is associated with the CC30/USA200 lineage, but its role in CC30 pathogenicity awaits detailed investigation. It is interesting that present isolates of the CC30 lineage carry mutations in the agrC and hla (α-toxin) loci, which significantly reduce acute virulence (DeLeo et al., 2011). As CC30 is the second most predominant HA-MRSA lineage in the U. S., it may be concluded that sustained establishment of an MRSA clone in hospital settings involves reduced aggressive toxicity. Nevertheless, the agrC mutation in contemporary CC30 clones (such as EMRSA-16, representative clone Sanger 252) only slightly reduces Agr activity; and EMRSA-16 still produces toxins such as δ-toxin at considerable levels (Cheung et al., 2011). Notably, EMRSA-16 also produces significant amounts of PSM-mec.
PSM-mec is a member of the PSM class of S. aureus peptide toxins, but in contrast to all other known PSMs it is encoded on MGEs, namely SCCmec elements of types 2,3, and 8, and thus correlated with specific MRSA lineages (Chatterjee et al., 2011, Queck et al., 2009). PSM-mec has cytolytic activity toward neutrophils and erythrocytes. It is a significant determinant of S. aureus infection, at least in strains that produce high relative amounts of PSM-mec compared to other potent PSM cytolysins.
Surface proteins
Surface proteins have multiple important roles in S. aureus pathogenesis. In addition to key functions in bacterial cell wall metabolism, they serve to bind to host tissue, facilitate internalization and immune evasion, and are involved in bacterial aggregation and biofilm formation (Foster et al., 1998). Most surface proteins are encoded on the core genome. Therefore, the specific success of an MRSA clone has not been linked to a specific surface protein. The SasX protein already mentioned above is an exception, inasmuch as it is encoded on an MGE and in addition to its role in colonization, has a significant impact on virulence of SasX-positive MRSA. Mechanistically, the impact of SasX on virulence may at least in part be due to promoting bacterial aggregation, leading to decreased neutrophil phagocytosis (Li et al., 2012).
Regulators
Global regulatory systems, such as Agr, SarA, SaeRS, etc. govern a multitude of different aspects of staphylococcal physiology, including many virulence traits. Agr has been known for a long time as a pivotal regulator of virulence, with a positive effect on toxin and a negative effect on the expression of surface proteins (Queck et al., 2009). However, more recent data indicate that Agr may also positively regulate surface proteins, possibly in a strain-dependent fashion (Cheung et al., 2011).
International guidelines define methicillin resistance as resistance above 4 mg/l (using oxacillin), and susceptible below 2 mg/l. However, resistance levels may differ significantly between strains. The mechanisms leading to these strongly varying resistance levels in the presence of mecA are poorly understood. Recent results suggest that core genome-encoded regulatory genes may influence mecA expression, such as for example Agr (Cheung et al., 2011). On the other hand, Agr expression is impacted by the mec locus (Rudkin et al., 2012).
Community-associated MRSA
Until the 1990s, MRSA was exclusively a hospital-associated pathogen. Then, cases of MRSA community infections were documented in individuals in the U.S. without any history of hospitalization (CDC, 1999). These first cases were fatal, but most CA-MRSA infections present as moderately severe infections of the skin and soft tissues. Notably, CA-MRSA may infect healthy adult individuals without predisposing conditions. Nowadays, CA-MRSA has emerged all over the world. Global CA-MRSA lineages cover the whole diversity of S. aureus as a species (DeLeo et al., 2009). Notably, the U. S. is experiencing the most pronounced CA-MRSA epidemic, which is almost entirely due to strain USA300. In 2006, one specific clone of USA300 was reported to be the single most frequent cause of skin and soft tissue infections reporting to U.S. emergency departments. The extraordinary success of CA-MRSA strains, in particular USA300, is believed to be due to higher virulence and increased transmissibility characteristics, as compared to traditional HA-MRSA. While not much is known about transmissibility, there has been intensive research in the last decade on CA-MRSA virulence (Otto, 2010). However, the molecular basis of CA-MRSA virulence is still a matter of controversy.
CA-MRSA transmissibility
The sustained spread of CA-MRSA may in principle be due only to the shear number of infections and direct transmission from infected patients, and thus to infectivity or virulence. However, CA-MRSA may also show increased transmissibility and colonization characteristics. CA-MRSA isolates carry SCCmec elements of type 4 or 5, which as a result of their smaller sizes compared to other SCCmec elements may be associated with lower fitness costs. Otherwise, there are only limited data assigning potentially increased colonization capacity to a molecular factor uniquely present in CA-MRSA. The arginine catabolic mobile genetic element (ACME) of USA300 harbors a spermidin acetyltransferase gene (speG) that transfers resistance to spermidin and other polyamines, molecules, which are produced by most living cells except for S. aureus (Joshi et al., 2011). The exceptional sensitivity that S. aureus has to polyamines is only abolished in ACME-containing USA300 strains. Thus, speG may serve to explain augmented colonization capacity in USA300. However, other CA-MRSA, including some South American clones of USA300, lack ACME. Moreover, ACME harbors an arginine deiminase and an oligopeptide gene cluster, which have been implicated in colonization capacity, but there are no clear experimental data supporting this hypothesis (Otto, 2010). Finally, CA-MRSA strains may express and regulate surface adhesins in a manner different from other strains, but whether this translates to increased colonization capacity is now known (Cheung et al., 2011).
CA-MRSA virulence
The theory that increased virulence, as compared to HA-MRSA strains, is accountable for the ability of CA-MRSA strains to infect healthy people was confirmed in animal infection models and is generally accepted. Increased virulence of CA-MRSA strains may be due to a considerable extent to the fact that they exhibit high capacity to circumvent killing by human neutrophils, the first line of defense of the human body against staphylococcal infections. However, there has been much debate about which mechanisms are responsible for the exceptional ability of CA-MRSA strains to evade human immune defenses and lead to infection in healthy hosts. Two main hypotheses have been discussed. The first hypothesis attributes increased CA-MRSA virulence to the acquisition of MGEs, namely that containing Panton-Valentine leukocidin (PVL) (Vandenesch et al., 2003), while the other explains this by increased expression of core genome-encoded virulence genes, such as phenol-soluble modulin (PSM) cytolysins, α-toxin, and other virulence determinants (Li et al., 2009). Notably, these two explanations are not mutually exclusive.
PVL
PVL is a member of the bi-component family of staphylococcal leukocidins. It has been associated early with specific forms of skin infections, such as furunculosis. At the beginning of the CA-MRSA epidemic, the PVL genes lukS and lukF were found in virtually all CA-MRSA clones, while PVL is typically absent from HA-MRSA (Vandenesch et al., 2003). Thus, researchers focused on PVL as a potential cause of increased CA-MRSA virulence. However, two main findings have cast severe doubt on a significant role of PVL as a virulence factor in CA-MRSA disease (Otto, 2010). First, CA-MRSA clones have emerged in the meantime that lack PVL genes and still show significant virulence. Second, analysis of isogenic PVL gene deletion mutants did not confirm a substantial impact of PVL on CA-MRSA virulence in several animal models.
As among neutrophils of commonly used model animals, only those of rabbits show considerable sensitivity to PVL, rabbit models are now used to investigate the contribution of PVL to virulence. Results from rabbit models of severe lung infection and osteomyelitis indicated a significant role of PVL in the pathogenesis of these disease types (Diep et al., 2010, Cremieux et al., 2009). Contrastingly, two studies failed to detect an impact of PVL to CA-MRSA skin infection in rabbits. In the first study, which used isogenic USA300 deletion mutants, PVL failed to show an impact on virulence, whereas other toxins (PSMα, α-toxin) showed strong effects (Kobayashi et al., 2011). In the second study, PVL-negative CA-MRSA strains showed virulence characteristics similar to those of PVL-positive strains (Li et al., 2010). Then again, another group reported a moderate yet significant impact of PVL on virulence in rabbit skin infection, but they did not compare with the effects of other toxins (Lipinska et al., 2011).
Overall, PVL does not appear to exert the dramatic effects on CA-MRSA virulence that it was first believed to have. Furthermore, given the only minor or absent impact it has on skin infections, it is hard to imagine how PVL could be a significant factor driving the CA-MRSA epidemic. Nevertheless, because different groups have achieved contrasting results until very recently, the role of PVL in CA-MRSA infection is still a matter of debate.
PSMs, α-toxin, and other core-genome encoded toxins
PSMs are a family of amphipathic peptides, some of which show pronounced cytolytic capacities, in particular to human neutrophils (Wang et al., 2007). The PSMα peptides of S. aureus have a considerable impact on virulence of CA-MRSA strains in mouse and rabbit skin infection models. While psm genes are present in all S. aureus strains, CA-MRSA strains in average show increased PSM production compared to HA-MRSA strains (Li et al., 2009). Further, the cytolysin α-toxin significantly affects CA-MRSA virulence in skin and lung infection models (Bubeck Wardenburg et al., 2007, Kobayashi et al., 2011). While not cytolytic toward human neutrophils, α-toxin lyses a series of other cells, such as erythrocytes and macrophages, and facilitates breach of the epithelial barrier, at least in part mediated by interaction with the ADAM10 receptor (Hanberger et al., 2011). Finally, a core genome-encoded superantigen, SElX, was recently described, which contributes to CA-MRSA necrotizing pneumonia, as demonstrated in a rabbit lung infection model (Wilson et al., 2011).
As most S. aureus strains carry genes for these toxins, increased virulence that is dependent on those factors must be mediated by increased expression. High expression of PSMs, α-toxin, and additional virulence determinants such as proteases has been shown to occur in the lineage that leads to the USA300 clone, which includes the USA300 predecessor USA500 (Li et al., 2009). Likely, one reason for increased virulence factor expression in that lineage is increased activity of Agr. However, the exceptional virulence potential of USA300 and other CA-MRSA clones can certainly not be explained by increased activity of Agr alone. Presumably, genetic rearrangements that are yet poorly understood have led to clones that are highly virulent and exceptionally good colonizers. Most research has been performed on strain USA300, but it is possible that other CA-MRSA clones underwent different changes to obtain these phenotypic features. Additionally, at least some CA-MRSA clones such as USA300 may have been optimized in virulence and colonization capacities by acquiring molecular determinants on MGEs, such as ACME and the genes coding for PVL (Fig. 2).
Fig. 2. Potential factors contributing to the pathogenic success of CA-MRSA.
Molecular factors being discussed as potential contributors to the sustained spread and pathogenic success of CA-MRSA, especially the USA300 clone, can be categorized into those facilitating pathogen virulence, fitness, or colonization. CA-MRSA may show unique gene presence after acquisition of genes via MGEs (shown in blue) or increased expression of core genome-encoded genes (shown in red). Increased expression of virulence determinants may at least in part be due to higher activity of the virulence regulator Agr. The PVL genes were acquired by many CA-MRSA strains via acquisition of an MGE, but PVL expression is also dependent on Agr. Both mechanisms may thus account for the discussed exceptional role of PVL in CA-MRSA virulence (shown in purple). In animal models, Agr, α-toxin, PSMα peptides, and SelX showed significant contributions to virulence. In the case of PVL, virulence contribution may depend on the type of infection. The contribution of PVL to skin infections as the most frequent type of CA-MRSA disease is controversial. SCCmec type 4 does not appear to lead to increased fitness compared to other SCCmec types during infection, but its contribution to increased fitness during colonization has not yet been investigated in appropriate models. No animal data are available for the role of speG in infection or colonization.
Concluding remarks
Novel MRSA clones keep occurring in hospitals and more recently in the community, often causing sustained epidemics. We are now beginning to understand how S. aureus optimizes gene content and expression to create such new strains with optimized virulence and colonization capacities. In addition, MRSA strains are constantly accumulating further antibiotic resistance genes, creating highly virulent and hardly treatable “superbugs”. It is therefore an important task of current S. aureus pathogenesis research to delineate factors that define virulence of the entire range of infectious MRSA strains, in order to generate drugs with as broad and long-lasting an anti-staphylococcal potential as possible. This should happen simultaneously to maintained efforts aiming to find new, efficient antibiotics.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), NIH.
References
- Abraham EP, Chain E. An enzyme from bacteria able to destroy penicillin. Nature. 1940;146:837. [PubMed] [Google Scholar]
- Amaral MM, Coelho LR, Flores RP, Souza RR, Silva-Carvalho MC, Teixeira LA, et al. The predominant variant of the Brazilian epidemic clonal complex of methicillin-resistant Staphylococcus aureus has an enhanced ability to produce biofilm and to adhere to and invade airway epithelial cells. J Infect Dis. 2005;192:801–810. doi: 10.1086/432515. [DOI] [PubMed] [Google Scholar]
- Ammerlaan HS, Kluytmans JA, Wertheim HF, Nouwen JL, Bonten MJ. Eradication of methicillin-resistant Staphylococcus aureus carriage: a systematic review. Clin Infect Dis. 2009;48:922–930. doi: 10.1086/597291. [DOI] [PubMed] [Google Scholar]
- Barber M, Rozwadowska–Dowzenko M. Infection by penicillin-resistant staphylococci. Lancet. 1948;2:641–644. doi: 10.1016/s0140-6736(48)92166-7. [DOI] [PubMed] [Google Scholar]
- Bubeck Wardenburg J, Bae T, Otto M, DeLeo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- CDC. From the Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus--Minnesota and North Dakota, 1997–1999. Jama. 1999;282:1123–1125. [PubMed] [Google Scholar]
- CDC. Staphylococcus aureus resistant to vancomycin--United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:565–567. [PubMed] [Google Scholar]
- Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee SS, Chen L, Joo HS, Cheung GY, Kreiswirth BN, Otto M. Distribution and regulation of the mobile genetic element-encoded phenol-soluble modulin PSM-mec in methicillin-resistant Staphylococcus aureus. PLoS One. 2011;6:e28781. doi: 10.1371/journal.pone.0028781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun. 2011;79:1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremieux AC, Dumitrescu O, Lina G, Vallee C, Cote JF, Muffat-Joly M, et al. Panton-valentine leukocidin enhances the severity of community-associated methicillin-resistant Staphylococcus aureus rabbit osteomyelitis. PLoS One. 2009;4:e7204. doi: 10.1371/journal.pone.0007204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo FR, Chambers HF. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clin Invest. 2009;119:2464–2474. doi: 10.1172/JCI38226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo FR, Kennedy AD, Chen L, Bubeck Wardenburg J, Kobayashi SD, Mathema B, et al. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc Natl Acad Sci U S A. 2011;108:18091–18096. doi: 10.1073/pnas.1111084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, Beach M. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32(Suppl 2):S114–132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- Diep BA, Chambers HF, Graber CJ, Szumowski JD, Miller LG, Han LL, et al. Emergence of Multidrug-Resistant, Community-Associated, Methicillin-Resistant Staphylococcus aureus Clone USA300 in Men Who Have Sex with Men. Ann Intern Med. 2008;148:249–257. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A. 2010;107:5587–5592. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TJ, Hook M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–488. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- Hanberger H, Walther S, Leone M, Barie PS, Rello J, Lipman J, et al. Increased mortality associated with meticillin-resistant Staphylococcus aureus (MRSA) infection in the intensive care unit: results from the EPIC II study. Int J Antimicrob Agents. 2011;38:331–335. doi: 10.1016/j.ijantimicag.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Hartman B, Tomasz A. Altered penicillin-binding proteins in methicillin-resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1981;19:726–735. doi: 10.1128/aac.19.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K, Cui L, Kuroda M, Ito T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001;9:486–493. doi: 10.1016/s0966-842x(01)02175-8. [DOI] [PubMed] [Google Scholar]
- Jevons MP, Coe AW, Parker MT. Methicillin resistance in staphylococci. Lancet. 1963;1:904–907. doi: 10.1016/s0140-6736(63)91687-8. [DOI] [PubMed] [Google Scholar]
- Joshi GS, Spontak JS, Klapper DG, Richardson AR. Arginine catabolic mobile element encoded speG abrogates the unique hypersensitivity of Staphylococcus aureus to exogenous polyamines. Mol Microbiol. 2011;82:9–20. doi: 10.1111/j.1365-2958.2011.07809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2000;44:1549–1555. doi: 10.1128/aac.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Jama. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, et al. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis. 2011;204:937–941. doi: 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Cheung GY, Hu J, Wang D, Joo HS, Deleo FR, Otto M. Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis. 2010;202:1866–1876. doi: 10.1086/657419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A. 2009;106:5883–5888. doi: 10.1073/pnas.0900743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Du X, Villaruz AE, Diep BA, Wang D, Song Y, et al. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat Med. 2012 doi: 10.1038/nm.2692. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska U, Hermans K, Meulemans L, Dumitrescu O, Badiou C, Duchateau L, et al. Panton-Valentine leukocidin does play a role in the early stage of Staphylococcus aureus skin infections: a rabbit model. PLoS One. 2011;6:e22864. doi: 10.1371/journal.pone.0022864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Miyake K, Misawa S, Kuno Y, Horii T, Hori S, et al. Association between antimicrobial consumption and clinical isolates of methicillin-resistant Staphylococcus aureus: a 14-year study. J Infect Chemother. 2012;18:90–95. doi: 10.1007/s10156-011-0302-6. [DOI] [PubMed] [Google Scholar]
- Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. Embo J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien LM, Walsh EJ, Massey RC, Peacock SJ, Foster TJ. Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: implications for nasal colonization. Cell Microbiol. 2002;4:759–770. doi: 10.1046/j.1462-5822.2002.00231.x. [DOI] [PubMed] [Google Scholar]
- Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008;322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. Basis of Virulence in Community–Associated Methicillin-Resistant Staphylococcus aureus. Annu Rev Microbiol. 2010;64:143–162. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- Peschel A. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 2002;10:179–186. doi: 10.1016/s0966-842x(02)02333-8. [DOI] [PubMed] [Google Scholar]
- Queck SY, Khan BA, Wang R, Bach TH, Kretschmer D, Chen L, et al. Mobile genetic element-encoded cytolysin connects virulence to methicillin resistance in MRSA. PLoS Pathog. 2009;5:e1000533. doi: 10.1371/journal.ppat.1000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammelkamp CH, Maxon T. Resistance of Staphylococcus aureus to the action of penicillin. Proc Royal Soc Exper Biol Med. 1942;51:386–389. [Google Scholar]
- Roche FM, Meehan M, Foster TJ. The Staphylococcus aureus surface protein SasG and its homologues promote bacterial adherence to human desquamated nasal epithelial cells. Microbiology. 2003;149:2759–2767. doi: 10.1099/mic.0.26412-0. [DOI] [PubMed] [Google Scholar]
- Roundtree PM, Freeman BM. Infections caused by a particular phage type of Staphylococcus aureus. Med J Aust. 1956;42:157–161. [PubMed] [Google Scholar]
- Rudkin JK, Edwards AM, Bowden MG, Brown EL, Pozzi C, Waters EM, et al. Methicillin resistance reduces the virulence of healthcare-associated methicillin-resistant Staphylococcus aureus by interfering with the agr quorum sensing system. J Infect Dis. 2012;205:798–806. doi: 10.1093/infdis/jir845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shopsin B, Drlica-Wagner A, Mathema B, Adhikari RP, Kreiswirth BN, Novick RP. Prevalence of agr dysfunction among colonizing Staphylococcus aureus strains. J Infect Dis. 2008;198:1171–1174. doi: 10.1086/592051. [DOI] [PubMed] [Google Scholar]
- Shorr AF. Epidemiology of staphylococcal resistance. Clin Infect Dis. 2007;45(Suppl 3):S171–176. doi: 10.1086/519473. [DOI] [PubMed] [Google Scholar]
- Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, Gross M, et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med. 2004;10:243–245. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004;364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- Wilson GJ, Seo KS, Cartwright RA, Connelley T, Chuang-Smith ON, Merriman JA, et al. A novel core genome-encoded superantigen contributes to lethality of community- associated MRSA necrotizing pneumonia. PLoS Pathog. 2011;7:e1002271. doi: 10.1371/journal.ppat.1002271. [DOI] [PMC free article] [PubMed] [Google Scholar]