Abstract
Porcine circovirus associated disease (PCVAD) is currently one of the most economically important diseases in the global swine industry. Porcine circovirus type 2 (PCV2) is the primary causative agent, however co-infection with other swine pathogens such as porcine reproductive and respiratory syndrome virus (PRRSV) is often required to induce the full spectrum of clinical PCVAD. While the specific mechanisms of viral co-infection that lead to clinical disease are not fully understood, immune modulation by the co-infecting viruses likely plays a critical role. We evaluated the ability of dendritic cells (DC) infected with PRRSV, PCV2, or both to induce regulatory T cells (Tregs) in vitro. DCs infected with PCV2 significantly increased CD4+CD25+FoxP3+ Tregs (p < 0.05) and DCs co-infected with PRRSV and PCV2 induced significantly higher numbers of Tregs than with PCV2 alone (p < 0.05). Cytokine analysis indicated that the induction of Tregs by co-infected DCs may be dependent on TGF-β and not IL-10. Our data support the immunomodulatory role of PCV2/PRRSV co-infection in the pathogenesis of PCVAD, specifically via Treg-mediated immunosuppression.
Keywords: Porcine reproductive and respiratory syndrome virus (PRRSV), Porcine circovirus type 2 (PCV2), Dendritic cells, Porcine circovirus associated disease (PCVAD), Regulatory T cell, Co-infection
1. Introduction
Porcine circovirus associated disease (PCVAD), commonly known as porcine circovirus disease (PCVD) in Europe, is arguably the most economically significant disease facing the global swine industry today. This disease has devastated every major swine producing country in the world and has culminated in losses of up to twenty dollars per pig in the United States swine population (Gillespie et al., 2009). While PCVAD encompasses a spectrum of clinical syndromes, all are unified by the presence of porcine circovirus type 2 (PCV2) as the primary causative agent. PCV2 is a small, non-enveloped, single-stranded, circular DNA virus belonging to the family Circoviridae (Todd et al., 2005). PCVAD requires the presence of PCV2, but PCV2 infection alone rarely produces the full spectrum or severity of clinical disease. Other co-infecting pathogens, such as porcine reproductive and respiratory syndrome virus (PRRSV), porcine parvovirus, swine influenza virus, Mycoplasma hyopneumoniae, and Torque teno sus virus (TTSuV) augment the severity of clinical disease and result in increased PCV2 viral load in infected pigs (Opriessnig and Halbur, 2012). The exact mechanisms by which viral or bacterial co-infection with PCV2 potentiates clinical PCVAD are unknown, but modulation of the host immune system is likely a key event in the pathogenesis of this disease (Gillespie et al., 2009, Krakowka et al., 2001).
Virus-mediated induction of regulatory T cells (Tregs) is one specific mechanism of modulating the host immune response in favor of maintaining viral infection (Belkaid, 2007). Tregs are broadly divided into natural Tregs, originating from the thymus, and inducible (adaptive) Tregs, derived outside the thymus from naïve CD4+ T cells (Askenasy et al., 2008). Although the phenotype of Tregs is variable, CD4+CD25+FoxP3+ cells exhibiting suppressor activity by a variety of mechanisms have been identified in pigs (Kaser et al., 2008a, Kaser et al., 2008b, Kaser et al., 2011). By altering the host immune response to viral infection, Tregs contribute to persistent infection of many viruses including Friend virus, herpes simplex virus, hepatitis C virus, hepatitis B virus, human immunodeficiency virus, feline immunodeficiency virus, simian immunodeficiency virus, cytomegalovirus, and Epstein–Barr virus (Belkaid, 2007, Li et al., 2008, Rouse et al., 2006).
Recently, PRRSV has been shown to induce Tregs both in vitro and in vivo (LeRoith et al., 2011, Silva-Campa et al., 2009, Wongyanin et al., 2010, Wongyanin et al., 2012), although, to our knowledge, Treg induction by PCV2 has not been described. PRRSV-mediated Treg induction appears to vary depending on the virus genotype (Silva-Campa et al., 2010). Since co-infection with PCV2 and PRRSV is very common in the swine population, and since pigs co-infected with PCV2 and PRRSV have reduced IFN-γ and increased IL-10 expression in peripheral blood mononuclear cells (PBMC) (Shi et al., 2010), we hypothesize that co-infection should induce higher numbers of Tregs in vitro.
2. Materials and methods
2.1. Viruses
PRRSV isolate ATCC VR2385 (Meng et al., 1994) and PCV2a isolate ISU-40895 (Fenaux et al., 2000) were used in this study. PRRSV VR2385 was propagated in confluent monolayers of MARC-145 cells. The viral titer was determined by an immunofluorescent assay (IFA) with an anti-PRRSV N antibody (SDOW17) and quantified in fluorescent focus-forming units (FFU) as described previously (Fang et al., 2006). Infectious virus stocks were generated for PCV2a by transfection of PK-15 cells in T25 flasks with Lipofectamine LTX (Invitrogen) using a dimerized infectious DNA clone as described previously (Fenaux et al., 2002, Fenaux et al., 2003). The 50% tissue culture infective dose (TCID50) per ml was calculated according to the method of Reed and Muench (1938).
2.2. Isolation of PBMCs and generation of monocyte-derived dendritic cells
Peripheral blood samples from five PRRSV- and PCV2-free pigs were collected into heparinized syringes, diluted 1:2 with sterile PBS, overlaid on Ficoll-Paque™ (GE Healthcare, Piscataway, NJ) and PBMCs were collected as previously described (Silva-Campa et al., 2009). Porcine monocyte-derived dendritic cells (DC) were generated as previously described for pig 1 (Wang et al., 2007). Briefly, CD14-positive monocytes were purified from PBMCs by immunomagnetic labeling of cells using mouse anti-swine CD14 monoclonal antibody (R&D Systems, Minneapolis, MN) and goat-anti-mouse IgG microbeads (Miltenyi Biotec, Auburn, CA). The CD14-positive monocytes were cultured for 5 days in complete medium supplemented with 20 ng/ml recombinant porcine IL-4 and 20 ng/ml recombinant porcine GM-CSF (Cell Sciences, Canton, MA). Due to low DC yields by this method, an alternative technique for generating DCs was employed for pigs 2–5 as previously reported (Carrasco et al., 2001, Flores-Mendoza et al., 2008). Freshly isolated PBMCs were seeded in T75 tissue culture flasks and incubated overnight in complete medium at 37 °C with 5% CO2 to allow monocytes to adhere. Non-adherent cells were removed and frozen in fetal bovine serum containing 10% dimethyl sulfoxide (DMSO). Adherent cells were cultured at 37 °C with 5% CO2 in complete medium supplemented with 20 ng/ml recombinant porcine IL-4 and 20 ng/ml recombinant porcine GM-CSF. After 5 days, DCs were harvested using Cellstripper™, an enzyme-free cell dissociation medium (Cellgro, Manassas, VA). DC differentiation from both methods was confirmed by typical veiled morphology and phenotyping with monoclonal antibodies specific for MHC I, MHC II, CD 172 (SWC3), CD 14, and CD 1 (Wang et al., 2007).
2.3. Infection of DCs by PRRSV and PCV2
DCs were inoculated with PRRSV at a multiplicity of infection (m.o.i.) of 0.1 as previously described (Silva-Campa et al., 2009), and with PCV2a at a m.o.i. of 0.01 (Vincent et al., 2003), or with both viruses and incubated for 1 h at 37 °C. Cells were then washed twice with complete medium and seeded into 96-well tissue culture plates at 5 × 104 cells per well. After 24 h of incubation at 37 °C with 5% CO2, approximately 5 × 105 lymphocytes (CD14-negative cell fraction for pig 1, non-adherent cells from an overnight culture of PBMCs for pigs 2–5) were added and co-cultured for 3 days. DC-lymphocyte co-cultures were performed in triplicate for each treatment group per pig.
2.4. Immunofluorescence assay (IFA) to determine PCV2 and PRRSV infectivity
DCs were inoculated with PCV2, PRRSV, or both as described above and infected cells were seeded in 35 mm glass bottom dishes (MatTek Corporation, Ashland, MA). DCs were incubated for 36 h at 37 °C with 5% CO2, and the cells were then fixed with 80% acetone. After washing with PBS buffer, cells were sequentially incubated with an anti-PRRSV N monoclonal antibody SDOW17 and swine anti-PCV2 polyclonal serum followed by FITC conjugated goat anti-swine antibody (KPL, Gaithersburg, MD) and Alexa-fluor647 conjugated goat anti-mouse antibody (Invitrogen, Carlsbad, CA). The cells were then washed with PBS, covered with VECTASHIELD mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and imaged using a Nikon TE2000-E confocal microscope.
2.5. Flow cytometry
Following 3 days of co-culture with virus-infected or uninfected control DCs, lymphocytes were evaluated for their expression of CD4, CD25, and FoxP3 using flow cytometry. Briefly, cells were sequentially stained with mouse anti-porcine CD4 (VMRD, Pullman, WA), goat anti-mouse IgG2b:Alexa-fluor647 (Invitrogen, Carlsbad, CA), mouse anti-porcine CD25 (AbD Serotec, Raleigh, NC), and goat anti-mouse IgG:FITC (AbD Serotec, Raleigh, NC). For intracellular staining, cells were permeabilized with a FoxP3 permeabilization/fixation buffer kit followed by staining with anti-mouse/rat FoxP3:PE that reacts with porcine FoxP3 (eBioscience Inc., San Diego, CA). Flow cytometric analysis was conducted on the lymphocytes using a FACSCalibur cytometer (Becton-Dickinson Biosciences, San Jose, CA) and analyzed using FlowJo 7.6.3 software. Dendritic cells were excluded based on forward and side scatter and 10,000 events (lymphocytes) were examined per replicate sample.
2.6. ELISA
Cell culture supernatants were collected following three-day co-culture of lymphocytes and virus-infected DCs. Levels of secreted IL-10 and TGF-β were quantified using commercial ELISA kits according to the manufacturer's recommendations (R&D Systems, Minneapolis, MN).
2.7. Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA). Differences among treatment groups were determined by Tukey–Kramer or paired t-test. Data analysis was performed using JMP 8.0 (SAS Institute Inc., Cary, NC). Differences were considered to be statistically significant where p < 0.05.
3. Results
3.1. Detection of viral antigen in DCs inoculated with PCV2 and PRRSV
An IFA assay was employed to confirm PRRSV and PCV2 infectivity of porcine DCs. PCV2 antigen was visualized as diffuse cytoplasmic staining with no viral antigen visible in the nucleus (Fig. 1A and D). Punctate cytoplasmic and nuclear staining were observed using a PRRSV N-protein-specific monoclonal antibody (Fig. 1F and H). Positive staining for PCV2 and PRRSV was visualized in DCs from both single virus and co-infected groups. In the co-infected group, no DCs exhibiting concurrent staining for both viruses were seen.
Fig. 1.
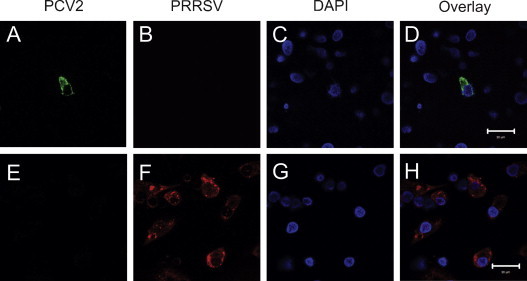
Porcine monocyte-derived dendritic cells 36 h post-inoculation with PCV2 and PRRSV. DCs were immunostained with anti-PCV2 polyclonal antibody (green) and diffuse cytoplasmic immunoreactivity was observed (A and D). DCs were stained with a PRRSV N-protein-specific monoclonal antibody (red) and punctate cytoplasmic and nuclear immunoreactivity were seen (F and H).
3.2. DCs infected with PRRSV, PCV2, or both viruses induce CD4+CD25+FoxP3+ Tregs
Following three days co-culture with virus-infected DCs, lymphocytes were evaluated for CD4, CD25, and FoxP3 expression via tri-color flow cytometry. Following exposure to PCV2-infected DCs, CD4+CD25+FoxP3+ Tregs were significantly increased in two pigs compared to uninfected controls (Fig. 2B). PRRSV-infected DCs induced a significant increase in Tregs compared to uninfected controls in only one pig. DCs co-infected with PRRSV and PCV2 significantly induced Tregs compared to the uninfected controls in all pigs (Fig. 2A and B). In pig 4, one-way analysis of variance revealed no statistically significant difference among treatment groups (p < 0.05), which is likely due to high variance in the control triplicate samples. However, when the PRRSV/PCV2 co-infected group was compared directly to the uninfected control group using a paired t-test, the mean percentage of CD4+CD25+FoxP3+ Tregs in the co-infected group was significantly higher than that in the uninfected control group (p = 0.015). Furthermore, PRRSV/PCV2 co-infected DCs induced significantly higher CD4+CD25+FoxP3+ Treg percentages than either virus alone in three of five pigs (Fig. 2B).
Fig. 2.
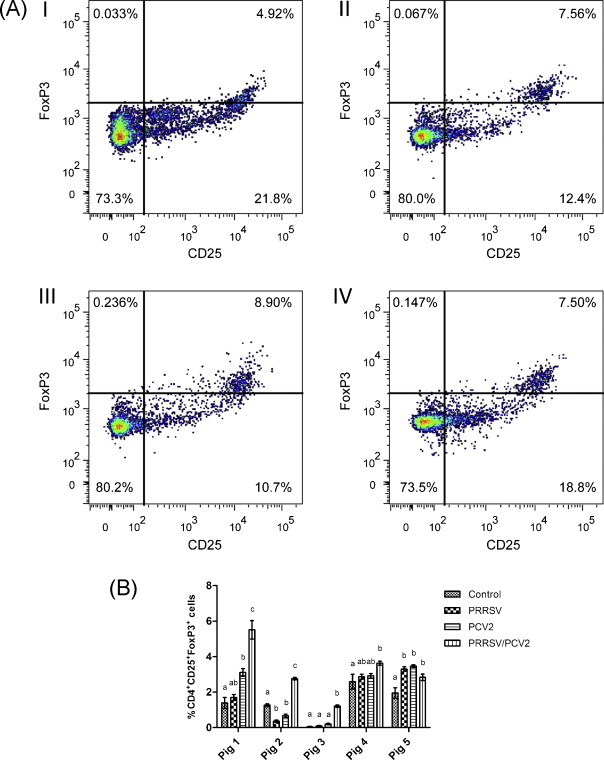
(A) Representative flow cytometry profile of lymphocytes following 3-day co-culture with virus infected DCs. CD4-gated lymphocytes expressing CD25+ and FoxP3+ are shown. (I) Uninfected control DCs; (II) PRRSV-infected DCs; (III) PCV2-infected DCs; and (IV) PRRSV/PCV2 co-infected DCs. (B) Mean CD4+CD25+FoxP3+ T cells ± standard error of the mean as a percentage of lymphocytes co-cultured with DCs infected with PRRSV, PCV2 or both viruses. Data represent three replicates per group from five independent experiments (n = 5 pigs). Data not connected by the same letter are significantly different, p < 0.05.
3.3. TGF-β is up-regulated following co-infection with PCV2 and PRRSV
IL-10 and TGF-β in cell culture supernatant from co-cultured DCs and lymphocytes were quantified using commercial ELISA kits. Compared to infection with either PRRSV or PCV2 alone, co-culture of lymphocytes with PRRSV/PCV2 co-infected DCs induced significantly (p < 0.05) higher levels of TGF-β (Fig. 3 A). Due to high levels of latent TGF-β in the cell culture media resulting from the addition of FBS, there was variability in TGF-β among pigs resulting from different lots of FBS used between experiments. Therefore, the quantity of TGF-β in cell-culture supernatant from the virus-infected groups was standardized against the uninfected control group for each pig and reported as relative levels. No significant difference in IL-10 levels among uninfected control or treatment groups was seen (Fig. 3B).
Fig. 3.
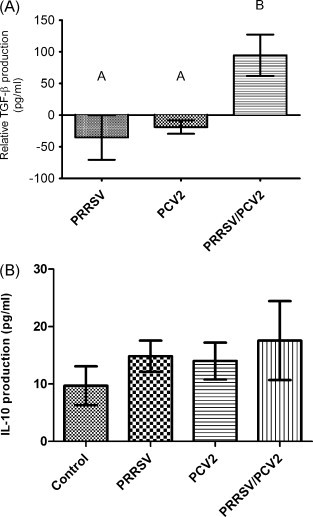
Cytokine levels of TGF-β (A) and IL-10 (B) from cell culture supernatants following 3-day co-culture of lymphocytes with virus-infected DCs were quantified by ELISA. The data is from five independent experiments (n = 5 pigs). TGF-β levels are expressed relative to the uninfected control group for each experiment. Data not connected by the same letter are significantly different, p < 0.05.
4. Discussion
Our results further confirm a previous report that following in vitro PCV2 infection of DCs, PCV2 antigen was detectable only in the cytoplasm but not in the nucleus (Vincent et al., 2003). This indicates that PCV2 persists in DCs although reportedly there is no evidence of viral replication, transmission of virus to activated syngeneic T lymphocytes or cell death (Steiner et al., 2008, Vincent et al., 2003, Vincent et al., 2005). Similarly, PRRSV antigen was detected following in vitro infection of DCs as previously described (Wang et al., 2007). In the present study no DCs were visible that were concurrently expressing PCV2 and PRRSV antigen. However, given the low m.o.i. of PCV2 this does not completely preclude the possibility that PCV2 and PRRSV can co-infect the same DC.
Modulation of the immune system is considered to play a critical role in the pathogenesis of PCVAD (Gillespie et al., 2009, Opriessnig et al., 2007). In addition, co-infection with PCV2 and PRRSV is one of the major contributors to development of clinical PCVAD (Opriessnig et al., 2007). However, the specific means by which PRRSV/PCV2 co-infection modulates the immune response in PCVAD is currently unknown. One potential mechanism is virus-mediated induction of Tregs. It has been reported that pigs co-infected with PCV2 and PRRSV have more severe lymphoid depletion and enhanced PCV2 replication and tissue distribution (Allan and Ellis, 2000, Harms et al., 2001, Rovira et al., 2002). While protective immunity against PCV2 is associated with neutralizing antibody and IFN-γ production (Meerts et al., 2005), Tregs decrease the IFN-γ response, block migration and proliferation of effector T cells, and inhibit IL-2 production (Askenasy et al., 2008). Therefore, PCV2/PRRSV-mediated activation of Tregs, as demonstrated in this study, may dampen the immune responses to PCV2, resulting in increased viral replication and clinical disease. In support of this model, PRRSV/PCV2 co-infection has been shown to upregulate IL-10 expression while suppressing IL-2, IL-4, IL-6, IL-12p40 and IFN-γ (Shi et al., 2010).
In the present study, 3-day co-culture of lymphocytes with virus-infected DCs was chosen, as opposed to a 5-day co-culture as previously described (Silva-Campa et al., 2009). This was due to morphologic evidence of cytolysis of DCs beginning approximately 5 days post-inoculation with PRRSV strain VR-2385. DCs did not exhibit morphological evidence of necrosis or apoptosis at the time lymphocytes were harvested for flow cytometry (following 3-day co-culture), but cell viability assays were not performed. Therefore PRRSV mediated cytopathic effect on DCs and the abbreviated co-culture time could potentially have impacted the results seen in this study.
PRRSV has been shown to induce IL-10 and it is thought that the potent immunosuppressive properties of this cytokine significantly contribute to modulation of the host immune system in the pathogenesis of PRRSV infection (Charerntantanakul et al., 2006, Feng et al., 2003, Suradhat and Thanawongnuwech, 2003). However, a highly virulent PRRSV strain did not induce IL-10 in vitro or in vivo, and there is evidence that the ability of PRRSV to induce IL-10 varies depending on the strain (Darwich et al., 2011, Gimeno et al., 2011, Subramaniam et al., 2011). There are conflicting data in the literature regarding the relationship between PRRSV-mediated IL-10 production and Treg induction. In one study PRRSV mediated induction of Tregs was shown to be dependent on TGF-β, but not IL-10 (Silva-Campa et al., 2009). However, in a more recent study DCs pulsed with PRRSV N protein induced IL-10 producing cells and Tregs, suggesting a correlation between IL-10 production and development of PRRSV-induced Tregs (Wongyanin et al., 2012). In the present study co-infection of DCs with PCV2 and PRRSV was associated with an up-regulation of TGF-β compared to infection with either virus alone, but no increase in IL-10 was seen following infection with either virus alone or co-infection with both viruses compared to uninfected controls. These results are similar to previous reports of PRRSV-mediated Treg induction (Silva-Campa et al., 2009). Because cytokine levels were quantified in the cell culture supernatant, the increase in TGF-β could reflect production by DCs or T lymphocytes. Individual pig TGF-β levels in the PRRSV/PCV2 co-infected groups did not directly correlate with the magnitude of CD4+CD25+FoxP3+ Treg induction in (data not shown), which further suggests that TGF-β production is not solely attributable to Tregs. In addition, DCs were not treated with LPS or an unrelated virus (i.e. influenza virus or transmissible gastroenteritis coronavirus). Therefore, it is uncertain whether the observed increase in Tregs occurs in a virus-specific manner or reflects a more general homeostatic response to inflammation.
Although induction of porcine Tregs by PRRSV has previously been reported in vitro and in vivo (LeRoith et al., 2011, Silva-Campa et al., 2009, Wongyanin et al., 2010, Wongyanin et al., 2012), the evidence for PRRSV induction of Tregs is inconsistent and some have speculated that this may be due to variation in viral genotype, since Treg induction was seen with North American but not European PRRSV strains (Silva-Campa et al., 2009, Silva-Campa et al., 2010). In the present study, there was considerable individual variation among pigs in the Treg response to infection with PRRSV, PCV2, or both viruses, even though the same genotype II North American PRRSV and PCV2a strains were used in all replicates. This may be due in part to genetic variation among pigs contributing to relative resistance or susceptibility of the host immune response, as has been described for PRRSV infection (Lunney and Chen, 2010). Although the results from this study suggest that Treg induction may play a role in modulating the immune response to co-infection with PCV2 and PRRSV in vitro, additional studies investigating the Treg response to PRRSV/PCV2 co-infection in vivo are needed to address the biological significance of virus-mediated Treg induction in the pathogenesis of PCVAD.
Acknowledgements
The authors would like to thank Melissa Makris for her help with FACS analysis, Pete Jobst for providing the pig blood for the in vitro studies, Will Cecere for statistical support, and Dr. Scott Kenney for technical assistance with confocal microscopy. This work was supported by National Pork Board grant #08-156 and Virginia NC-229 funds. T. E. Cecere is supported by a grant from the National Institutes of Health (5T32RR021819).
References
- Allan G.M., Ellis J.A. Porcine circoviruses: a review. J. Vet. Diagn. Invest. 2000;12:3–14. doi: 10.1177/104063870001200102. [DOI] [PubMed] [Google Scholar]
- Askenasy N., Kaminitz A., Yarkoni S. Mechanisms of T regulatory cell function. Autoimmun. Rev. 2008;7:370–375. doi: 10.1016/j.autrev.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- Carrasco C.P., Rigden R.C., Schaffner R., Gerber H., Neuhaus V., Inumaru S., Takamatsu H., Bertoni G., McCullough K.C., Summerfield A. Porcine dendritic cells generated in vitro: morphological, phenotypic and functional properties. Immunology. 2001;104:175–184. doi: 10.1046/j.0019-2805.2001.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charerntantanakul W., Platt R., Roth J.A. Effects of porcine reproductive and respiratory syndrome virus-infected antigen-presenting cells on T cell activation and antiviral cytokine production. Viral Immunol. 2006;19:646–661. doi: 10.1089/vim.2006.19.646. [DOI] [PubMed] [Google Scholar]
- Darwich L., Gimeno M., Sibila M., Diaz I., de la Torre E., Dotti S., Kuzemtseva L., Martin M., Pujols J., Mateu E. Genetic and immunobiological diversities of porcine reproductive and respiratory syndrome genotype I strains. Vet. Microbiol. 2011;150:49–62. doi: 10.1016/j.vetmic.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Fang Y., Rowland R.R., Roof M., Lunney J.K., Christopher-Hennings J., Nelson E.A. A full-length cDNA infectious clone of North American type 1 porcine reproductive and respiratory syndrome virus: expression of green fluorescent protein in the Nsp2 region. J. Virol. 2006;80:11447–11455. doi: 10.1128/JVI.01032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenaux M., Halbur P.G., Gill M., Toth T.E., Meng X.J. Genetic characterization of type 2 porcine circovirus (PCV-2) from pigs with postweaning multisystemic wasting syndrome in different geographic regions of North America and development of a differential PCR-restriction fragment length polymorphism assay to detect and differentiate between infections with PCV-1 and PCV-2. J. Clin. Microbiol. 2000;38:2494–2503. doi: 10.1128/jcm.38.7.2494-2503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenaux M., Halbur P.G., Haqshenas G., Royer R., Thomas P., Nawagitgul P., Gill M., Toth T.E., Meng X.J. Cloned genomic DNA of type 2 porcine circovirus is infectious when injected directly into the liver and lymph nodes of pigs: characterization of clinical disease, virus distribution, and pathologic lesions. J. Virol. 2002;76:541–551. doi: 10.1128/JVI.76.2.541-551.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenaux M., Opriessnig T., Halbur P.G., Meng X.J. Immunogenicity and pathogenicity of chimeric infectious DNA clones of pathogenic porcine circovirus type 2 (PCV2) and nonpathogenic PCV1 in weanling pigs. J. Virol. 2003;77:11232–11243. doi: 10.1128/JVI.77.20.11232-11243.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W.H., Tompkins M.B., Xu J.S., Zhang H.X., McCaw M.B. Analysis of constitutive cytokine expression by pigs infected in-utero with porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 2003;94:35–45. doi: 10.1016/s0165-2427(03)00059-x. [DOI] [PubMed] [Google Scholar]
- Flores-Mendoza L., Silva-Campa E., Resendiz M., Osorio F.A., Hernandez J. Porcine reproductive and respiratory syndrome virus infects mature porcine dendritic cells and up-regulates interleukin-10 production. Clin. Vaccine Immunol. 2008;15:720–725. doi: 10.1128/CVI.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J., Opriessnig T., Meng X.J., Pelzer K., Buechner-Maxwell V. Porcine circovirus type 2 and porcine circovirus-associated disease. J. Vet. Intern. Med. 2009;23:1151–1163. doi: 10.1111/j.1939-1676.2009.0389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno M., Darwich L., Diaz I., de la Torre E., Pujols J., Martin M., Inumaru S., Cano E., Domingo M., Montoya M., Mateu E. Cytokine profiles and phenotype regulation of antigen presenting cells by genotype-I porcine reproductive and respiratory syndrome virus isolates. Vet. Res. 2011;42:9. doi: 10.1186/1297-9716-42-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms P.A., Sorden S.D., Halbur P.G., Bolin S.R., Lager K.M., Morozov I., Paul P.S. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet. Pathol. 2001;38:528–539. doi: 10.1354/vp.38-5-528. [DOI] [PubMed] [Google Scholar]
- Kaser T., Gerner W., Hammer S.E., Patzl M., Saalmuller A. Detection of Foxp3 protein expression in porcine T lymphocytes. Vet. Immunol. Immunopathol. 2008;125:92–101. doi: 10.1016/j.vetimm.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Kaser T., Gerner W., Hammer S.E., Patzl M., Saalmuller A. Phenotypic and functional characterisation of porcine CD4(+)CD25(high) regulatory T cells. Vet. Immunol. Immunopathol. 2008;122:153–158. doi: 10.1016/j.vetimm.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Kaser T., Gerner W., Saalmuller A. Porcine regulatory T cells: mechanisms and T-cell targets of suppression. Dev. Comp. Immunol. 2011;35:1166–1172. doi: 10.1016/j.dci.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Krakowka S., Ellis J.A., McNeilly F., Ringler S., Rings D.M., Allan G. Activation of the immune system is the pivotal event in the production of wasting disease in pigs infected with porcine circovirus-2 (PCV-2) Vet. Pathol. 2001;38:31–42. doi: 10.1354/vp.38-1-31. [DOI] [PubMed] [Google Scholar]
- LeRoith T., Hammond S., Todd S.M., Ni Y., Cecere T., Pelzer K.D. A modified live PRRSV vaccine and the pathogenic parent strain induce regulatory T cells in pigs naturally infected with Mycoplasma hyopneumoniae. Vet. Immunol. Immunopathol. 2011;140:312–316. doi: 10.1016/j.vetimm.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Li S., Gowans E.J., Chougnet C., Plebanski M., Dittmer U. Natural regulatory T cells and persistent viral infection. J. Virol. 2008;82:21–30. doi: 10.1128/JVI.01768-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunney J.K., Chen H. Genetic control of host resistance to porcine reproductive and respiratory syndrome virus (PRRSV) infection. Virus Res. 2010;154:161–169. doi: 10.1016/j.virusres.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Meerts P., Van Gucht S., Cox E., Vandebosch A., Nauwynck H.J. Correlation between type of adaptive immune response against porcine circovirus type 2 and level of virus replication. Viral Immunol. 2005;18:333–341. doi: 10.1089/vim.2005.18.333. [DOI] [PubMed] [Google Scholar]
- Meng X.J., Paul P.S., Halbur P.G. Molecular cloning and nucleotide sequencing of the 3′-terminal genomic RNA of the porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 1994;75(Pt 7):1795–1801. doi: 10.1099/0022-1317-75-7-1795. [DOI] [PubMed] [Google Scholar]
- Opriessnig T., Halbur P.G. Concurrent infections are important for expression of porcine circovirus associated disease. Virus Res. 2012;164:20–32. doi: 10.1016/j.virusres.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opriessnig T., Meng X.J., Halbur P.G. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J. Vet. Diagn. Invest. 2007;19:591–615. doi: 10.1177/104063870701900601. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Hygiene. 1938;27:493–497. [Google Scholar]
- Rouse B.T., Sarangi P.P., Suvas S. Regulatory T cells in virus infections. Immunol. Rev. 2006;212:272–286. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Rovira A., Balasch M., Segales J., Garcia L., Plana-Duran J., Rosell C., Ellerbrok H., Mankertz A., Domingo M. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. J. Virol. 2002;76:3232–3239. doi: 10.1128/JVI.76.7.3232-3239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi K.C., Ge G.X.X.N., Liu Q., Yang H.C. Cytokine mRNA expression profiles in peripheral blood mononuclear cells from piglets experimentally co-infected with porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. Vet. Microbiol. 2010;140:155–160. doi: 10.1016/j.vetmic.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Silva-Campa E., Cordoba L., Fraile L., Flores-Mendoza L., Montoya M., Hernandez J. European genotype of porcine reproductive and respiratory syndrome (PRRSV) infects monocyte-derived dendritic cells but does not induce Treg cells. Virology. 2010;396:264–271. doi: 10.1016/j.virol.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Silva-Campa E., Flores-Mendoza L., Resendiz M., Pinelli-Saavedra A., Mata-Haro V., Mwangi W., Hernandez J. Induction of T helper 3 regulatory cells by dendritic cells infected with porcine reproductive and respiratory syndrome virus. Virology. 2009;387:373–379. doi: 10.1016/j.virol.2009.02.033. [DOI] [PubMed] [Google Scholar]
- Steiner E., Balmelli C., Herrmann B., Summerfield A., McCullough K. Porcine circovirus type 2 displays pluripotency in cell targeting. Virology. 2008;378:311–322. doi: 10.1016/j.virol.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Sur J.H., Kwon B., Pattnaik A.K., Osorio F.A. A virulent strain of porcine reproductive and respiratory syndrome virus does not up-regulate interleukin-10 levels in vitro or in vivo. Virus Res. 2011;155:415–422. doi: 10.1016/j.virusres.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Suradhat S., Thanawongnuwech R. Upregulation of interleukin-10 gene expression in the leukocytes of pigs infected with porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2003;84:2755–2760. doi: 10.1099/vir.0.19230-0. [DOI] [PubMed] [Google Scholar]
- Todd D., Bendinelli M., Biagini P., Hino S., Mamkertz S., Mishiro S., Niel C., Okamoto H., Raidal S., Ritchie B.W., Teo G.C. Circoviridae. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press; San Diego: 2005. pp. 327–334. [Google Scholar]
- Vincent I.E., Carrasco C.P., Guzylack-Piriou L., Herrmann B., McNeilly F., Allan G.M., Summerfield A., McCullough K.C. Subset-dependent modulation of dendritic cell activity by circovirus type 2. Immunology. 2005;115:388–398. doi: 10.1111/j.1365-2567.2005.02165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent I.E., Carrasco C.P., Herrmann B., Meehan B.M., Allan G.M., Summerfield A., McCullough K.C. Dendritic cells harbor infectious porcine circovirus type 2 in the absence of apparent cell modulation or replication of the virus. J. Virol. 2003;77:13288–13300. doi: 10.1128/JVI.77.24.13288-13300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Eaton M., Mayer M., Li H., He D., Nelson E., Christopher-Hennings J. Porcine reproductive and respiratory syndrome virus productively infects monocyte-derived dendritic cells and compromises their antigen-presenting ability. Arch. Virol. 2007;152:289–303. doi: 10.1007/s00705-006-0857-1. [DOI] [PubMed] [Google Scholar]
- Wongyanin P., Buranapraditkul S., Yoo D., Thanawongnuwech R., Roth J.A., Suradhat S. Role of porcine reproductive and respiratory syndrome virus nucleocapsid protein in induction of interleukin-10 and regulatory T-lymphocytes (Treg) J. Gen. Virol. 2012;93:1236–1246. doi: 10.1099/vir.0.040287-0. [DOI] [PubMed] [Google Scholar]
- Wongyanin P., Buranapraditkun S., Chokeshai-Usaha K., Thanawonguwech R., Suradhat S. Induction of inducible CD4+CD25+Foxp3+ regulatory T lymphocytes by porcine reproductive and respiratory syndrome virus (PRRSV) Vet. Immunol. Immunopathol. 2010;133:170–182. doi: 10.1016/j.vetimm.2009.07.012. [DOI] [PubMed] [Google Scholar]


