Abstract
We studied whether allospecific CD4+ effector memory T cells (TEM) could induce graft-versus-host disease (GVHD) using a novel GVHD model solely induced by CD4+ T cell receptor transgenic TEa cells. Allospecific TEM generated in a lymphopenic host bore a typical memory phenotype. Moreover, these cells were able to elicit a faster and more effective proliferative response upon challenge with alloantigen in vitro and to mediate “second-set” skin graft rejection in vivo. However, these allospecific TEM were unable to induce GVHD. Allospecific TEM recipients became tolerant to alloantigen as a result of clonal deletion. Even though allospecific TEM were able to respond to alloantigen initially, the expansion of these cells and inflammatory cytokine production during GVHD were dramatically decreased. The inability of allospecific TEM to sustain the alloresponse may be a result of enhanced activation induced cell death. These observations provide insights into how allospecific CD4+ TEM respond to alloantigen during GVHD and underscore the fundamental differences of alloresponses mediated by allospecific TEM in graft rejection and GVHD settings.
INTRODUCTION
Memory immune response is specific to the antigen that has been encountered previously and is mediated by memory lymphocytes (1). In organ transplantation, memory response is also called “second set” rejection (2, 3). In unprimed recipients, one could also detect non-allospecific memory T cells that cross-react with alloantigens (4). Based on the observations in organ transplantation (4), it was presumed that memory T cells from both primed and unprimed donors would induce more severe graft versus host disease (GVHD) than naïve T cells do. This view was recently challenged by the results published by several groups demonstrating that effector memory T cells (TEM) from non-alloantigen-sensitized donors do not induce GVHD in different animal models (5–9). These published data indicate that, TEM isolated from unprimed donors and containing mostly non-alloreactive T cells but likely some crossreactive T cells, are unable to induce GVHD.
Because this approach has the potential to transfer memory T cell immunity against infectious agents and tumors to the host without inducing GVHD, there is great enthusiasm for translating these findings into clinic. One of the major concerns is the existence of crossreactive and even true allospecific memory T cells in humans (4, 10). Humans do not live in a specific pathogen free environment and likely contain more crossreactive T cells (4, 10). In parous females or the donors who have had transfusion or transplantation previously, memory T cells may contain true allospecific T cells (10). Allospecific memory T cells could also be generated as a result of homeostatic proliferation of allospecific naïve T cells (11). Because both crossreactive and allospecific memory T cells have been shown to mediate quicker and more effective immune responses in organ transplantation setting (10), it would be critical to determine how allospecific memory T cells respond in GVHD setting. Dutt et al demonstrated that TEM from alloantigen primed donors induced a chronic form of GVHD in contrast to acute GVHD induced by naïve T cells (12). A recent publication also suggested that the allo-primed TEM induce only mild GVHD (13). In these models, a heterogeneous population of memory T cells in which allospecific memory T cells represent only a small portion of total cells. In order to test the role of allospecific TEM in GVHD more definitely, we established a GVHD model induced solely by TEa CD4+ T cell receptor transgeneic T cells. TEa cells are TCR-transgenic CD4+ T cells with a single defined specificity against IEα 52–68 peptide in the context of I-Ab (14). Using this model, we demonstrated that allospecific TEM were unable to induce GVHD. Because all TEa cells have single antigen specificity and can be tracked in vivo, the mechanisms by which allospecific TEM were unable to induce GVHD were also studied in detail.
MATERIAL AND METHODS
Mice
C57BL/6 (H2b, CD229.1−), B6.129S7-Rag1tm1Mom/J (Rag-1−/−, H2b, CD229.1−) and CB6F1 (H2b/d, CD229.1+) mice were purchased from The Jackson Laboratories (Bar Harbor, ME). TEa transgenic breeders (C57BL/6 background, CD229.1−, generous gifts from Dr. Alexander Rudensky, University of Washington, Seatle, WA), which express a TCR that recognizes a peptide that is expressed in all antigen presenting cells from H2b/I-E+ strains such as CB6F1 (14), were bred at Duke University. Female mice were primarily used in this study. Most of the unprimed donor mice were between 6 and 10 weeks of age at the beginning of the study. Recipient mice were 7–12 weeks old at the time of transplantation. The mice were housed in a specific pathogen-free facility throughout the study.
T-cell depletion from bone marrow
T cells were depleted from bone marrow using anti-Thy1.2 antibody and complement according to a published protocol from our laboratory (15). Briefly, bone marrow cells were flushed out from femurs, tibias and humeri and strained through a 70 µm cell strainer (Becton Dickinson labware, Franklin Lakes, NJ). Cells were then resuspended in Cytotoxicity Medium (Cedarlane, Hornby, ON, Canada) at a concentration of 1×107/ml. Anti-Thy1.2 monoclonal antibody (0.1 µg per 1×107 cells, clone 30H12; BD Pharmingen, San Diego, CA) was added and mixed. After a 60-minute incubation at 4 °C, the cells were washed once and then resuspended in cytotoxicity Medium (Cedarlane) containing 1:10 Low-Tox-M Rabbit Complement (Cedarlane). The cells were then incubated at 37°C for 60 minutes and washed twice before use. For the bone marrow from Rag-1−/− mice, whole bone marrow was used directly without further T-cell depletion.
T cell separation
Purified CD4+ T cells were separated from splenocytes using antibodies against CD11b, CD45R, DX5, Ter-119, and CD8 (Miltenyi Biotec) through negative selection. Effector memory phenotype TEa cells were obtained after depletion of CD62L+ cells using anti-CD62L microbeads (Miltenyi Biotec) from purified CD4+ T cells. The purity of the cell population was always checked by flow cytometry after magnetic separation. The cell doses used in the experiments were always adjusted based on T-cell content.
Skin transplantation
This procedure was based on a protocol published by Markees et al (16). Briefly, tail skin was removed from sacrificed donors and cut into ~0.5×0.5 cm2 pieces. The dorsal surface of anesthetized recipient mice was shaved and washed with iodine solution, and rinsed with an alcohol swab. A graft bed was prepared with fine scissors by removing an area of epidermis and dermis down to the level of the intrinsic muscle. Grafts were fitted to the prepared bed, sutured with 6-0 surgical suture, and then covered with an adhesive plastic bandage. After 4 days, the bandage was removed. Skin graft survival was assessed everyday by visual and tactile examination. Rejection was defined as the first day when the entire epidermal surface of the graft was less than 10%.
Flow cytomeric analysis
Blood (50µl) or 1×106 cells were incubated with titrated antibodies for 15 minutes at room temperature in the dark, red cells were lysed by FACS lysing solution (BD Bioscience). Cells were stained with antibody cocktail and then analyzed using a BD-FASCanto flow cytometer equipped with FACSDiva software (BD Bioscience). The absolute counts were determined by using Flow-Count fluorospheres (Beckmen Coulter). The antibodies included fluoresce inisothio-cyanate (FITC)–conjugated anti-CD62L (clone MEL-14); R-phycoerythrin (PE)–conjugated anti-CD45.1 (clone A20); cy-chrome–conjugated anti-CD44 (clone IM7), and their isotype controls from BD PharMingen (San Diego, CA); FITC-conjugated anti–H-2Db (clone CTDb); PE-conjugated anti-CD4 (clone CT-CD4), B220 (clone RA3-6B2); PE-Texasred–conjugated anti-CD4 (clone RM4–5); tri-color–conjugated anti-CD4 (clone CT-CD4), CD8α (clone CT-CD8α), and their isotype controls from Invitrogen (Carlsbad, CA).
Generation of allospecific TEM
1×107 Naïve TEa cells were transferred into Rag-1−/− mice. On the second day, 5×107 irradiated (20 Gy) host-type splenocytes were given to recipients through intraperitoneal injection. More than 8 weeks later, some of the animals were transplanted with allospecific antigen original skin grafts to observe second-set rejection. Other animals were used to isolate effector memory T cells by depleting CD62L+ cells using magnetic beads. Many of the cells that were transferred into the Rag-1−/− mice but were not challenged with alloantigens also obtained memory phenotype, these cells were also included as a control and were termed “TEM-like”.
Analysis of serum cytokines
Serum was analyzed for 23 cytokines and chemokines using the Protein Multiplex Immunoassay kit (Invitrogen) as described before (17, 18). Briefly, Biosource’s Multiplex beads were vortexed and sonicated for 30 seconds and 25 µl was added to each well and washed twice with wash buffer. The samples were diluted 1:2 with assay diluent and loaded onto a Millipore Multiscreen BV 96-well filter plate; 50 µl of incubation buffer had been added previously to each well. Serial dilutions of cytokine standards were prepared in parallel and added to the plate. Samples were incubated on a plate shaker at 600 revolution/min in the dark at room temperature for 2 hours. The plate was applied to a Millipore Multiscreen Vacuum Manifold and washed twice with 200 µl of wash buffer. 100 µl of biotinylated Anti-Multi-Cytokine Reporter was added to each well. The plate was incubated on a plate shaker at 600 revolutions/minute in the dark at room temperature for 1 hour. The plate was applied to a Millipore Multiscreen Vacuum Manifold and washed twice with 200 µl of wash buffer. Streptavidin-phycoerythrin was diluted 1:10 in wash buffer, and 100 µl was added directly to each well. The plate was incubated on a plate shaker at 600 revolutions /minute in the dark at room temperature for 30 minutes. The plate was applied to the vacuum manifold, washed twice, and each well was resuspended in 100 µl wash buffer and shaken for 1 minute. The assay plate was transferred to the Bio-Plex Luminex 100 XYP instrument for analysis. Cytokine concentrations were calculated using Bio-Plex Manager 3.0 software with a five parameter curve-fitting algorithm applied for standard curve calculations. Specifically, we assayed the serum for interleukin (IL)-1a, IL-1b, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-17, Eotaxin, granulocyte-colony stimulating factor (G-CSF), granulocyte/macrophage-colony stimulating factor (GM-CSF), tumor necrosis factor (TNF)-α, kerotinocyte chemoattractant (KC), monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1a, MIP-1b, regulated upon activation, normal T-cell expressed, and secreted (RANTES), and interferon (IFN)γ.
Proliferation assay
Proliferation assay has been described previously (5). Graded numbers of purified T cells (0.0156–2.5×105) were plated in 96-well, flat-bottom culture plates with 5×105 irradiated (20Gy) allogeneic splenocytes in a final volume of 200 µl. After incubation at 37°C and 5% CO2 for a certain period of time (96 hours, except where indicated), cultures were pulsed with 3H-thymidine (1 µCi [0.037 MBq]/well). After another 16 hours of incubation, the cultures were harvested and counted in a MicroBeta Trilux liquidscintillation counter (EG&GWallac, Turku, Finland). Triplicate cultures were set up for every cell population tested.
CFSE assay
CFSE staining was based on Lyons A’s protocol (19). Briefly, mononuclear cells were resuspended in PBS+0.1% fetal bovine serum (FBS), a 10 mM stock solution of 5-(and -6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) in dimethyl sulfoxide (DMSO) was added to a final concentration of 5 µM and incubated at 37 °C for 5 minutes. At the end of incubation period, the cells were immediately washed three times in cold RPMI-1640 + 10% FBS cell culture medium. After the last wash, the samples were analyzed by flow cytometry.
GVHD model
Lethally irradiated (10.5 Gy) mice were injected intravenously through the tail vein with 1×107 T-cell-depleted bone marrow (TCD BM) cells and purified T cells. Survival and clinical evidences of GVHD, such as weight change, skin changes (hair loss and erythema), diarrhea, and hunched posture, were monitored daily. Moribund mice were humanely killed according to an animal protocol approved by the Duke University Institutional Animal Care and Use Committee. Except where indicated, the experiments were performed in the TEa→CB6F1 system.
Statistical analysis
Data are represented as mean±SD or mean+SD. Kaplan-Meier survival curve was made by JMP software (SAS Corporation). Statistical differences in animal survival were analyzed by log-rank test. Differences in cpm in MLR system and recipients body weight change were analyzed using two-tailed student T-test. For all tests, p <0.05 was considered significant.
RESULTS
A novel GVHD model induced by CD4+ TCR transgenic T cells alone
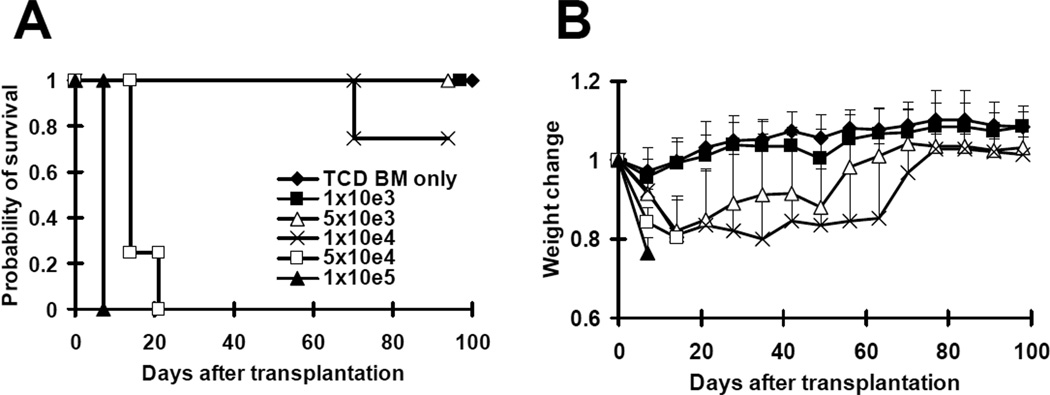
In previously published studies (12, 13), a heterogeneous population of memory T cells (where allospecific memory T cells represented only a small portion of total cells) was used to study the effects of allospecific memory T cells on GVHD. In order to investigate the role of allospecific memory T cells in GVHD more definitely, we developed a GVHD model induced solely by TEa cells. This model was previously described by Gonzalez et al (20). In the published study, the recipient CB6F1 mice were irradiated with a sub-lethal dose of radiation (6.5 Gy) and approximately 60% of the recipients developed lethal GVHD despite a large dose of TEa cells (4×106). We anticipated that we would not be able to generate such high numbers of memory TEa cells. In order to develop such a model, we increased the dose of irradiation to CB6F1 mice from sub-lethal (6.5 Gy) to lethal dose (10.5 Gy). After irradiation, graded numbers (1×103 to 1×105) of purified TEa cells from naïve TEa mice were transplanted into CB6F1 recipients together with 1×107 C57BL/6 TCD bone marrow cells. As demonstrated in Figure 1, as few as 5×104 purified TEa cells were able to induce lethal GVHD, resulting in the death of all recipients within 20 days. Despite significant weight loss observed in the recipients of 5×103 and 1×104 purified TEa cells, the majority of them survived more than 100 days after transplantation. None of the recipients of 1×103 purified TEa cells developed GVHD and all of them survived more than 100 days after transplantation. Because only 5×104 purified TEa cells are required to induce lethal GVHD, this would be an ideal model to study the role of allospecific TEM in GVHD.
Figure 1. A novel GVHD model induced by TEa CD4+ TCR transgenic T cells alone.
Graded numbers (1×103 to 1×105) of purified TEa cells were transplanted into lethally irradiated CB6F1 recipients along with 1×107 T-cell-depleted BM cells. Mice were monitored for the development of GVHD. Each groups contained 4–5 mice. Consistent results were obtained using 1×104 and 1×105 TEa cells in more than 3 other independent experiments. (A) Survival. p<0.005 (5×104 or 1×105 versus TCD BM only, 1×103, 5×103, and 1×104). (B) Body weight.. p<0.001, 1×105 versus TCD BM only, 1×103, 5×103, and 1×104 on day 7 and14; p<0.05, 1×105 versus TCD BM only, 1×103, and 5×103 on day 21.
Generation of allospecific TEM
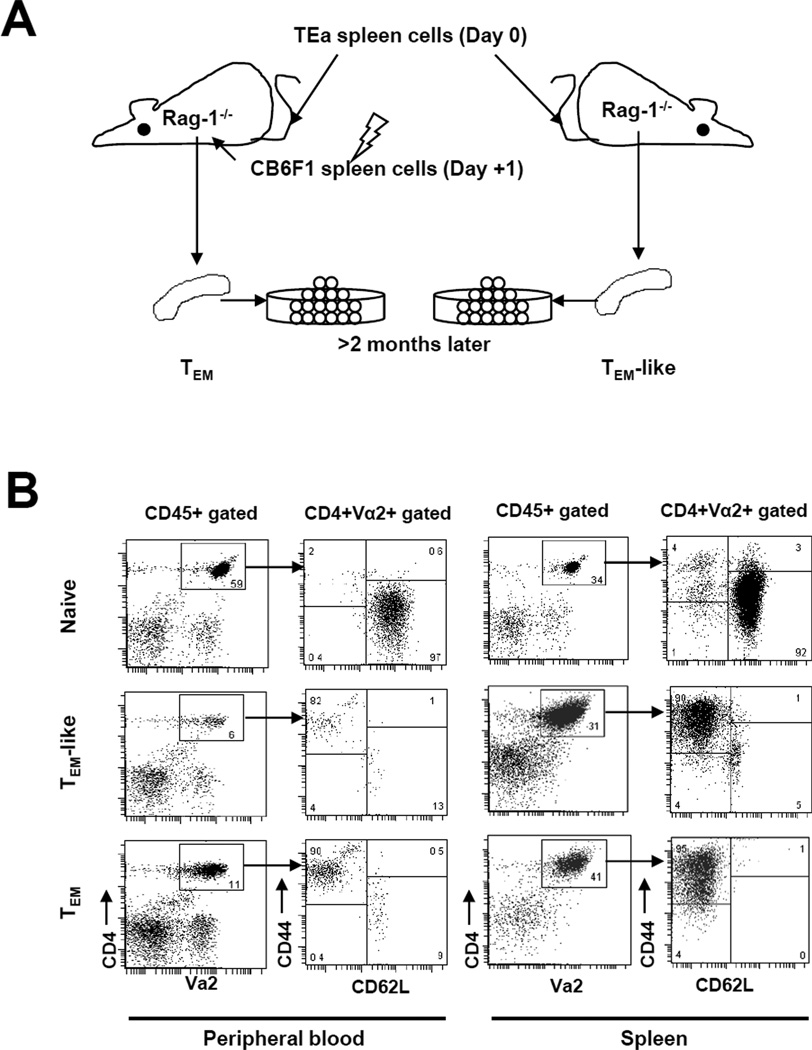
Using the novel GVHD model induced by TEa cells, we next studied the ability of allospecific TEM to induce GVHD. In order to obtain enough purified allospecific TEM for testing, we used the T and B cell deficient Rag-1−/− mouse model, which has been used successfully by several investigators to generate memory T cells (21). We first injected spleen cells from naïve TEa mice containing 1×107 TEa cells into Rag-1−/− mice intraveneously (Figure 2A). Eighteen to twenty-four hours later, these mice were immunized with 5×107 CB6F1 irradiated (20 Gy) spleen cells through intraperitoneal injection. Effector memory T cells were harvested from these mice at least 8 weeks after priming. Many of the T cells that were transferred into the Rag-1−/− mice but were not challenged with alloantigen also obtained memory phenotype through homeostatic proliferation (4). These cells were included as a control and were termed “TEM-like cells”. At least eight weeks after adoptive transfer and priming, we were able to detect 0.3–27.7% and 4.2–41% of TEa cells in peripheral blood and spleen respectively (Figure 2B). As shown in both peripheral blood and spleen, more than 90% of TEa cells became TEM and only ~1% of TEa cells were TCM in TEM donors. Similar results were obtained in TEM-like cell donors.
Figure 2. Generation of allospecific TEM.
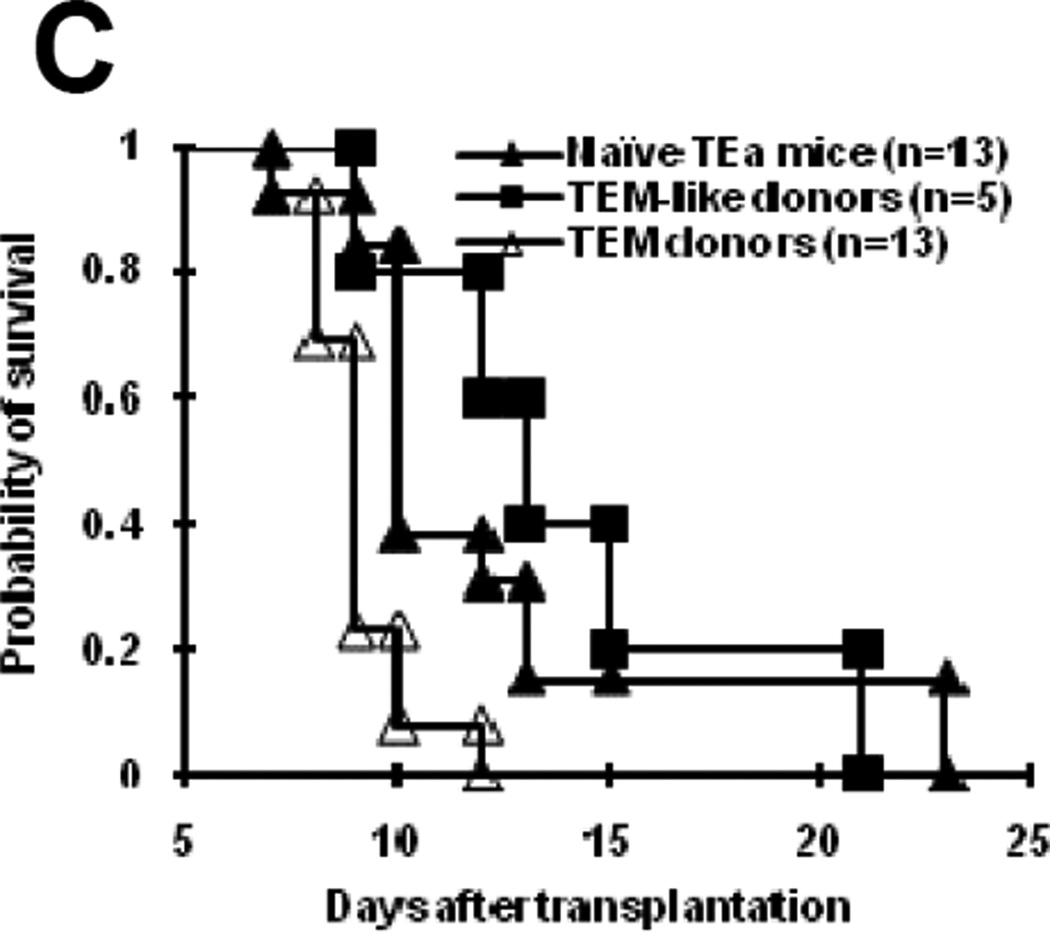
To generate allospecfic TEM, TEa spleen cells containing 1×107 TEa T cells were first transferred into Rag-1−/− mice. These mice were subsequently immunized with irradiated CB6F1 splenocytes 18–24 hour later. (A) A schema showing how effector memory TEa cells were generated. The details of how allospecific TEM were obtained are described in the Material and Methods section. (B) Phenotypes of TEa cells TEM donor mice. Flow cytometric analysis was performed in TEM donor mice at least 8 weeks after priming. (C) TEM donor mice mediated “second-set” skin graft rejection. At least 8 weeks after priming, TEM donor mice were transplanted with CB6F1 skin grafts. Graft survival was observed daily. P<0.01, TEM donors vs. other groups. P=0.057, TEM-like donors vs. naïve TEa mice.
To ensure that functional memory T cells were generated in the TEM donors, we transplanted CB6F1 skin grafts into these mice. As shown in Figure 2C, TEM donors rejected skin grafts faster than naïve TEa mice did (P<0.01), indicating that TEM donors are able to mediate memory responses. Because the vast majority of memory phenotype T cells in allospecific TEM donors were TEM, it is most likely that the accelerating rejection of skin graft was mediated by TEM, suggesting that TEM generated in this model are truely functional memory T cells. Despite TEM-like cell donors also contained mostly memory phenotype TEa cells, they did not reject CB6F1 skin faster than naïve TEa mice did, suggesting that TEM-like cells may not be fully functional. Even though further analyses will be needed to confirm the functional differences between these cells, these data indicate that both TEM-like cells and TEM are unequivocally functional.
Purification and characterization of allospecific TEM
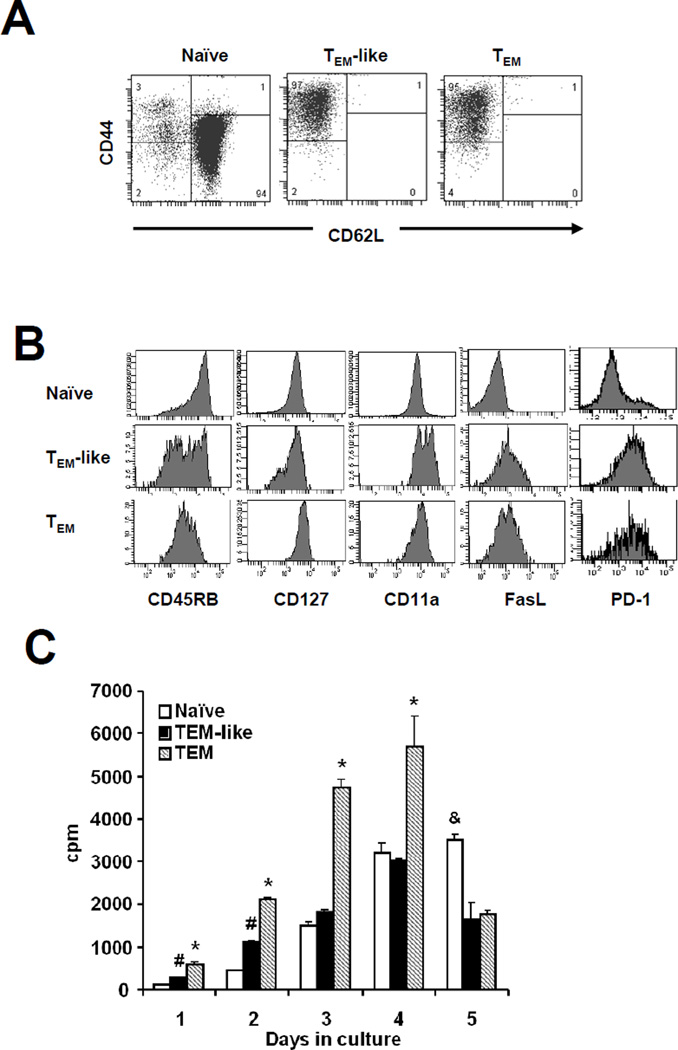
In order to study the effect of allospecific TEM, we first sorted purified naïve and effector memory phenotype TEa cells by magnetic bead selection. Naïve TEa cells were purified after depletion of all non-CD4+ cells from naïve TEa spleen cells. Both TEM-like cells and TEM were purified after depletion of all non-CD4+ cells followed by deletion of all CD62L+ cells. The purity of naïve TEa cells (CD62L+CD44low) was 94%. Contamination of naïve (CD62L+CD44low) and TCM (CD62L+CD44high) in both TEM-like cells and TEM was 1% (Figure 3A). To further ensure that these cells are true memory T cells, we stained them with additional memory T cell markers. As shown in Figure 3B, both TEM-like cells and TEM had a typical memory T cell phenotype.
Figure 3. Purification and characterization of allospecific TEM.
At least 8 weeks after priming, spleen cells were first harvested from Rag-1−/− mice that contained TEa cells. CD4+ cells were then negatively selected using magnetic beads. Purified effector memory TEa cells were finally isolated after depletion of CD62L+ cells using magnetic beads. A representative of at least two similar experiments is shown. (A) Phenotypes of purified TEa cells used for the subsequent experiments. (B) Additional memory T cell markers. (C) Mixed lymphocyte reaction. Purified TEa cells (62,500 cells/well) from different groups were cultured with 5×105 irradiated CB6F1 spleen cells. Proliferation was determined by 3H-thymidine incorporation. *P<0.05, TEM vs. others; #P<0.05, TEM-like vs. naïve; &P<0.05, naïve vs. others
This approach has also been used by many other groups (21). Moreover, we demonstrated in our model that primed TEM donors mediated memory T cell responses (Figure 2C) and the purified allospecific TEM from these mice bore typical memory T cell phenotype (Figure 3B). Despite these strong evidences, we wanted to further ensure that TEM generated using this model are truly functional memory T cells by testing them using an in vitro mixed lymphocyte reaction assay. Primed TEM were cultured with irradiated CB6F1 spleen cells and the proliferation potential was assessed over time. As shown in Figure 3C, TEM mediated more rapid and stronger proliferative responses upon challenge with alloantigens compared with both naïve TEa cells and TEM-like cells controls. Taken together, these data indicated that allospecific TEM used in our studies were truly functional memory T cells.
Allospecific primed TEM do not induce GVHD
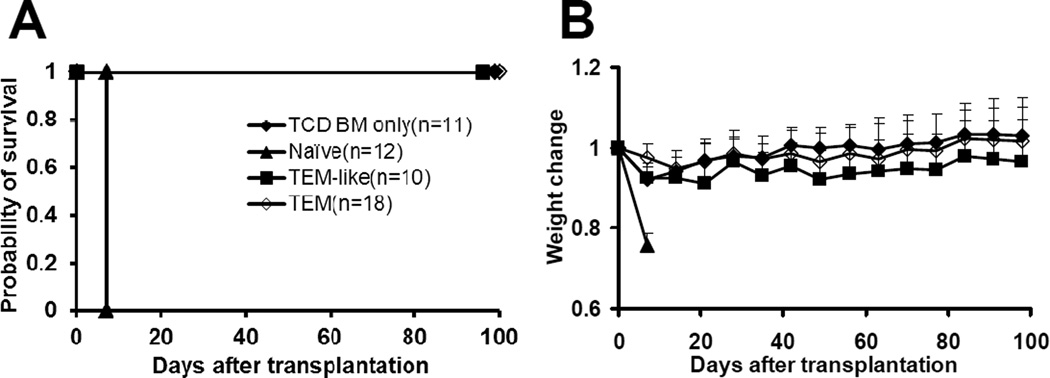
We then tested the ability of primed TEM to induce GVHD. Purified TEa cells (1×105/mouse) from different groups were transplanted into lethally irradiated CB6F1 mice along with 1×107 TCD bone marrow cells. All naïve TEa cell recipients developed GVHD rapidly after transplantation and all of them died within one week after transplantation (Figure 4). By contrast, none of TEM recipients developed GVHD and all of them survived more than 100 days after transplantation. Similarly, neither clinical nor histological GVHD was observed in TEM-like cell recipients. Although it is not statistically significant, body weights did trend to be lower in TEM-like cell group compared with TCD bone marrow only group, suggesting mild GVHD might have developed in these animals. Nevertheless, all TEM-like cell recipients survived more than 100 days after transplantation. Based on the data presented in Figure 1 and 4, both allospecific TEM and TEM-like cells are at least 20 times less potent in inducing GVHD. These data clearly demonstrated that allospecific TEM from both primed and unprimed donors had dramatically decreased ability to induce GVHD.
Figure 4. Allospecific TEM do not induce GVHD.
Purified TEa cells (1×105) from each group were transplanted into lethally irradiated CB6F1 recipients along with 1×107 T-cell-depleted BM cells. The data were pooled from 3 independent experiments with similar results. Mice were then monitored for the development of GVHD. Survival was monitored daily. (A) Survival. p<0.0001, Naïve vs. other groups. (B) Body weight. P<0.001, TEM vs. naïve at day +7, +14, and +21.
T cell expansion and cytokine secretion in allospecific primed TEM recipients
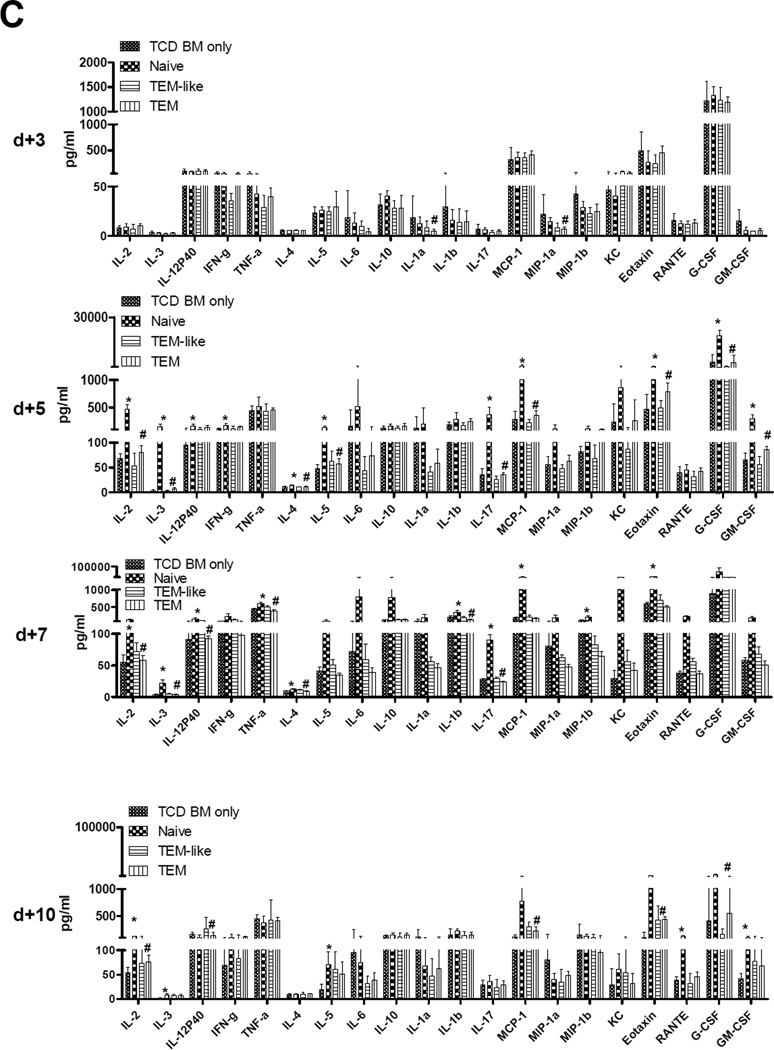
To investigate why primed TEM did not induce GVHD, we monitored T cell expansion in primed TEM recipents post transplantation. In order to track TEa cells in vivo during GVHD, the same model as what we used in Figure 4 was used except that the bone marrow was from Rag-1−/− mice rather than wild-type C57BL/6 mice. Because CD229.1 is only expressed in the recipient CB6F1 cells and Rag-1−/− mice were used as bone marrow donors, TEa cells (CD229.1−CD4+Vα2+) could be monitored by flow cytometry as shown in Figure 5A. The mice were sacrificed at day1, 3, 5, and 7 after transplantation and TEa cells were quantified in peripheral blood. Unfortunately, TEa cells could not be detected in any groups at any time points before day +7. On day +7, blood and spleen TEa cell numbers in both TEM and TEM-like cell recipients were significantly lower than that in naïve TEa cell recipients (P<0.05, Figure 5B). Similar results were obtained in liver and bone marrow (data not shown). These data indicated that T cell expansion was severely inhibited in both TEM-like cell and TEM recipients during GVHD at least at the peak of naïve T cell expansion. Because allospecific TEM are able to proliferate and expand in response to alloantigen as indicated in the mixed lymphocyte reaction assay (Figure 3C), the in vivo T cell expansion data suggested that, similar to memory T cells from unprimed donors (22, 23), allospecific TEM also mediated an abortive alloresponse after hematopoietic stem cell transplantation. Cytokine storm is a downstream cascade after T cell activation and clonal expansion during GVHD (24). Multiple inflammatory cytokines are involved in the pathogenesis of GVHD. To determine whether an abortive T cell response mediated by allospecific primed TEM leads to a disruption in inflammatory cytokine production after transplantation, we measured cytokine changes in allospecifc TEM recipients. Plasma was harvested at day 3, 5, 7, and 10 after transplantation and the levels of multiple cytokines and chemokines were measured by luminex technology. As shown in Figure 5C, multiple cytokines in Th1 (IL-2, IL-3, IL-12P40, IFNγ, TNFα), Th2 (IL-4, IL-5, IL-10), Th17 (IL-1α, IL-1β, IL-6, IL-17), hematopoietic families (G-CSF and GM-CSF), and chemokines (MIP-1a, MIP-1b, RANTE, MCP-1, KC, Eotaxin), were increased in naïve TEa cell recipients compared with TCD bone marrow only control starting at day +5 (P<0.05). This trend lasted at least until day +10. By contrast, none of these cytokines were increased in both TEM-like cells and TEM recipients. These data demonstrated that cytokine storm was attenuated in allospecific TEM cells recipients after hematopoietic stem cell transplantation.
Figure 5. T cell expansion and cytokine secretion in allospecific TEM recipients.
Purified TEa cells (1×105/mouse) from each group were transplanted into lethally irradiated CB6F1 recipients along with 1×107 T-cell-depleted BM cells. Numbers of TEa cells were measured at day +7 by using flow cytometry. Cytokines were measured at day +3, +5, +7, and +10 by using luminex assay. (A) Representative histograms showing how TEa cells were measured in transplantation recipients using flow cytometry. (B) T cell expansion in vivo. Similar results were obtained in spleen, liver, and bone marrow. *P<0.05, naïve vs. TEM. (C) Cytokine changes in plasma. P<0.05, naïve vs. TEM in all cytokines; P<0.05, in IL-12P40, TNFα, RANTE. *P<0.05, TCD BM only vs. Naïve; #P<0.05, Naïve vs. TEM; P=NS, TEM vs. TCD BM only except GM-CSF, IFNγ, MIP-1b on day +5, IL-17 on day +7, IL-2, IL-3, IL-5, GM-CSF, and MCP-1 on day +10; P=NS, TEM–like vs. TCD BM only except RANTE on day +7, IL-3, IL-5, eotaxin, and MCP-1 on day +10.
Allospecific TEM undergo apoptosis and die faster than naïve T cells do
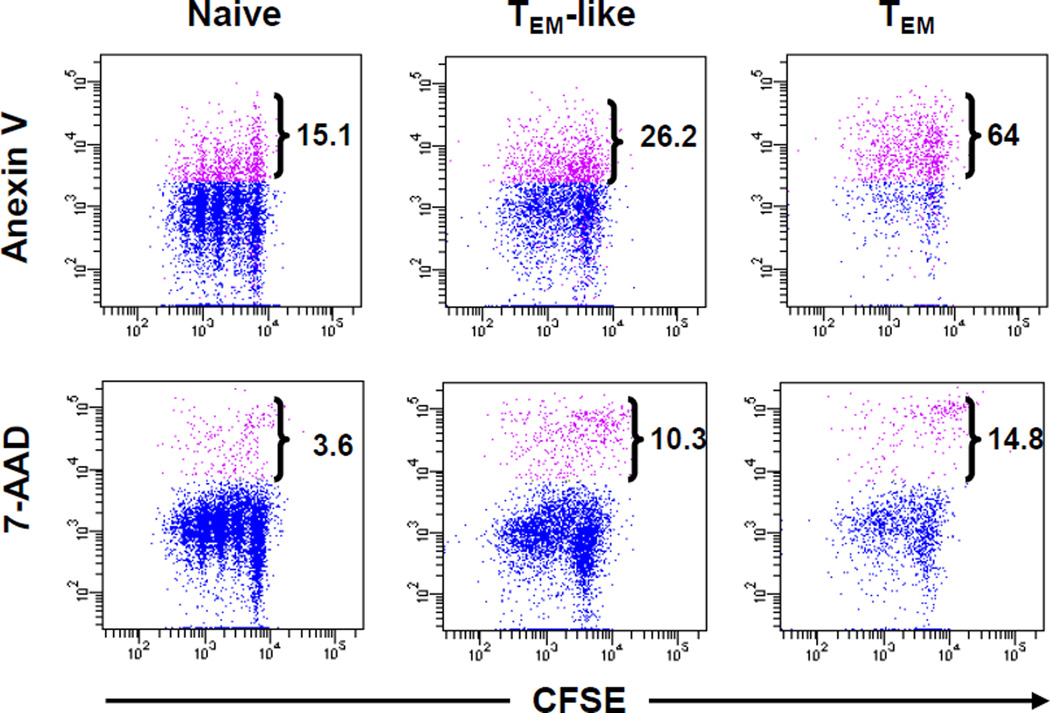
Previously published data from our group suggest that inability of memory T cells from unprimed donors is a result of an abortive alloresponse (22). The data from this study as presented in Figure 3C and 4 also suggest that this may be the same case with allospecifc TEM. In order to understand why allospecific TEM is able to respond to alloantigen initially but fail to sustain the response, we studied proliferation and cell apoptosis/death of allospecific TEM simultaneously. T cells were first labeled with CFSE and then cultured them with irradiatied CB6F1 cells in vitro. After 72 hours in culture, cells were harvested and the percentages of apoptotic and dead cells in each cell generation were determined by flow cytometry (Figure 6). The results (Figure 6) indicated that more TEM-like cells and TEM underwent apoptosis in all generations during alloresponses compared to naïve TEa cells.
Figure 6. Allospecific TEM undergo apoptosis and die faster than naïve T cells do in vitro.
Purified TEa cells from different groups were labeled with CFSE. Labeled TEa cells (1.25×105/well) were cultured with irradiated CB6F1 spleen cells (5×105/well) for 3 days. At the end of the culture, cells were stained with Anexin V and 7-AAD. The dot plots were gated on 7-AAD− (top panel) or total (bottom panel) CD229.1−CD4+Vα2+ (TEa) cells. The values represent percent apoptotic (AnexinV+7-AAD−) or dead cells (7-AAD−) among TEa cells. A representative of three similar experiments with similar results is shown.
Allospecific TEM are deleted in “GVHD” but not skin graft recipients
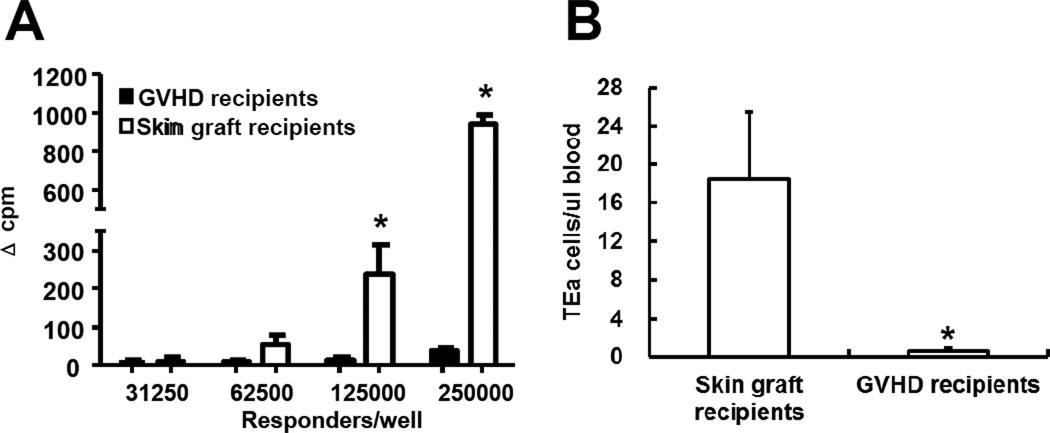
The data presented here demonstrated that, despite the ability of allospecific TEM to respond to alloantigen initially (Figure 3C and 6), they failed to induce GVHD after allogeneic stem cell transplantation (Figure 4). These data suggested that allospecific primed TEM had become non responsive to alloantigen in “GVHD” recipients. To determine whether this occured, we isolated spleen cells from allospecific TEM recipients and re-challenged them with irradiated CB6F1 spleen cells in vitro. Because all the naïve TEa cell recipients died of GVHD early after transplantation and were not available as a positive control, we included TEa-containing Rag-1−/− mice that had rejected CB6F1 skin grafts previously as a positive control (termed “skin graft recipients”). As shown in Figure 7A, spleen cells from TEM recipients were unable to respond upon challenge with alloantigen while those from skin graft recipients responded vigorously against alloantigen as expected. These data demonstrated that, upon transfer to irradiated allogeneic recipients, allospecific TEM became tolerant to alloantigen.
Figure 7. Allospecific TEM are deleted in ‘GVHD’ but not skin graft recipients.
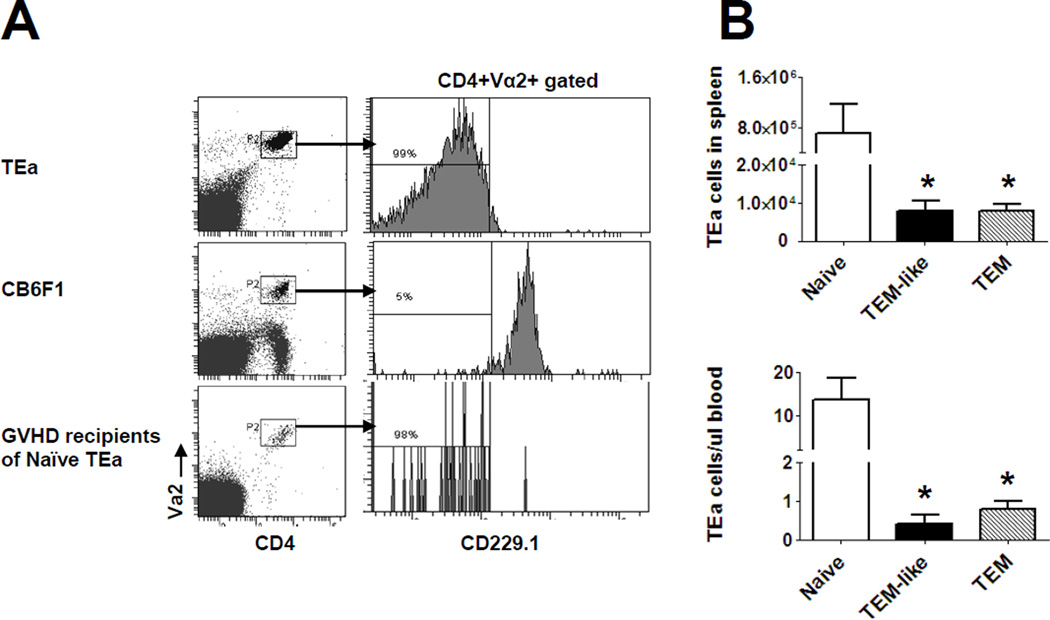
Spleen cells from skin graft recipients (Figure 2C) and “GVHD” recipients (Figure 4) were harvested 84 days after transplantation. Proliferative responses against alloantigen and the numbers of TEa cells in peripheral blood were determined. (A) Proliferation against alloantigens. Various numbers of spleen cells were cultured with irradiated CB6F1 spleen cells (5×105/well) for 3 days. Proliferation was determined by 3H-thymidine incorporation. Results are represented as mean±SD of triplicate wells. All experiments were repeated at least twice and yielded similar results. *P<0.05. (B) Peripheral TEa cell counts. Absolute counts of TEa cells were determined by flow cytometer. Each group contained at least 5 animals. *P<0.01.
Clonal deletion is one of the central mechanisms responsible for tolerance induction (25). To determine whether this mechanism is responsible for tolerance observed in allospecific TEM recipients, we measured the numbers of allospecific cells (TEa) in long-term allospecific TEM recipients. The same experimental model as described in Figure 5 was used in this experiment so that we could track TEa cells by flow cytometry in vivo. The results presented in Figure 7B demonstrated that virtually no TEa cells (<1 cell/µl blood) could be detected in TEM GVHD recipients. In contract, TEa cells were readily detectable in skin graft recipients. These data demonstrated that allospecific TEM were clonally deleted in GVHD recipients. Colonal deletion of allospecific TEa cells might have occurred very early because very small number of TEa cells could be detected in blood on day 7 (Figure 5B).
DISCUSSION
Several different groups have independently demonstrated that TEM from unprimed donors have dramatically decreased ability to induce GVHD (5–9). While this approach holds great translational potential, concerns remain. One of the major concerns is that, unlike memory T cells from experimental animals that are usually housed at clean facilities, human memory T cells contain cells that can crossreact with alloantigens (4). In some human donors, allospecific naïve T cells may acquire memory phenotype after homeostatic proliferation or after exposure to alloantigens in the form of blood transfusion, prior transplantation, or pregnancy (4). Both crossreactive and allospecific memory T cells may induce GVHD. In order to address this concern, we studied the effects of allospecific TEM on GVHD by using a novel GVHD model induced solely by TCR transgenic T cells. Because antigen specific TCR transgenic T cells can be easily tracked in vivo, we also hoped that studies using this model would also help us better understand the mechanisms by which TEM have decreased ability to induce GVHD. The results from the current studies have demonstrated that allospecific TEM are unable to induce GVHD (Figure 4). Based on the published data which suggest that alloreactive TEM have decreased ability to induce acute GVHD (12, 13), these results are not completely surprising. However, these data are against the current dogma that memory T cells mediate stronger and more effective immune responses (1).
Because a T cell transgenic model was used to generate allospecific TEM in our study, an immediate concern is whether the allospecific TEM used in our experiments are true memory T cells. Although the model that we used in this study have been used by many other groups to generate memory T cells (21), concern remains whether memory T cells generated in this system differ from those generated in a physiological condition (i.e., T cell replete host). To be certain that the cells used in our study were true memory T cells, we took several measures. First, since longevity is a hallmark of memory cells, we had waited at least 8 weeks after priming before we harvested the cells to minimize the contamination of effector cells. Secondly, the donors, in whom the vast majority of memory T cells bore effector memory phenotype (Figure 2B), were able to mediate “second-set” skin rejection (Figure 2C), indicating that the donors did contain functional memory T cells. Thirdly, the cells we used in our study bore typical TEM phenotype such as high expression of CD44 and low expression of CD62L, as shown in Figure 2B and Figure 3A and B. Finally, the TEM that we used in our study mediate faster and stronger proliferative response against alloantigen (Figure 3C). All these data demonstrate that the cells utilized are fully functional memory T cells and the observations regarding the role of allospecific primed TEM in GVHD in the current study are due to true memory T cells. Why are these functional allospecific TEM unable to induce GVHD? Clonal expansion of allospecific T cells is essential for the induction of GVHD (24). In our model, clonal expansion of allospecific TEa cells can be easily tracked by flow cytometry in vivo (Figure 5A). The data obtained from this analysis demonstrate that allospecific TEM recipients have dramatically decreased numbers of TEa cells as compared with naïve TEa recipients at day +7, demonstrating that allospecific TEM do not expand to the full potential in GVHD recipients. As a result, the “cytokine storm”, a critical step in the GVHD cascade (24), might have been attenuated with allospecific TEM recipients (Figure 5C). Inability of allospecific TEM to proliferate and fully expand and the subsequent failure to produce inflammatory cytokines may explain why both TEM-like cells and TEM could not induce GVHD. Long-term follow-up of TEa cells reveals that allospecific TEa cells are clonally deleted in allospecific TEM recipients (Figure 7B), further confirming why allospecific TEM recipents become tolerant to alloantigen, never develop GVHD, and survive long-term after allogeneic hematopoietic stem cell transplantation.
Even though there is abundant evidence demonstrating weak immune responses of allospecific TEM during GVHD (Figure 4, 5, and 7), it is currently not clear why allospecific TEM do not respond well in GVHD. One of the hypotheses based on our published data using memory T cells from unprimed donors (5) is that alloresponses mediated by TEM can not be sustained (“abortive response” hypothesis) to induce GVHD. MLR data presented in Figure 3C are consistent with this hypothesis. Simultaneous detection of cell proliferation and apoptotic and necrotic death after activation (Figure 6) further demonstrate that allospecific TEM are more prone to apoptosis than naïve T cells after activation. The susceptibility of allospecific TEM to apoptosis could be a result of T cell exhaustion (26) because high expression of PD-1 is observed with these cells (Figure 3B).
However, the “abortive response” hypothesis cannot explain why allospecific TEM are able to mediate recall immune response in skin rejection setting (Figure 2C) but are unable to mediate meaningful immune response in GVHD setting. One of the major differences in these two settings is that abundant alloantigens persist long-term in GVHD setting but not in graft rejection setting. Prolonged exposure to antigens has been reported to induce clonal deletion or clonal anergy in GVHD (20, 27) and other settings (28, 29). In GVHD setting, allospecific TEM could have been deleted due to the prolonged exposure to alloantigen, leading to antigen specific tolerance (Figure 7). In graft rejection setting, alloantigens do not exist after skin grafts are rejected by allogeneic TEM. Theses allospecific TEM survive long-term. This explanation is supported by the data presented in Figure 7B. In fact, allospecific naïve T cells have also been demonstrated to become anergic or deleted after prolonged exposure to antigen (20, 27, 30, 31).
Because TEM do not express homing molecule CD62L, it was initially thought that the inability of TEM to induce GVHD was due to their inability to home to lymph node and Peyer patches (“homing” hypothesis). We were unable to test this hypothesis in the current model because very low T cell dose was used and allospecific T cells could not be detected in all recipients early after transplantation. However, published data from other and our own groups suggest that this is not the main mechanism to explain why memory T cells do not induce GVHD. First, we have demonstrated that TCM, which are able to home to secondary lymphoid organs, do not induce GVHD (22). Secondly, Anderson et al. showed that enforced constitutive expression of CD62L on TEM do not endow them with the ability to cause GVHD (32). Thirdly, secondary lymphoid organs are not required for naïve T cells to induce GVHD (33). Based on these observations, it is unlikely that the inability of allospecific TEM to induce GVHD is a result of homing defect.
In summary, we have demonstrated that allospecifc TEM are unable to induce GVHD using a novel GVHD model induced by CD4+ TCR transgenic T cells. Allospecific TEM recipients become tolerant to alloantigen as a result of clonal deletion. Even though allospecific TEM are able to respond to alloantigen initially, the expansion of these cells and inflammatory cytokine production during GVHD is dramatically decreased. The inability of allospecific TEM to sustain the alloresponse may be a result of enhanced activation induced cell death. These observations provide insights into how allospecific TEM respond to alloantigen during GVHD and underscore the fundamental difference of alloresponses mediated by allospecific TEM in graft rejection and GVHD settings.
ACKNOWLEDGMENTS
We thank Dr. Alexander Rudensky for kindly providing TEa transgenic mice. Cytokine analyses were performed in the Duke Human Vaccine Institute Immune Reconstitution & Biomarker Shared Resource Facility (Durham, NC) under the direction of Dr. Gregory D. Sempowski.
Financial support: This study was supported by National Institutes of Health grants P01-HL67314 and P01-CA47741.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure statement: All authors declare no competing financial interests.
REFERENCES
- 1.Sprent J, Tough DF. T cell death and memory. Science. 2001;293:245–248. doi: 10.1126/science.1062416. [DOI] [PubMed] [Google Scholar]
- 2.Billingham RE, Brent L, Medawar PB. Quantitative studies on tissue transplantation immunity. II. The origin, strength and duration of actively and adoptively acquired immunity. Proc R Soc Lond B Biol Sci. 1954;143:58–80. doi: 10.1098/rspb.1954.0054. [DOI] [PubMed] [Google Scholar]
- 3.Hall BM, Dorsch S, Roser B. The cellular basis of allograft rejection in vivo. II. The nature of memory cells mediating second set heart graft rejection. J Exp Med. 1978;148:890–902. doi: 10.1084/jem.148.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bingaman AW, Farber DL. Memory T cells in transplantation: Generation, function, and potential role in rejection. American Journal of Transplantation. 2004;4:846–852. doi: 10.1111/j.1600-6143.2004.00453.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L-memory T cells without graft-versus-host disease. Blood. 2004;103:1534–1541. doi: 10.1182/blood-2003-08-2987. [DOI] [PubMed] [Google Scholar]
- 6.Anderson BE, McNiff J, Yan J, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112:101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Joe G, Zhu J, et al. Dendritic cell-activated CD44hiCD8+ T cells are defective in mediating acute graft-versus-host disease but retain graft-versus-leukemia activity. Blood. 2004;103:3970–3978. doi: 10.1182/blood-2003-09-3135. [DOI] [PubMed] [Google Scholar]
- 8.Xystrakis E, Bernard I, Dejean AS, Alsaati T, Druet P, Saoudi A. Alloreactive CD4 T lymphocytes responsible for acute and chronic graft-versus-host disease are contained within the CD45RChigh but not the CD45RClow subset. Eur J Immunol. 2004;34:408–417. doi: 10.1002/eji.200324528. [DOI] [PubMed] [Google Scholar]
- 9.Spickett GP, Brandon MR, Mason DW, Williams AF, Woollett GR. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J Exp Med. 1983;158:795–810. doi: 10.1084/jem.158.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selin LK, Welsh RM. Plasticity of T cell memory responses to viruses. Immunity. 2004;20:5–16. doi: 10.1016/S1074-7613(03)00356-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Dutt S, Tseng D, Ermann J, et al. Naive and memory T cells induce different types of graft-versus-host disease. J Immunol. 2007;179:6547–6554. doi: 10.4049/jimmunol.179.10.6547. [DOI] [PubMed] [Google Scholar]
- 13.Anderson BE, Tang AL, Wang Y, et al. Enhancing alloreactivity does not restore GVHD induction but augments skin graft rejection by CD4 effector memory T cells. European journal of immunology. 2011 doi: 10.1002/eji.201141678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 15.Chen BJ, Cui X, Sempowski GD, Domen J, Chao NJ. Hematopoietic stem cell dose correlates with the speed of immune reconstitution after stem cell transplantation. Blood. 2004;103:4344–4352. doi: 10.1182/blood-2003-07-2534. [DOI] [PubMed] [Google Scholar]
- 16.Markees TG, Phillips NE, Noelle RJ, et al. Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation. 1997;64:329–335. doi: 10.1097/00007890-199707270-00026. [DOI] [PubMed] [Google Scholar]
- 17.Chang DH, Osman K, Connolly J, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams DL, Li C, Ha T, et al. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–456. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- 19.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez M, Quezada SA, Blazar BR, Panoskaltsis-Mortari A, Rudensky AY, Noelle RJ. The balance between donor T cell anergy and suppression versus lethal graft-versus-host disease is determined by host conditioning 2. J Immunol. 2002;169:5581–5589. doi: 10.4049/jimmunol.169.10.5581. [DOI] [PubMed] [Google Scholar]
- 21.Cho BK, Wang C, Sugawa S, Eisen HN, Chen J. Functional differences between memory and naive CD8 T cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2976–2981. doi: 10.1073/pnas.96.6.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen BJ, Deoliveira D, Cui X, et al. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse 1. Blood. 2007;109:3115–3123. doi: 10.1182/blood-2006-04-016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Barefoot BE, Mo W, et al. CD62L- memory T cells enhance T-cell regeneration after allogeneic stem cell transplantation by eliminating host resistance in mice. Blood. 2012 doi: 10.1182/blood-2011-03-342055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao NJ, Chen BJ. Prophylaxis and treatment of acute graft-versus-host disease. Seminars in hematology. 2006;43:32–41. doi: 10.1053/j.seminhematol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 26.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 27.Flutter B, Edwards N, Fallah-Arani F, et al. Nonhematopoietic antigen blocks memory programming of alloreactive CD8+ T cells and drives their eventual exhaustion in mouse models of bone marrow transplantation. The Journal of clinical investigation. 2010;120:3855–3868. doi: 10.1172/JCI41446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang X, Yang Y. The fate of effector CD8 T cells in vivo is controlled by the duration of antigen stimulation. Immunology. 2006;118:361–371. doi: 10.1111/j.1365-2567.2006.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 30.Choi EY, Christianson GJ, Yoshimura Y, et al. Real-time T-cell profiling identifies H60 as a major minor histocompatibility antigen in murine graft-versus-host disease. Blood. 2002;100:4259–4265. doi: 10.1182/blood-2002-05-1299. [DOI] [PubMed] [Google Scholar]
- 31.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. The Journal of experimental medicine. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson BE, Taylor PA, McNiff JM, et al. Effects of donor T-cell trafficking and priming site on graft-versus-host disease induction by naive and memory phenotype CD4 T cells. Blood. 2008;111:5242–5251. doi: 10.1182/blood-2007-09-107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welniak LA, Kuprash DV, Tumanov AV, et al. Peyer patches are not required for acute graft-versus-host disease after myeloablative conditioning and murine allogeneic bone marrow transplantation. Blood. 2006;107:410–412. doi: 10.1182/blood-2004-11-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]